Abstract
Background
Assessment of myocardial infarct (MI) size is important for therapeutic and prognostic reasons. We used body surface potential mapping (BSPM) to evaluate whether single‐lead electrocardiographic variables can assess MI size.
Methods
We performed BSPM with 120 leads covering the front and back chest (plus limb leads) on 57 patients at different phases of MI: acutely, during healing, and in the chronic phase. Final MI size was determined by contrast‐enhanced cardiac magnetic resonance imaging (DE‐CMR) and correlated with various computed depolarization‐ and repolarization‐phase BSPM variables. We also calculated correlations between BSPM variables and enzymatic MI size (peak CK‐MBm).
Results
BSPM variables reflecting the Q‐ and R wave showed strong correlations with MI size at all stages of MI. R width performed the best, showing its strongest correlation with MI size on the upper right back, there representing the width of the “reciprocal Q wave” (r = 0.64–0.71 for DE‐CMR, r = 0.57–0.64 for CK‐MBm, P < 0.0001). Repolarization‐phase variables showed only weak correlations with MI size in the acute phase, but these correlations improved during MI healing. T‐wave variables and the QRSSTT integral showed their best correlations with DE‐CMR defined MI size on the precordial area, at best r = −0.57, P < 0.0001 in the chronic phase. The best performing BSPM variables could differentiate between large and small infarcts at all stages of MI.
Conclusions
Computed, single‐lead electrocardiographic variables can estimate the final infarct size at all stages of MI, and differentiate large infarcts from small.
Keywords: electrocardiography, body surface potential mapping, cardiac magnetic resonance imaging, myocardial infarction, myocardial infarct size
After acute myocardial infarction (MI), an important prognostic factor for both patient outcome and left ventricular (LV) function is the size of the MI. Accurate and precise noninvasive methods are nowadays available for determination of MI size and location. Yet these methods, such as cardiac magnetic resonance imaging (CMR), myocardial perfusion, and metabolic imaging techniques, are not readily available. Electrocardiography (ECG), in contrast, is easy and quick to perform even at the bedside; 12‐lead ECG is routinely performed on patients with cardiac symptoms, and is the most important clinical tool in MI diagnosis.
Body surface potential mapping (BSPM), an electrocardiographic measurement that uses multiple leads covering the front and back chest, records electrical activation of the heart from a much larger thoracic surface area than does the conventional 12‐lead ECG.1 BSPM has been shown to be more sensitive in the detection of acute as well as chronic MI.2, 3 A variety of ECG variables, such as amplitudes and time‐voltage integrals from depolarization as well as repolarization phases, can be computed and automatically analyzed from BSPM data. Our group found earlier that computed ECG variables improve sensitivity in detection of chronic and subacute MI as compared to Q‐wave analysis from 12‐lead ECG.4 We have also shown that in acute MI patients, computed ECG variables can predict recovery of myocardial function.5 Although ECG is routine in MI diagnostics, assessment of MI size by ECG is not current clinical practice. Our aim was to learn whether single‐lead ECG variables computed from BSPM recordings could serve in MI quantification. In different stages of MI, from the acute phase to complete healing, we sought the best ECG variables and the best thoracic recording locations for assessment of MI size.
METHODS
Patients
Patients with acute coronary syndrome were recruited during office hours at the Coronary Care Unit of Helsinki University Central Hospital. The inclusion criterion was chest pain lasting a minimum of 20 minutes within 48 hours of recruitment, associated with findings suggestive of acute ischemia in the initial 12‐lead ECG (ST‐segment elevation, ‐depression, or T‐wave inversions in ≥2 contiguous leads). Exclusion criteria were bundle branch block, atrial fibrillation, or pacemaker. Of the 79 patients initially recruited, only the data of those 57 participating in the final control were included in analyses.
