Abstract
Brugada phenocopy describes conditions with Brugada‐like ECG pattern but without true congenital Brugada syndrome. We report a case of 44‐year‐old man with no known medical history who presented with loss of consciousness. Toxicology screening was positive for opiates and high serum alcohol level. His initial ECG showed Brugada type 1 pattern which resolved after several hours of observation and treatment with continuous naloxone infusion. Patient regained his consciousness and disclosed heroin abuse and drinking alcohol. This case highlights the heroin overdose as a possible cause of Brugada phenocopy.
Keywords: electrophysiology, Brugada syndrome, noninvasive techniques, electrocardiography
BACKGROUND
Brugada syndrome is a group of genetic disorders with distinct electrocardiographic (ECG) pattern, which may lead to malignant arrhythmias and sudden cardiac death.1 Brugada syndrome is mostly reported in young individuals without structural heart disease. The characteristic ECG finding in Brugada syndrome shows coved ST‐segment elevation ≥2 mm accompanied with inverted T wave in at least two precordial leads (Brugada type 1 ECG pattern). The ECG pattern in Brugada syndrome is usually dynamic so that ECG may not show the classic pattern in most of the times. However, certain medications may unmask the ECG pattern in individuals with true congenital Brugada syndrome. Another growing entity is Brugada‐like ECG pattern in individuals without true congenital Brugada syndrome. This entity is recently called as Brugada phenocopy.2–4 In this article, we report a case of Brugada phenocopy in a patient with concomitant alcohol and heroin overdose.
CASE PRESENTATION
A 44‐year‐old man with no known medical history was found unconscious and unresponsive by Emergency Medical Services. The patient regained his consciousness temporarily en route following administration of naloxone by paramedics. Upon arrival to emergency department, patient was again stuporous with limited responsiveness. The patient vital signs upon arrival were: temperature 37.2°C, pulse rate 92/min, blood pressure 122/85 mmHg, respiratory rate 18/min. Pupils were 3 mm bilaterally, round and reactive to light. His urine toxicology screen was positive for opiates, but negative for cannabinoids, barbiturates, amphetamines, benzodiazepines, and cocaine. Acetaminophen and salicylate levels were <2 and <17 mcg/ml, respectively. Serum alcohol level upon arrival to hospital was 267 mg/dl. The initial ECG in the emergency department showed normal sinus rhythm, rightward axis, and coved‐type down‐sloping ST‐segment elevation in leads V1 and V2 as well as elevated J‐point in V3 consistent with Brugada type 1 pattern (Fig. 1). Cardiac biomarker CK‐MB every 8 hours was 6.9, 3.5, and 3.0 ng/ml (normal range 0 to 3.5 ng/ml); however, troponin I was <0.02 ng/ml in all three consecutive measurements. Serum sodium and potassium levels were 136 and 3.6 mmol/l, respectively. Patient received an additional 2 mg of naloxone, and was placed on naloxone infusion 4 mg/h, subsequently. He regained his consciousness and remained clinically stable throughout hospital observation period. Following improvement of his level of consciousness, he disclosed chronic heroin use. Patient denied any prior history of syncope, or any family history of syncope or sudden death. Serial ECG demonstrated improvement of ST elevation in V1 and V2 and decrease in J‐point elevation in leads V1 and V3 (Fig. 2).
Figure 1.
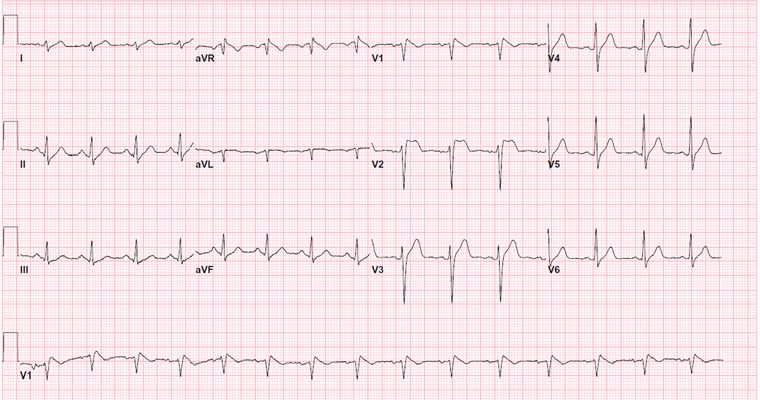
ECG on admission showing characteristic changes of Brugada type 1 ECG pattern.
