Abstract
Background
Anterolateral myocardial infarction (MI) is traditionally defined on the electrocardiogram by ST‐elevation (STE) in I, aVL, and the precordial leads. Traditional literature holds STE in lead aVL to be associated with occlusion proximal to the first diagonal branch of the left anterior descending coronary artery. However, concomitant ischemia of the inferior myocardium may theoretically lead to attenuation of STE in aVL. We compared segmental distribution of myocardial area at risk (MaR) in patients with and without STE in aVL.
Methods
We identified patients in the MITOCARE study presenting with a first acute MI and new STE in two contiguous anterior leads from V1 to V6, with or without aVL STE. Patients underwent cardiac magnetic resonance imaging 3–5 days after acute infarction for quantitative assessment of MaR.
Results
A total of 32 patients met inclusion criteria; 13 patients with and 19 without STE in lead aVL. MaR > 20% at the basal anterior segment was seen in 54% of patients with aVL STE, and 11% of those without (p = 0.011). MaR > 20% at the apical inferior segment was seen in 62% and 95% of patients with and without aVL STE, respectively (p = 0.029). The total MaR was not different between groups (44% ± 10% and 39% ± 8.3% respectively, p = 0.15).
Conclusion
Patients with anterior STEMI and concomitant STE in aVL have less MaR in the apical inferior segment and more MaR in the basal anterior segment.
1. INTRODUCTION
Acute anterolateral ST‐elevation myocardial infarction (STEMI) is defined by ST‐elevation (STE) in the precordial leads V1–V6 in addition to leads I and aVL. STE in lead aVL has been associated with infarct‐related occlusion proximal to the first diagonal branch (D1) of the left anterior descending coronary artery (LAD; Arbane & Goy, 2000; Birnbaum et al., 1993; Yoshino et al., 2000).
It has been recognized that anterior STEMI may also affect the ST segment in the inferior leads. Sasaki, Yotsukura, Sakata, Yoshino, and Ishikawa (2001) using coronary angiography, suggested that ST‐segment changes in the inferior leads in acute anterior myocardial infarction (MI) may result from competition between the reciprocal changes of the anterolateral wall STEMI due to proximal LAD artery occlusion, causing ST segment depression, and inferoapical wall ischemia due to a long, wrap‐around LAD artery causing STE, corroborating prior work (Birnbaum et al., 1994; Sapin, Musselman, Dehmer, & Cascio, 1992; Tamura, Kataoka, Nagase, Mikuriya, & Nasu, 1995).
Therefore, STE may not be present in aVL in the case of concurrent ischemia of the anterolateral and the inferior myocardium. In the case of anterolateral STEMI, we hypothesize that an ischemic vector directed toward the inferior myocardium may attenuate the ischemic vector directed toward lead aVL. Hence, it is possible that absence of STE in lead aVL represents either proximal occlusion of a wrap‐around LAD artery supplying the inferior myocardium or distal occlusion of a short LAD artery (Sasaki et al., 2001).
This question has heretofore not been investigated using direct imaging of the ischemic myocardium, and we set out to examine the relationship between the presence of STE in aVL during acute anterior STEMI and myocardium at risk (MaR) assessed by cardiac magnetic resonance imaging (CMR).
2. METHODS
2.1. Patients
This analysis is a substudy of the MITOCARE study, a European based phase II, multicenter, randomized, double‐blind, placebo‐controlled study assessing the use of TRO40303 for reduction of reperfusion injury in patients undergoing percutaneous coronary intervention (PCI) for acute STEMI (Atar et al., 2015; MITOCARE Study Group, 2012).
All patients had new STE at the J‐point in ≥ two contiguous leads with cutoff points: >0.2 mV in males or > 0.15 mV in females in leads V2–V3 and/or > 0.1 mV in other leads. Additional details of the MITOCARE study have been previously published in full (Atar et al., 2015; MITOCARE Study Group, 2012).