For study subject characteristics, see Table 1. The clinicians’ initial 12‐lead ECG interpretation was ST‐elevation MI (STEMI) in 41 patients, and acute coronary syndrome without ST elevations in 16. Most of the patients, 50, had a release of cardiac biomarkers indicating acute MI (CK‐MBm > 5 μg/L or TnT > 0.03 μg/L). All patients underwent coronary angiography in the acute phase, and those 55 with significant stenosis (>50% of luminal diameter) were revascularized by percutaneous coronary intervention (PCI) or coronary artery bypass grafting. In patients with STEMI, time to thrombolysis or primary PCI from onset of symptoms was 4.9 ± 6.4 hours.
Table 1.
Patient Baseline Characteristics
| Patients (n) | 57 (male 45) |
|---|---|
| Age (years) | 60.1 ± 10.0 |
| BMI (kg/m2) | 27.0 ± 4.2 |
| Previous MI in clinical history (n) | 8 |
| Hypertension (n) | 23 |
| Maximal CK‐MBm (μg/L) | 137 ± 163 |
| Culprit artery (n) | |
| LAD | 33 |
| RCA | 16 |
| LCX | 8 |
Data presented as number (n) or mean ± SD.
BMI = body mass index; MI = myocardial infarction; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; RCA = right coronary artery.
All study subjects gave their written informed consent. The research protocol was approved by the local ethics committee and complied with the Declaration of Helsinki.
Body Surface Potential Mapping
Recording and Analysis of BSPM
BSPM was recorded at inclusion, within 48 hours (10.7 ± 14.4) after the onset of chest pain. The acute BSPM measurement was done in 38 patients following urgent reperfusion therapy (thrombolysis or PCI), while 19 patients had stabilized spontaneously before the recording. Recording of BSPM was repeated during recovery at 16 ± 14 days, and then again in the chronic phase after complete healing of the MI, at 331 ± 111 days after the index event.
Resting‐BSPM for 5 minutes was recorded with 120 unipolar leads covering the whole thorax (plus limb leads) using one Mark 6 and one ActiveTwo biopotential amplifier from Biosemi (Amsterdam, The Netherlands). Wilson's central terminal, defined from the limb leads, served as the reference potential for the chest leads. The electrodes were mounted on 18 strips, 5 cm apart, placed on the subject's thorax vertically with horizontal spacing determined by upper body dimensions (Fig. 1).
Figure 1.
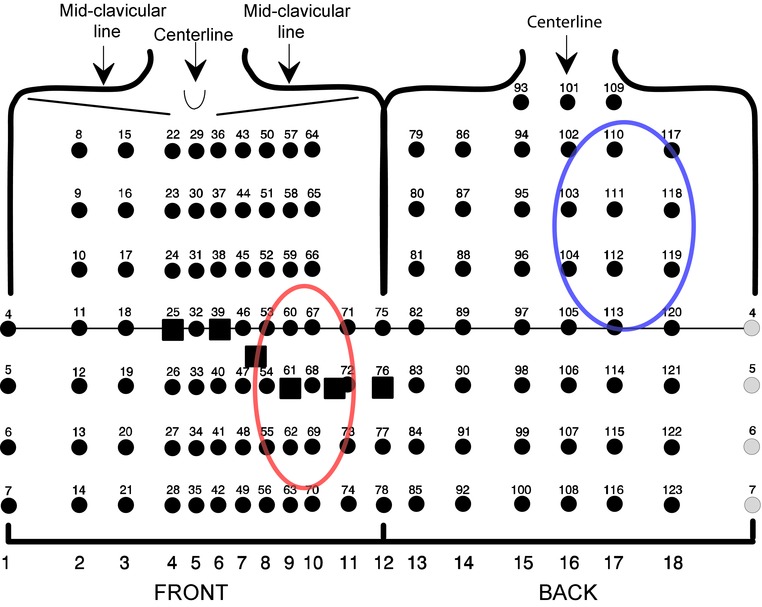
Body surface potential mapping layout of the 120 thoracic leads, mounted on 18 adhesive strips and placed according to anatomical landmarks as indicated. Horizontal line marks 4th intercostal space. Standard ECG chest leads V1 to V6 marked with a square for reference. The best recording sites yielding the highest correlations with computed ECG variables and left ventricular infarct percentage are highlighted with red and blue circles.