Figure 2.
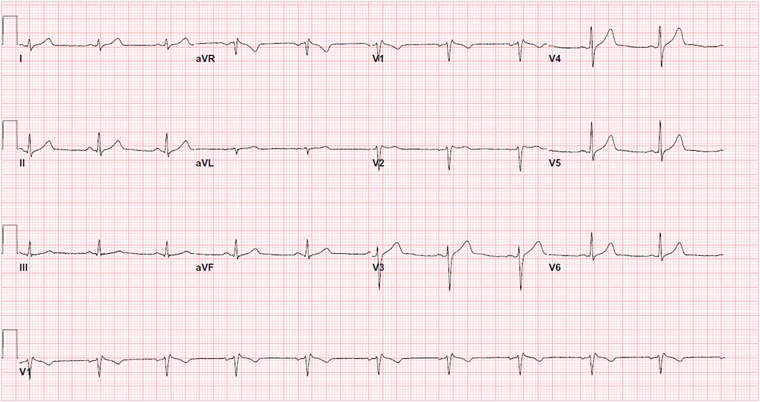
Follow‐up ECG prior to discharge, showing resolution of coved ST elevation in V1 and V2 that are characteristic of Brugada ECG pattern.
DISCUSSION
We report of a case of Brugada phenocopy in a young man with heroin overdose and alcohol toxicity. The patient did not have any medical and/or surgical history and denied any history of syncope, or a family history of syncope or sudden cardiac death. The ECG pattern resolved after several hours of observation and treatment with naloxone.
Brugada phenocopy is a new terminology that is proposed recently by Baranchuk and colleagues.2 The authors classified the Brugada‐like pattern ECG in three categories:1 manifested Brugada syndrome,2 concealed Brugada syndrome,3 and Brugada phenocopy. The latter group consists of patient with Brugada type 1 or 2 ECG pattern, which is induced by exposure to drugs or pathological conditions that are not known to be unmasking agent. The ECG pattern in Brugada phenocopy resolves once underlying condition is treated or resolved; and family history of syncope or sudden cardiac death is typically negative.2
The causes of Brugada phenocopy include metabolic conditions, ischemia, mechanical compression, and myocardial and pericardial disease. List of agents that cause Brugada phenocopy is growing consistently. This list, however, is not limited to antiarrhythmic drugs (Na+ channel blockers, Ca++ channel blockers, and beta‐blockers), and antianginal and psychotropic drugs. Few reports have suggested association between Brugada phenocopy and agents such as dimenhydrinate, cocaine, alcohol, and opioid intoxication.
Heroin‐induced ECG changes had been reported in up to 55% of heroin abusers.5 These ECG changes were mostly QTc prolongation and significant bradyarrhythmia.5, 6 Significantly high concentrations of opioids may cause alterations in action potential configuration. Krantz et al. reported association of torsades de pointes with very high‐dose methadone.6 Cole et al. reported an asymptomatic Brugada‐like ECG pattern associated with isolated tramadol overdose.7 Authors hypothesize that tramadol blockade of sodium channels might be the cause of observed ECG changes.