Electrocardiograms (ECG) were recorded at hospital admission, with analyses performed in a core laboratory (Rigshospitalet, Copenhagen, Denmark). ST‐segment deviation was measured manually to the nearest 0.5 mm at the J‐point in all 12 ECG leads. Three to five days after STEMI, CMR was performed on whole‐body 1.5 T magnetic resonance scanners with cardiac applications used for standard clinical CMR.
In this study, we included patients with STE in two or more of the precordial leads V1–V3 with or without STE in additional precordial leads. Patients with wide QRS or pacemaker rhythm on the admission ECG were excluded.
2.2. Cardiac magnetic resonance imaging
2.2.1. Image acquisition
The CMR images were acquired in multiple centers (n = 10) across Europe on either a 1.5 T or 3 T scanner. Contrast‐enhanced steady‐state free precession (CE‐SSFP) short‐axis cine images of the left ventricle (LV) were obtained 5 min after intravenous injection of a gadolinium‐based contrast agent (0.2 mmol/kg). Approximately 15 min after the contrast injection, late gadolinium enhancement (LGE) images were acquired in the same image planes as the CE‐SSFP (Atar et al., 2015).
2.2.2. Image analysis
Left ventricular function and MaR were assessed from the CE‐SSFP short‐axis cine images. Endo‐ and epicardial borders and MaR were manually delineated in both end‐systole and end‐diastole as previously described (Sorensson et al., 2010). MaR was expressed both as a percentage of the LV and as a percentage of each of the 17 segments pertaining to the American Heart Association 17‐segment model for uniform description of myocardial segments (Cerqueira et al., 2002). Infarct size was assessed on LGE images using an automated algorithm (Heiberg et al., 2008). Manual corrections were done if required. All CMR analyses were performed by a core laboratory (Imacor AB, Lund, Sweden) blinded to clinical data using the software Segment 1.9 (Medviso AB, Lund Sweden; Heiberg et al., 2010).
2.3. Statistical methods
Categorical variables were compared with Fisher's exact test, and continuous variables were compared with two‐sample Student's t test. A p‐value < 0.05 was considered to be statistically significant. Where appropriate, values are expressed as mean ± standard deviation (SD). Categorical variables are described as frequencies and percentages.
3. RESULTS
3.1. Patient characteristics
A total of 32 patients met the inclusion criteria. About 19 patients (59%) were male and 17 patients (53%) had a history of prior or present tobacco use. The mean age of patients was 72.7 ± 11.3 years. Table 1 summarizes the demographic characteristics of the patients with and without STE in aVL, which did not differ significantly between the two groups.
Table 1.
Patient characteristics in the anterior ST‐elevation distributions
| ECG STE distribution | p value | ||
|---|---|---|---|
| aVL STE | No aVL STE | ||
| Number of patients | 13 | 19 | – |
| Age (years) | 60 ± 12 | 66 ± 9.6 | 0.18 |
| Male, n (%) | 9 (69%) | 10 (53%) | 0.47 |
| Tobacco use history, n (%) | 9 (69%) | 8 (42%) | 0.17 |
| Admission peak troponin I (ng/ml) | 162 ± 124 | 98 ± 77 | 0.058 |
| Myocardium at risk (%) | 44 ± 10 | 39 ± 8.3 | 0.15 |
| Infarct size (g) | 30 ± 19 | 24 ± 8.2 | 0.22 |
| IS/MaR (%) | 46 ± 21 | 49 ± 16 | 0.66 |
| LVEF (%) | 45 ± 9.4 | 47 ± 11 | 0.57 |
ECG: electrocardiogram; IS: infarct size; MaR: myocardium at risk; LVEF: left ventricular ejection fraction; STE: ST‐elevation.
3.2. STE pattern versus LV ischemia, infarction, and function
Patients were categorized according to the presence of STE > 0.1 mV in lead aVL on the admission ECG. About 13 patients (41%) demonstrated STE in aVL, and 19 patients (59%) did not.