BSPM data were visually inspected for validity of the recording and signal‐averaged according to criteria of a 0.9 or greater correlation of the QRS complex with a selected template and noise <30 μV. The T wave was required to fit in an envelope of 110–150 μV around the template.6 The baseline was estimated using a third‐order spline function fitted to consecutive PQ segments. Invalid leads were replaced by interpolation of data from surrounding leads according to a method modified from that of Oostendorp et al.7 The QRS start and end were determined automatically from the vector magnitude of a representative precordial set of high‐pass filtered leads, following the guidelines of Simson.8 The J point was determined for each lead separately as the time instant of the maximum curvature of the signal returning to the ST level around the QRS end.9 Time points of the apex and the end of the T wave were determined automatically as described elsewhere.10
ECG Variables Calculated from BSPM
From BSPM data, ECG variables were calculated automatically using special software developed at the Department of Biomedical Engineering and Computational Science, Aalto University. The computed variables comprise amplitudes, slopes, time intervals, time‐voltage integrals, and time‐voltage areas from both de‐ and repolarization phases of the QRSSTT complex. The ECG variables are listed and defined in Table 2. The best performing ECG variables are illustrated in Figure 2.
Table 2.
BSPM‐Derived Computed ECG Variables
| Q width | Time interval from QRS start to the first positive peak, to the moment when signal amplitude increases 30 μV above minimum value after QRS start. |
| R width | Time interval from QRS start to the first negative peak, to the moment when signal amplitude decreases 30 μV below maximum value after QRS start. |
| Ramp | Peak (negative or positive) amplitude during first 15–44.75% of the QRS. |
| Samp | Amplitude of the first opposite‐sign peak after the Ramp. |
| 1st QRSint | Integral of the first quartile of QRS. |
| 2nd QRSint | Integral of the second quartile of QRS. |
| 3rd QRSint | Integral of the third quartile of QRS. |
| 4th QRSint | Integral of the fourth quartile of QRS. |
| QRSint | Integral of entire QRS complex. |
| QRSSTTint | Integral from onset of QRS to end of T wave. |
| QRSTTarea | Integral of the absolute values from onset of QRS to end of T wave. |
| Jamp | Amplitude at J point. |
| ST60amp | Average amplitude at 58–62 ms from QRS end. |
| ST80amp | Average amplitude at 78–82 ms from QRS end. |
| ST60slope | Slope from QRS end to 60 ms from QRS end. |
| ST80slope | Slope from QRS end to 80 ms from QRS end. |
| ST60int | Integral between QRS end and 60 ms from the QRS end. |
| ST80int | Integral between QRS end and 80 ms from the QRS end. |
| STint | Integral of the first half of interval from QRS end to median T‐wave end. |
| STTint | Integral from QRS end to T‐wave end. |
| Tamp | T‐apex amplitude. |
| T80int | Integral from 80 ms before to 80 ms after the T‐wave apex. |
| TPEint | Integral from T‐wave apex to T‐wave end. |
Figure 2.
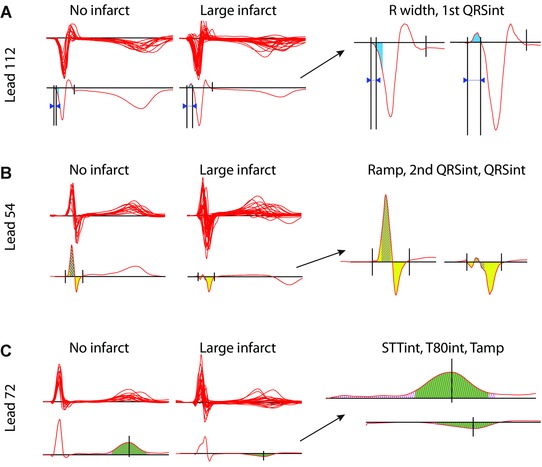
(A–C) QRSSTT complexes in the chronic phase in patients with no infarct or with large infarct. In the left column, above, are piled complexes of all patients, and below, single representative examples within each group (no infarct vs large infarct). The optimal leads differentiating the groups are displayed. The single QRSSTT examples highlight the best performing BSPM variables, the highlighted regions are further enlarged in the column to the right: (A) Lead 112, 1st QRSint (blue) is negative in a patient without infarct, and positive in a patient with a large infarct; R width (width of the “reciprocal Q wave”) between arrows is wider in the patient with a large infarct. (B) Lead 54, 2nd QRSint (hatched) and QRSint (yellow) are positive and greater, and Ramp is higher in a patient without infarct compared to a patient with a large infarct. (C) Lead 72, T80int (green) and STTint (hatched) are positive and greater, and Tamp positive in a patient without infarct compared to a patient with a large infarct. Lead numbers refer to the BSPM layout in Figure 1. ECG variables are defined in Table 2.