Although sodium channel blockade is, in general, an established method to uncover Brugada ECG changes,8 inhibition of calcium channels by alcohol may be the main mechanism of Brugada‐type ECG in alcohol intoxication.9 There are at least two documented cases of Brugada‐type ECG reported in alcohol intoxication: a sustained monomorphic ventricular tachycardia in a patient with diagnosed Brugada syndrome,10 and a Brugada‐like ECG pattern in a case of concomitant alcohol and fluoxetine intoxication.11
Our case report should be qualified with regard to its strengths and limitations. Our patient reportedly did not have a prior history of syncope or family history of sudden death and/or syncope. These features although are not exclusive, but are highly suggestive of Brugada phenocopy, rather than true Brugada syndrome. We also observed the patient for several hours while receiving continuous naloxone infusion until the ECG pattern resolved. Lack of echocardiogram, genetic studies, and provocative testing with sodium blocker agents, are the main limitations of our case report. A negative result of provocative testing with sodium channel blockers (e.g., flecainide, procainamide, or ajmaline) is another criterion in the updated definition of Brugada phenocopy.3, 4 Moreover, the concomitant overdose of heroin and alcohol makes it difficult to ascertain that the Brugada phenocopy is solely due to heroin overdose.
Heroin intoxication is associated with sudden death syndrome.12 Fatal arrhythmia has been reported as one of the causes of sudden death in heroin users irrespective of the dose and serum concentration.5 Brugada phenocopy seen in association with heroin overdose may be another cause of ECG abnormalities seen in heroin overdose and might be linked to sudden cardiac death. The role of Brugada phenocopy in heroin overdose and its association with clinical outcomes, such as sudden cardiac death, is unclear; however, physicians, especially in emergency department, should be particularly vigilant about such ECG changes, and to report any suspicious case of ECG changes to evaluate “clinical reproducibility” of our observation.4 This should be regarded in milieu of steadily increasing prevalence of heroin abuse that has risen from 373,000 in 2007 to 669,000 in 2012.13 The White House Office of National Drug Control Policy recently reported that the number of overdose deaths involving heroin increased by 45% between 2006 and 2010.
REFERENCES
- 1. Bayes de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol 2012;45(5):433–442. [DOI] [PubMed] [Google Scholar]
- 2. Baranchuk A, Nguyen T, Ryu MH, et al. Brugada phenocopy: New terminology and proposed classification. Ann Noninvasive Electrocardiol 2012;17(4):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anselm DD, Baranchuk A. Brugada phenocopy: Redefinition and updated classification. Am J Cardiol 2013;111(3):453. [DOI] [PubMed] [Google Scholar]
- 4. Genaro NR, Anselm DD, Cervino N, et al. Brugada phenocopy clinical reproducibility demonstrated by recurrent hypokalemia. Ann Noninvasive Electrocardiol 2013. Oct 23 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lipski J, Stimmel B, Donoso E. The effect of heroin and multiple drug abuse on the electrocardiogram. Am Heart J 1973;86(5):663–668. [DOI] [PubMed] [Google Scholar]
- 6. Krantz MJ, Lewkowiez L, Hays H, et al. Torsade de pointes associated with very‐high‐dose methadone. Ann Intern Med 2002;137(6):501–504. [DOI] [PubMed] [Google Scholar]
- 7. Cole JB, Sattiraju S, Bilden EF, et al. Isolated tramadol overdose associated with Brugada ECG pattern. Pacing Clin Electrophysiol 2012;35(8):e219–e221. [DOI] [PubMed] [Google Scholar]
- 8. Badani RS, Sharada K, Rao HB. C N. Brugada syndrome unmasked by sodium channel blockade. Indian Heart J 2006;58(6):447–449. [PubMed] [Google Scholar]
- 9. Habuchi Y, Furukawa T, Tanaka H, et al. Ethanol inhibition of Ca2+ and Na+ currents in the guinea‐pig heart. Eur J Pharmacol 1995;292(2):143–149. [DOI] [PubMed] [Google Scholar]
- 10. Shimada M, Miyazaki T, Miyoshi S, et al. Sustained monomorphic ventricular tachycardia in a patient with Brugada syndrome. Jpn Circ J 1996;60(6):364–370. [DOI] [PubMed] [Google Scholar]
- 11. Goldgran‐Toledano D, Sideris G, Kevorkian JP. Overdose of cyclic antidepressants and the Brugada syndrome. N Engl J Med 2002;346(20):1591–1592. [DOI] [PubMed] [Google Scholar]
- 12. Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999‐2009. Drug Alcohol Depend 2013;131(3):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H‐46, HHS Publication No (SMA) 13‐4795. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2013. [Google Scholar]