MaR was 44% ± 10% of the LV in patients with STE in aVL and 39%±8.3% in patients without STE in aVL, respectively (p = 0.15). Infarct size (IS) was 30 g ± 19 g and 24 g ± 8.2 g in the same groups, respectively (p = 0.22). Mean left ventricular ejection fraction (LVEF) was 45%±9.4% and 47% ± 11% in these same groups (p = 0.57). Similarly, there was no statistically significant difference in admission peak troponin or myocardial salvage index (IS/MaR) between the two groups (Table 1).
The extent of MaR across the 17 myocardial segments is illustrated in Table 2. MaR > 20% was seen in seven (54%) and two (11%) of patients with and without aVL STE at the basal anterior segment (segment 1), respectively (p = 0.011). At the apical inferior segment (segment 15), eight (62%) and 18 (95%) of patients with and without aVL STE, respectively showed MaR > 20% (p = 0.029). No statistically significant difference in frequency of MaR > 20% was observed for the remainder of the myocardial segments.
Table 2.
Number of patients with > 20% myocardium at risk assessed by cardiac magnetic resonance imaging and grouped by electrocardiographic ST‐elevation distribution
| Segment number | Myocardial segment | ECG STE distribution | p value | |
|---|---|---|---|---|
| With aVL STE (n, %) | Without aVL STE (n, %) | |||
| 1 | Basal anterior | 7 (54) | 2 (11) | 0.011 |
| 2 | Basal anteroseptal | 7 (54) | 3 (16) | 0.05 |
| 3 | Basal inferoseptal | 1 (8) | 1 (5) | 0.66 |
| 4 | Basal inferior | 0 (0) | 0 (0) | 1 |
| 5 | Basal inferolateral | 0 (0) | 0 (0) | 1 |
| 6 | Basal anterolateral | 0 (0) | 0 (0) | 1 |
| 7 | Mid anterior | 12 (92) | 15 (79) | 0.31 |
| 8 | Mid anteroseptal | 12 (92) | 18 (95) | 0.66 |
| 9 | Mid inferoseptal | 9 (69) | 11 (58) | 0.71 |
| 10 | Mid inferior | 0 (0%) | 1 (5) | 1 |
| 11 | Mid inferolateral | 0 (0) | 0 (0) | 1 |
| 12 | Mid anterolateral | 5 (38) | 2 (11) | 0.08 |
| 13 | Apical anterior | 13 (100) | 18 (95) | 1 |
| 14 | Apical septal | 13 (100) | 19 (100) | 1 |
| 15 | Apical inferior | 8 (62) | 18 (95) | 0.029 |
| 16 | Apical lateral | 9 (69) | 7 (37) | 0.15 |
| 17 | Apex | 13 (100) | 19 (100) | 1 |
ECG: electrocardiogram; STE: ST‐elevation.
A comparison of all patients exhibiting > 20% MaR at the apical lateral segment revealed greater involvement of the apical inferior segment (segment 15) relative to the apical lateral segment (segment 16) in patients without aVL STE (Table 3). For patients without STE in aVL (n = 7) the ratio of apical inferior MaR/apical lateral MaR was 1.28 compared to 1.08 for those with STE in aVL (n = 9).
Table 3.
Comparison of mean myocardium at risk for all patients exhibiting > 20% myocardium at risk at the apical lateral segments
| Myocardial segment | ECG STE distribution | p value | |
|---|---|---|---|
| With aVL STE (%) (n = 9) | Without aVL STE (%) (n = 7) | ||
| Apical inferior | 51 ± 29 | 66 ± 25 | 0.28 |
| Apical lateral | 51 ± 19 | 52 ± 12 | 0.98 |
| Apical inferior MaR/Apical lateral MaR | 1.1 ± 0.72 | 1.3 ± 0.32 | 0.50 |
ECG: electrocardiogram; MaR: myocardium at risk; STE: ST‐elevation.
A comparison of infarct size in each of the 17 segments is presented in Table 4. Of note, the apical inferior segment (segment 15) demonstrated significantly greater infarct size in patients without STE in aVL (28%) compared to those with aVL STE (11%) (p = 0.02). Furthermore, >20% involvement of the apical inferior segment was seen in three patients (23%) with STE in aVL versus eight patients (47%) in those without STE in aVL (p = 0.26) and zero versus four (24%) had > 50% scar of the apical inferior segment, respectively (p = 0.11). There were no statistically significant differences in the extent of infarction between the group with and without STE in aVL in all the remaining myocardial segments.