Cardiac Magnetic Resonance Imaging
Cardiac magnetic resonance imaging with delayed contrast enhancement (DE‐CMR) was performed in all 57 patients after complete healing, 6–12 months after the index event. DE‐CMR was performed on a 1.5 T clinical scanner (Sonata, Siemens Medical Solutions, Helsinki University Hospital, Finland). MI location was determined by gadolinium delayed enhancement.11, 12 A bolus of 0.2 mmol/kg of contrast agent (gadodiamide, OminscanTM, GE Healthcare, Horten, Norway) was administered. About 5–10 minutes later short axis images (typically 6–10) were scanned by a breath‐hold T1‐weighted segmented inversion‐recovery turbo‐FLASH sequence (TR 750, TE 4,3, TI 300, matrix 256 × 256, slice thickness 6 mm). Identical sequence parameters were used on each system wherever possible. The myocardium was divided into 16 segments according to the recommendation of the American Heart Association.13 The presence, in each segment, of gadolinium delayed enhancement was considered indicative of myocardial scar. In each segment, determination of the area of the myocardial scar was by manual tracing. Total LV infarct size was determined as percentage of the LV, calculated as the sum of segmental myocardial scar areas divided by the total area of all segments.
CK‐MBm Measurements
CK‐MBm was measured according to routine clinical praxis: at arrival at the hospital, the following evening or morning, or both. The maximum value of these measurements served as a measure of final MI size. In most patients, measurement of this maximum value occurred 12–24 hours after onset of chest pain.
Statistical Analysis
Continuous variables are expressed as mean ± SD. Correlations between ECG variables and MI size were examined with the linear Pearson's correlation coefficient (r). A correlation coefficient ≥0.5 was considered strong. Patients were grouped according to the presence and size of MI, using the median value of infarction percentage to separate large and small infarcts. ECG variables in these groups were compared by one‐way ANOVA. A P‐value <0.05 was considered statistically significant.
RESULTS
Final MI Size by DE‐CMR and CK‐MBm
Final MI size was estimated as the percentage of LV infarct scar by DE‐CMR after complete healing of the MI, and by acute‐phase peak CK‐MBm. In those 44 patients with infarct scar evident on DE‐CMR, the LV infarct percentage was 14 ± 11% (range: 0.7–51), and EF 49 ± 11% (range: 24–70). In the 13 patients with no infarct scar, the EF was 59 ± 8% (range: 47–72). CK‐MBm and LV infarct percentage had a significant correlation, r = 0.64, P < 0.000.1. CK‐MBm was 88 ± 80 μg/L in patients with infarct percentage below the median, <11%, and 235 ± 194 μg/L in those with infarct percentage ≥ 11%; EF in these patients was 55 ± 7% and 45 ± 11%, respectively.
Assessment of LV Infarct Percentage by ECG Variables at Different Stages of MI
At all stages of MI from acute to chronic, the best recording sites—showing the highest correlations between the computed ECG variables and with final MI size as defined by DE‐CMR—were on the precordial area of the thorax, close to standard leads V3 to V6, and reciprocally on the right upper back. The correlations at these two recording sites were of different sign. These best recording sites are demonstrated in Figure 1.
Correlation of ECG Variables in the Chronic Phase with LV Infarct Percentage
Depolarization Variables
In the chronic phase, all QRS variables describing the Q‐ and the R wave showed good correlations with MI size, both on the precordial area and the upper back (Table 3). On the upper back, the polarity of QRS is of opposite sign of polarity on the precordial area. Thus, Q waves on the precordial area are seen as positive deflections on the upper back, while R waves on the precordial area are represented by negative deflections on the upper back. Interestingly, the variables describing the initial part of the QRS, hereafter called the “Q‐wave variables,” clearly performed best on the upper back; variables describing the second quartile of the QRS, hereafter called the “R‐wave variables,” performed slightly better on the precordial area. The R width on the upper back, what we herein call the width of the “reciprocal Q wave,” showed the strongest correlation with MI size of all ECG variables studied (r = 0.71, P < 0.0001), wider reciprocal Q waves meaning larger MI size (Fig. 3A). On the upper back, the 1st QRSint describes the reciprocal Q wave as well, and showed a strong, positive correlation with LV infarct percentage (r = 0.57, P < 0.0001) (Fig. 3B). The Q width on the precordial area had a strong correlation with MI size (r = 0.52, P < 0.0001), being clearly weaker, however, than that of the R width on the upper back. The 1st QRSint showed a moderate, negative correlation with MI size on the precordial area (r = −0.38, P = 0.004). The Ramp and the 2nd QRSint, describing the R wave, showed strong, negative correlations on the precordial area (r =−0.57 and r = −0.56 respectively, P < 0.0001) (Fig. 3C). The QRSint also showed a strong, negative correlation with MI size on the precordial area (r = −0.58, P < 0.0001). On the right upper back, the R‐wave variables had nearly as strong correlations as on the precordial area (r = 0.55 for Ramp and r = 0.53 for 2nd QRSint, P < 0.0001); the QRSint showed only a weak correlation on the upper back (r = 0.34, P = 0.01).
Table 3.
ECG Variables and Their Best Correlations with DE‐CMR‐Determined LV Infarct Percentage at Different Stages of MI
| Acute Phase | Recovery Phase | Chronic Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ECG Variable | r max | P‐Value | Lead | r max | P‐Value | Lead | r max | P‐Value | Lead |
| 1st QRSint | 0.56 | <0.0001 | 112 | 0.56 | <0.0001 | 112 | 0.57 | <0.0001 | 112 |
| 2nd QRSint | −0.59 | <0.0001 | 61 | −0.59 | <0.0001 | 55 | −0.56 | <0.0001 | 54 |
| 3rd QRSint | −0.43 | 0.0009 | 61 | −0.42 | 0.002 | 55 | −0.39 | 0.003 | 54 |
| 4th QRSint | 0.32 | 0.02 | 90 | n.s. | 0.34 | 0.01 | 82 | ||
| QRSint | −0.62 | <0.0001 | 54 | −0.58 | <0.0001 | 55 | −0.58 | <0.0001 | 54 |
| Ramp | −0.60 | <0.0001 | 67 | −0.59 | <0.0001 | 62 | −0.57 | <0.0001 | 61 |
| Samp | −0.51 | <0.0001 | 104 | −0.53 | <0.0001 | 113 | −0.42 | 0.001 | 105 |
| R width | 0.64 | <0.0001 | 112 | 0.65 | <0.0001 | 112 | 0.71 | <0.0001 | 112 |
| Q width | 0.59 | <0.0001 | 71 | 0.47 | 0.0003 | 61 | 0.51 | <0.0001 | 60 |
| Jamp | −0.30 | 0.02 | 5 | 0.28 | 0.04 | 54 | 0.28 | 0.03 | 112 |
| ST60amp | −0.30 | 0.02 | 5 | 0.36 | 0.008 | 63 | −0.30 | 0.02 | 88 |
| ST80amp | −0.33 | 0.01 | 5 | 0.29 | 0.04 | 63 | −0.35 | 0.008 | 80 |
| ST60int | −0.29 | 0.03 | 5 | 0.33 | 0.02 | 63 | n.s. | ||
| ST80int | −0.30 | 0.02 | 5 | 0.33 | 0.01 | 63 | n.s. | ||
| STint | −0.30 | 0.03 | 5 | 0.28 | 0.04 | 63 | −0.31 | 0.02 | 81 |
| ST60slope | 0.32 | 0.01 | 55 | −0.30 | 0.03 | 115 | 0.27 | 0.04 | 70 |
| ST80slope | 0.28 | 0.04 | 47 | −0.32 | 0.02 | 115 | −0.36 | 0.006 | 88 |
| Tamp | −0.40 | 0.002 | 75 | −0.54 | <0.0001 | 72 | −0.55 | <0.0001 | 72 |
| T80int | −0.36 | 0.006 | 75 | −0.52 | <0.0001 | 72 | −0.57 | <0.0001 | 72 |
| TPEint | −0.36 | 0.006 | 71 | −0.54 | <0.0001 | 72 | −0.56 | <0.0001 | 72 |
| STTint | n.s. | −0.38 | 0.004 | 72 | −0.52 | <0.0001 | 72 | ||
| QRSSTTint | −0.42 | 0.001 | 71 | −0.50 | 0.0001 | 69 | −0.54 | <0.0001 | 69 |
| QRSSTTarea | −0.35 | 0.007 | 119 | −0.51 | <0.0001 | 111 | −0.50 | <0.0001 | 111 |
Correlations ≥0.5 are highlighted.