Table 4.
Comparison of mean late gadolinium enhancement assessed by cardiac magnetic resonance imaging among the myocardial segments with and without avL ST‐elevation
| Segment number | Myocardial segment | ECG STE distribution | p value | |
|---|---|---|---|---|
| With aVL STE (LGE %) | Without aVL STE (LGE %) | |||
| 1 | Basal anterior | 4.6 ± 5.7 | 1.7 ± 3.6 | 0.10 |
| 2 | Basal anteroseptal | 11.9 ± 12.3 | 5.7 ± 11 | 0.16 |
| 3 | Basal inferoseptal | 2.2 ± 5.4 | 0.94 ± 3.5 | 0.45 |
| 4 | Basal inferior | 0 ± 0 | 0.26 ± 1.1 | 0.39 |
| 5 | Basal inferolateral | 0.036 ± 0.13 | 0 ± 0 | 0.26 |
| 6 | Basal anterolateral | 0.60 ± 1.6 | 0 ± 0 | 0.13 |
| 7 | Mid anterior | 32 ± 24 | 18 ± 16 | 0.06 |
| 8 | Mid anteroseptal | 37 ± 23 | 34 ± 21 | 0.66 |
| 9 | Mid inferoseptal | 9.7 ± 9.1 | 12 ± 16 | 0.65 |
| 10 | Mid inferior | 0.0033 ± 0.012 | 2.1 ± 5.2 | 0.18 |
| 11 | Mid inferolateral | 1.5 ± 5.0 | 0 ± 0 | 0.23 |
| 12 | Mid anterolateral | 8.1 ± 14 | 1.3 ± 2.8 | 0.06 |
| 13 | Apical anterior | 40 ± 22 | 32 ± 18 | 0.27 |
| 14 | Apical septal | 45 ± 23 | 55 ± 15 | 0.19 |
| 15 | Apical inferior | 11 ± 12 | 28 ± 21 | 0.02 |
| 16 | Apical lateral | 13 ± 18 | 6.2 ± 9.0 | 0.17 |
| 17 | Apex | 43 ± 24 | 52 ± 19 | 0.26 |
CMR: cardiac magnetic resonance; ECG: electrocardiogram; LGE: late gadolinium enhancement; STE: ST‐elevation.
4. DISCUSSION
4.1. Principal analysis
We investigated whether the absence of STE in lead aVL during acute anterior STEMI was associated with involvement of the inferior myocardium by CMR, which permits accurate evaluation of both MaR and myocardial infarction. Our study builds on prior work, as CMR better delineates the extent of myocardial ischemia and infarct in the acute setting, compared to echocardiography. Figure 1 depicts CMR images of two study patients: one with aVL STE and one without.
Figure 1.