DE‐CMR = contrast‐enhanced cardiac magnetic resonance imaging; LV = left ventricle.
For explanation of ECG variables, see Table 2.
Figure 3.
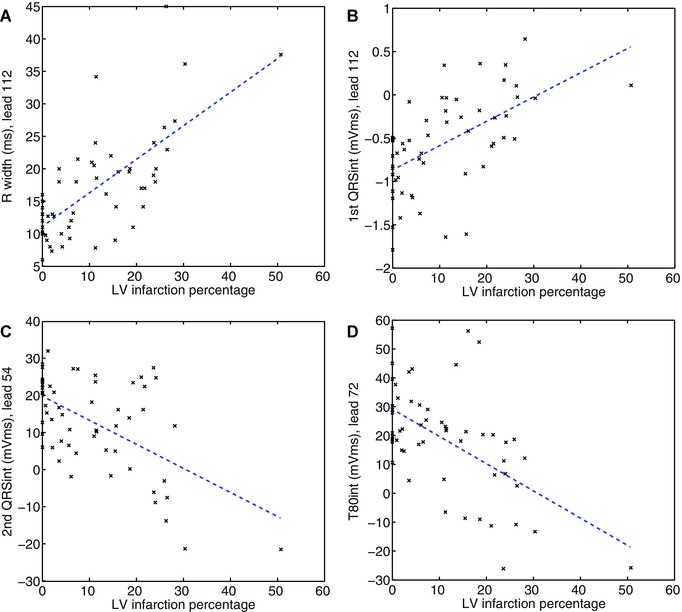
Correlations of the best performing ECG variables in the chronic phase with left ventricular infarction percentage determined by contrast‐enhanced magnetic resonance imaging.
The second part of the QRS was less informative with regards to MI size than was the first part (Table 3). The S wave seemed to shrink with increasing MI size. On the back, the S wave is a positive deflection, a mirror image of that on the precordial area. The Samp performed slightly better on the back than on the precordial area, having a moderate, negative correlation with LV infarct percentage (r = −0.42, P = 0.001) on the back. The 3rd QRSint and 4th QRSint, also describing, at least in part, the S wave, had weaker correlations.
Repolarization Variables
In the chronic phase, most of the repolarization variables had good, negative correlations with MI size on the precordial area (Table 3). The inverse correlations on the upper back were also significant, but clearly weaker. On the precordial area, the T‐wave variables performed the best; the smaller or the more negative the T wave, the larger the MI size (r = −0.55 to r = −0.57, P < 0.0001) (Fig. 3D). The STTint, QRSSTTint, and QRSSTTarea showed good negative correlations with MI size on the precordial area as well (r = −0.50 to r = −0.54, P < 0.0001). The correlations of the ST‐segment variables were weak, at best r = −0.35 for ST80amp, and r = −0.36 for ST80slope on the left upper back, P < 0.01.