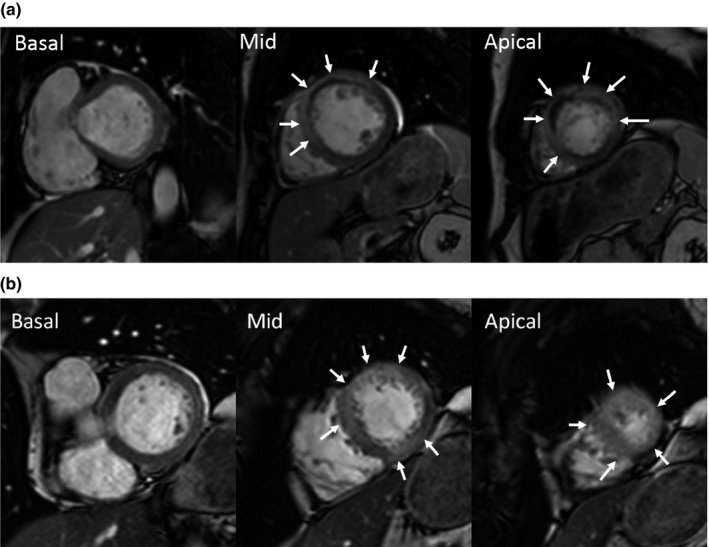
Contrast‐enhanced cine images from cardiac magnetic resonance in three short axis slices where the myocardium at risk (MaR) is indicated by arrows. In patient (a) with ST‐elevation in aVL there is MaR in the apical lateral segment but no inferior MaR. Conversely, in patient (b) without STE in the aVL there is MaR in the midventricular and apical inferior segment, corresponding to a large wrap‐around of the LAD coronary artery. However, there is no lateral MaR in the midventricular segment
The myocardium suffering from ischemia during an acute coronary occlusion (the MaR) will change its tissue properties, both in the irreversibly injured myocardium (infarction) and in the reversibly injured, salvaged myocardium. These changes include increased myocardial extracellular volume fraction (Jablonowski et al., 2015) and alterations in T1 and T2 properties which can be utilized for quantification of MaR using CE‐SSFP by MR (Nordlund et al., 2017; Sorensson et al., 2010). MaR by CE‐SSFP has been shown to be stable over the first week after the acute event (Nordlund, Klug, et al., 2016) and this technique enables detailed depiction of MaR for all the major coronary arteries (Nordlund, Heiberg, et al., 2016). Infarct size can be assessed by LGE MRI (Kim et al., 1999) and compared to the initial MaR for calculation of myocardial salvage. Advanced CMR techniques allow for assessment of MaR and infarction with higher sensitivity than other imaging modalities (Beek & van Rossum, 2010).
It is commonly believed that STE in aVL represents “high lateral” ischemia due to involvement of the first diagonal branch (Sapin et al., 1992; Tamura et al., 1995). Of note, no patients, including those with STE in aVL, showed MaR involving the basal anterolateral or basal inferolateral segments. Even involvement of the mid anterolateral segment was seen in only 38% of the patients with STE in aVL. In contrast, apical lateral involvement was seen in 69% of the patients with STE in aVL versus 37% of those without STE in aVL (Table 2), suggesting that STE in aVL signifies more distal lateral ischemia. Among patients with > 20% MaR involvement of the apical lateral segment, those without STE in aVL tended to have greater apical inferior MaR/apical lateral MaR. Although the comparison between these small subgroups was not statistically significant, this suggests that STE in aVL is attenuated in patients with more apical inferior involvement (Table 3).
Patients with STE in V1–V6 and no STE in aVL exhibited significantly greater MaR at the apical inferior segment (segment 15) than those with STE in aVL (Table 2). Furthermore, we observed a trend toward greater MaR involvement of the apical inferior segment (segment 15) in patients with apical lateral segment (segment 16) involvement and no STE in aVL (Table 3). Patients without aVL STE also developed greater infarct size solely in the apical inferior segment compared to those with aVL STE (Table 4). While there was a trend toward lesser involvement of the basal anteroseptal segment (segment 2) (p = 0.05), there was no demonstrable difference in MaR of the basal anterolateral segment (segment 6) (Table 2).
In contrast, significantly less frequent > 20% MaR of the basal anterior segment (segment 1) was seen in those without aVL STE (Table 2). However, there were no differences in eventual infarct size between groups in the basal anterior or anteroseptal segments (Table 4). The remainder of the myocardial segments exhibited no differences in myocardial edema or infarct size.
These data support the hypothesis that inferoapical ischemia occurring simultaneously with anterolateral wall ischemia may lead to the absence of STE in aVL, as the loss of electromotive forces in the inferoapical segment opposes the ischemic vector directed to the anterolateral territories. This parallels a previous study of ventriculography and ECGs in patients with proximal LAD occlusion (Takatsu, Osugi, & Nagaya, 1986) which suggested that the absence of Q waves in leads I and aVL could not be used to rule out extension to the high lateral wall in the patient with anterolateral MI. Our findings also complement investigations of inferior lead STE in the setting of anterior MI (Sapin et al., 1992; Sasaki et al., 2001; Tamura et al., 1995).
An explanation for these findings can be found in the coronary anatomy. Angiographically, STE in aVL is associated with proximal LAD artery occlusion before D1, (Birnbaum et al., 1993), and we accordingly observed a greater frequency of basal anterior segment (segment 1) involvement in patients with aVL STE. Infarct‐related lesions of the proximal LAD are associated with STE in high lateral leads, (Arbane & Goy, 2000; Birnbaum et al., 1993; Yoshino et al., 2000) but this may not hold true in patients with a long, wrap‐around LAD artery (Sasaki et al., 2001).
The LAD artery courses along the anterior ventricular groove toward the apex of the left ventricle, supplying branches to the septum and free wall. The LAD artery is considered to be long and wrapped if it observed to perfuse at least one quarter of the inferior myocardial wall of the LV (Lew, Hod, Cercek, Shah, & Ganz, 1987; Sasaki et al., 2001). Correlating echocardiographic and angiographic studies, Sasaki et al. found that patients with anterior STEMI and no STE in inferior leads were predominantly of two types: (a) patients with a proximal LAD lesion, wrap‐around LAD artery, and resultant large infarction and (b) patients with a distal LAD lesion, nonwrapped LAD artery, and resultant small infarction (Sasaki et al., 2001). This mixture of patients with both the most and least extensive infarctions was deemed to have resulted in the absence of a significant difference in the infarction extent in these patients compared to the group with inferior ST depressions. In our present investigation, the percentage of MaR, total infarct size, and mean LVEF did not differ significantly between patients with and without aVL STE (Table 1).
The clinician encountering a patient with anterior STEMI must recognize that although ST‐elevation in aVL traditionally suggests proximal LAD occlusion with more basal involvement, this is not a sensitive marker. Proximal occlusion of a long wrap‐around LAD with a large ischemic area at risk may lead to absence of ST‐elevation in aVL, as the ischemic vectors directed toward inferoapical myocardium attenuate those directed toward lead aVL.
4.2. Future research
Continued evaluations of traditional ECG nomenclature are needed, especially with regards to territorial descriptions of MI, and the field remains fertile (Beek & van Rossum, 2010). CMR is proving to be invaluable in the noninvasive assessment of infarction without the use of radiation. Perhaps wider availability and decreasing expense of this technology will permit increased use in clinical and research settings.
4.3. Limitations
Due to the specific ECG inclusion criteria, a small number of patients in this study were included. We were not able to explore other clinical factors such as the role of gender and differences in timing in the ischemic injury evolution (e.g., pain to balloon time).
5. CONCLUSION
Patients with anterior STEMI and no STE in aVL demonstrate significantly greater MaR and infarct size in the apical inferior myocardium compared to those with STE in aVL. This same group demonstrates significantly less frequent MaR at the basal anterior segment although there is no significant difference in infarct size at this segment. Anterior STEMI without aVL STE may represent either distal occlusion of the LAD or proximal occlusion of a long, wrap‐around LAD artery. In the latter scenario, we believe the ischemic vector of the consequently affected inferoapical myocardium offsets that directed toward lead aVL, resulting in STE diminution in this lead. Appreciating such potential variations in coronary anatomy is important in predicting the ultimate extent and location of affected myocardium during anterior MI.
CONFLICT OF INTEREST
Einar Heiberg is founder of Medviso AB, developing cardiovascular image processing software. Marcus Carlsson and Henrik Engblom are consultants at Imacor AB. Håkan Arheden is founder of and employed by Imacor AB. Maria Sejersten Ripa is employed full‐time by Novo Nordisk A/S.
ACKNOWLEDGMENTS
The MITOCARE project was supported by the European Union under the 7th Framework Programme—Grant Agreement HEALTH‐2010‐261034.