Correlation of ECG Variables in the Acute Phase with LV Infarct Percentage
The depolarization variables that performed well in the chronic phase showed good correlations with DE‐CMR already in the acute phase, in corresponding locations (Table 3). Also in the acute phase, the Q‐ and R‐wave variables showed strong correlations with final MI size, and performed better than the S‐wave variables. Again, the width of the reciprocal Q wave (R width) had the highest correlation with MI size of all variables (r = 0.64, P < 0.0001 on the right upper back). This correlation was somewhat lower than in the chronic phase. The other Q‐ and R‐wave variables showed similar or slightly stronger correlations in the acute than in the chronic phase, in the same or neighboring leads.
The repolarization variables showed their best correlations with MI size on the precordial area, as they did in the chronic phase, but these correlations were much weaker (Table 3). Clearly, the repolarization variables did not perform as well as the depolarization variables acutely. The T‐wave variables and QRSSTTint showed moderate, negative correlations with MI size on the precordial area (r = −0.36 to −0.42, P < 0.01); the correlations of the ST‐segment variables and the QRSSTTarea with MI size were weaker, and the correlation of the STTint was insignificant.
Correlation of ECG Variables in the Recovery Phase with LV Infarct Percentage
The depolarization and repolarization variables performed best in the same locations during recovery as in the acute and chronic phases, and the correlations fell between these two (Table 3). As in the acute and chronic phases, the Q‐ and R‐wave variables showed the strongest correlations with MI size, the best variable being the width of the reciprocal Q wave (R width) (r = 0.65, P < 0.0001). The repolarization variables showed better correlations during recovery than in the acute phase, but still did not perform quite as well as in the chronic phase.
Comparison of ECG Variables in Patients with Large, Small, and No Infarcts
Patients with large infarcts, as defined by an LV infarct percentage above the median (>11%), had significantly different values for their best performing ECG variables than did patients with small or no infarcts. BSPM could distinguish patients with a large infarct from those with a small or no infarct at all time points, from the acute phase to complete healing of MI (Table S1).
In the chronic phase, the width of the reciprocal Q wave (R width) was wider, and the 1st QRSint was positive, or at least less negative on the right upper back in patients with a large MI than in patients with a small or no MI (P < 0.001) (Figs. 4A and B). On the precordial area, the Ramp, 2nd QRSint, QRSint, Tamp, T80int, STTint, and QRSSTTint were smaller in patients with a large MI than in ones with a small or no MI (P ≤ 0.01) (Figs. 4C and D). Some of these best performing ECG variables could differentiate between large and small infarcts (R width, 1st QRSint, T80int, and QRSSTTint; P ≤ 0.03), but no statistically significant difference appeared between the groups of patients with small versus no infarcts. Figure 2 shows QRSSTT complexes in patients with large versus no infarct at the best recording sites.
Figure 4.
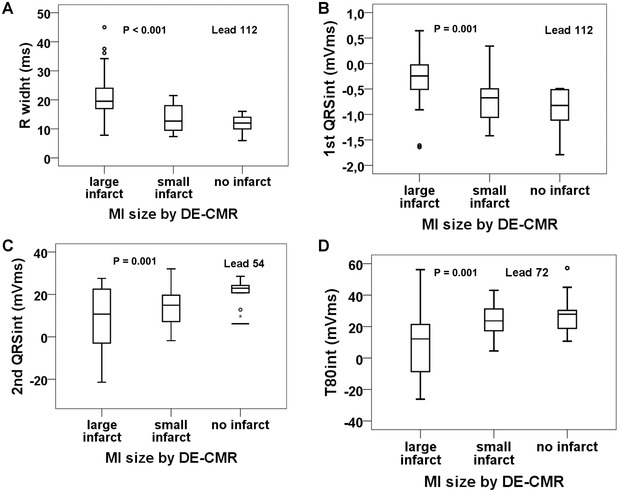
Values of the best performing ECG variables in the chronic phase in patients with large, small, or no infarct. Large and small infarcts separated by median infarct size determined by contrast‐enhanced cardiac magnetic resonance imaging: large infarcts ≥11%, small infarcts <11% of the left ventricle. P values are for large infarct versus small or no infarct. Boxes indicate medians, first and third quartiles; whiskers indicate minimum and maximum values, falling within 1.5× the interquartile range from the upper or lower edge of the box; outliers are indicated by points, falling outside 1.5× the interquartile range.