Allencherril J, Fakhri Y, Engblom H, et al. The significance of ST‐elevation in aVL in anterolateral myocardial infarction: An assessment by cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol. 2018;23:e12580 10.1111/anec.12580
REFERENCES
- Arbane, M. , & Goy, J.‐J. (2000). Prediction of the site of total occlusion in the left anterior descending coronary artery using admission electrocardiogram in anterior wall acute myocardial infarction. The American Journal of Cardiology, 85(4), 487–491. 10.1016/S0002-9149(99)00777-8 [DOI] [PubMed] [Google Scholar]
- Atar, D. , Arheden, H. , Berdeaux, A. , Bonnet, J. L. , Carlsson, M. , Clemmensen, P. , … Jensen, S. E. (2015). Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST‐elevation myocardial infarction: MITOCARE study results. European Heart Journal, 36(2), 112–119. 10.1093/eurheartj/ehu331. [DOI] [PubMed] [Google Scholar]
- Beek, A. M. , & van Rossum, A. C. (2010). Cardiovascular magnetic resonance imaging in patients with acute myocardial infarction. Heart (British Cardiac Society), 96(3), 237–243. 10.1136/hrt.2009.172296. [DOI] [PubMed] [Google Scholar]
- Birnbaum, Y. , Sclarovsky, S. , Solodky, A. , Tschori, J. , Herz, I. , Sulkes, J. , … Rechavia, E. (1993). Prediction of the level of left anterior descending coronary artery obstruction during anterior wall acute myocardial infarction by the admission electrocardiogram. The American Journal of Cardiology, 72(11), 823–826. 10.1016/0002-9149(93)91071-O [DOI] [PubMed] [Google Scholar]
- Birnbaum, Y. , Solodky, A. , Herz, I. , Kusniec, J. , Rechavia, E. , Sulkes, J. , & Sclarovsky, S. (1994). Implications of inferior ST‐segment depression in anterior acute myocardial infarction: Electrocardiographic and angiographic correlation. American Heart Journal, 127(6), 1467–1473. 10.1016/0002-8703(94)90372-7 [DOI] [PubMed] [Google Scholar]
- Cerqueira, M. D. , Weissman, N. J. , Dilsizian, V. , Jacobs, A. K. , Kaul, S. , Laskey, W. K. , … Verani, M. S. (2002). Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation, 105(4), 539–542. 10.1161/hc0402.102975 [DOI] [PubMed] [Google Scholar]
- Heiberg, E. , Sjögren, J. , Ugander, M. , Carlsson, M. , Engblom, H. , & Arheden, H. (2010). Design and validation of Segment‐freely available software for cardiovascular image analysis. BMC Medical Imaging, 10(1), 1 10.1186/1471-2342-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiberg, E. , Ugander, M. , Engblom, H. , Gotberg, M. , Olivecrona, G. K. , Erlinge, D. , & Arheden, H. (2008). Automated quantification of myocardial infarction from MR images by accounting for partial volume effects: Animal, phantom, and human study. Radiology, 246(2), 581–588. 10.1148/radiol.2461062164. [DOI] [PubMed] [Google Scholar]
- Jablonowski, R. , Engblom, H. , Kanski, M. , Nordlund, D. , Koul, S. , van der Pals, J. , … Carlsson, M. (2015). Contrast‐enhanced CMR overestimates early myocardial infarct size: Mechanistic insights using ECV measurements on day 1 and day 7. JACC: Cardiovascular Imaging, 8(12), 1379–1389. [DOI] [PubMed] [Google Scholar]
- Kim, R. J. , Fieno, D. S. , Parrish, T. B. , Harris, K. , Chen, E.‐L. , Simonetti, O. , … Judd, R. M. (1999). Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation, 100(19), 1992–2002. 10.1161/01.CIR.100.19.1992 [DOI] [PubMed] [Google Scholar]
- Lew, A. S. , Hod, H. , Cercek, B. , Shah, P. K. , & Ganz, W. (1987). Inferior ST segment changes during acute anterior myocardial infarction: A marker of the presence or absence of concomitant inferior wall ischemia. Journal of the American College of Cardiology, 10(3), 519–526. 10.1016/S0735-1097(87)80193-6 [DOI] [PubMed] [Google Scholar]
- MITOCARE Study Group (2012). Rationale and design of the ‘MITOCARE’ Study: A phase II, multicenter, randomized, double‐blind, placebo‐controlled study to assess the safety and efficacy of TRO40303 for the reduction of reperfusion injury in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Cardiology, 123(4), 201–207. 10.1159/000342981. [DOI] [PubMed] [Google Scholar]
- Nordlund, D. , Heiberg, E. , Carlsson, M. , Fründ, E.‐T. , Hoffmann, P. , Koul, S. , … Engblom, H. (2016). Extent of myocardium at risk for left anterior descending artery, right coronary artery, and left circumflex artery occlusion depicted by contrast‐enhanced steady state free precession and T2‐weighted short tau inversion recovery magnetic resonance Imaging CLINICAL PERSPECTIVE. Circulation: Cardiovascular Imaging, 9(7), e004376. [DOI] [PubMed] [Google Scholar]
- Nordlund, D. , Kanski, M. , Jablonowski, R. , Koul, S. , Erlinge, D. , Carlsson, M. , … Arheden, H. (2017). Experimental validation of contrast‐enhanced SSFP cine CMR for quantification of myocardium at risk in acute myocardial infarction. Journal of Cardiovascular Magnetic Resonance, 19(1), 12 10.1186/s12968-017-0325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund, D. , Klug, G. , Heiberg, E. , Koul, S. , Larsen, T. H. , Hoffmann, P. , … Aletras, A. H. (2016). Multi‐vendor, multicenter comparison of contrast‐enhanced SSFP and T2‐STIR CMR for determining myocardium at risk in ST‐elevation myocardial infarction. European Heart Journal‐Cardiovascular Imaging, 17(7), 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapin, P. M. , Musselman, D. R. , Dehmer, G. J. , & Cascio, W. E. (1992). Implications of inferior ST‐segment elevation accompanying anterior wall acute myocardial infarction for the angiographic morphology of the left anterior descending coronary artery morphology and site of occlusion. The American Journal of Cardiology, 69(9), 860–865. 10.1016/0002-9149(92)90783-U [DOI] [PubMed] [Google Scholar]
- Sasaki, K. , Yotsukura, M. , Sakata, K. , Yoshino, H. , & Ishikawa, K. (2001). Relation of ST‐segment changes in inferior leads during anterior wall acute myocardial infarction to length and occlusion site of the left anterior descending coronary artery. The American Journal of Cardiology, 87(12), 1340–1345. 10.1016/S0002-9149(01)01549-1 [DOI] [PubMed] [Google Scholar]
- Sorensson, P. , Heiberg, E. , Saleh, N. , Bouvier, F. , Caidahl, K. , Tornvall, P. , … Arheden, H. (2010). Assessment of myocardium at risk with contrast enhanced steady‐state free precession cine cardiovascular magnetic resonance compared to single‐photon emission computed tomography. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic Resonance, 12, 25 10.1186/1532-429X-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu, F. , Osugi, J. , & Nagaya, T. (1986). Is it possible to rule out extensive anterior myocardial infarction in the absence of abnormal Q waves in lead I and aVL? Effect of infero‐apical extension of infarction over apex. Japanese Circulation Journal, 50(7), 601–606. 10.1253/jcj.50.601 [DOI] [PubMed] [Google Scholar]
- Tamura, A. , Kataoka, H. , Nagase, K. , Mikuriya, Y. , & Nasu, M. (1995). Clinical significance of inferior ST elevation during acute anterior myocardial infarction. Heart (British Cardiac Society), 74(6), 611–614. 10.1136/hrt.74.6.611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino, H. , Kachi, E. , Shimizu, H. , Taniuchi, M. , Yano, K. , Udagawa, H. , … Ishikawa, K. (2000). Severity of residual stenosis of infarct‐related lesion and left ventricular function after single‐vessel anterior wall myocardial infarction: Implication of st‐segment elevation in lead avl of the admission electrocardiograms. Clinical Cardiology, 23(3), 175–180. 10.1002/clc.4960230309 [DOI] [PMC free article] [PubMed] [Google Scholar]