The depolarization variables could distinguish large from small infarcts already in the acute and recovery phases, in a similar fashion as in the chronic phase. Repolarization variables, too, could distinguish large from small infarcts in the recovery phase. However, in the acute phase, the QRSSTTint was the only repolarization variable showing a significant differentiating capacity.
Correlation of ECG Variables with CK‐MBm
The highest correlations between ECG variables and peak CM‐MBm values were close to the same recording sites yielding the best correlations between ECG variables and DE‐CMR. The best performing ECG variables showed strong correlations with CK‐MBm at all stages of MI: Width of the reciprocal Q wave (R width), Ramp, and 2nd QRSint all showed strong, positive correlations on the right upper back (r ≥ 0.54, P < 0.0001); the QRSint showed a strong, negative correlation on the precordial area (r ≤ – 0.53, P < 0.0001). In the acute phase, the Ramp and 2nd QRSint performed the best; during recovery and in the chronic phase, the width of the reciprocal Q wave (R width) showed the best correlation with CK‐MBm (Table S2).
The repolarization variables performed weaker than the depolarization variables at all stages of MI. Only in the acute phase did the ST60amp and ST80amp show strong, positive correlations with CK‐MBm on the precordial area (r = 0.52 and r = 0.51, respectively, P < 0.0001), performing better than the Jamp (r = 0.42, P = 0.001). During recovery, no repolarization variables performed well; the ST60amp and QRSSTTint showed moderate correlations with CK‐MBm on the precordial area. In the chronic phase, the QRSSTTint had a good correlation with CK‐MBm on the central upper back (r = 0.53, P < 0.0001), the other repolarization variables showing moderate correlations (Table S2).
DISCUSSION
Our results show that computed, single‐lead ECG variables can estimate final MI size acutely, during MI healing, and in its chronic phase. We found the best performing ECG variables and their best recording locations by using BSPM with 120 chest leads. The patients had acute coronary syndrome and were analyzed as one group irrespective of culprit artery.
Assessment of LV Infarct Percentage by BSPM
LV infarct percentage was defined by DE‐CMR after complete healing of the MI. Overall, the infarcts were small to moderate, reflecting the timely reperfusion therapy. The median value of LV infarct percentage was 11%. Of multiple depolarization as well as repolarization variables, the Q‐ and R‐wave variables, as well as the integral of the entire QRS, performed the best, having strong correlations with MI size at all stages of MI.
Depolarization Variables
At all stages of MI, the best variable was the width of the reciprocal Q wave, that is, R‐width on the upper back. Together with the 1st QRSint, the R width showed a strong, positive correlation with MI size on the right upper back. In healthy individuals, the initial deflection of the QRS is negative on the back, representing a mirror image of the R wave in precordial ECG leads; analogically, Q waves on the precordial area are reflected as R waves reciprocally on the back.5
What is well established is that Q waves indicate permanent myocardial damage after an infarction. Irrespective of MI age and location, classical Q waves (30–40 ms in width) are associated with larger MI size.14, 15, 16, 17 Even if small infarcts do not necessarily yield classical Q waves, we could show that even small computed Q waves correlated with MI size. Earlier, we found that in left anterior descending coronary artery (LAD)‐ and right coronary artery (RCA)‐related MIs, the 1st QRSint was the best depolarization variable to predict, after acute MI, recovery of myocardial function.5 In this study, the 1st QRSint was positive, or close to zero, at the best recording location on the right upper back in most patients with the largest infarcts. At this site, in all patients (but one) with a small or no infarct, the 1st QRSint was negative, similar to healthy individuals.
Ramp, 2nd QRSint, and QRSint showed strong, negative correlations with MI size on the precordial area, close to standard leads V3 to V5. In the acute phase, these variables showed correlations almost as strong as the R width; in the chronic phase, however, the R width clearly showed the strongest correlation of all variables. Studies using precordial or body surface mapping have correlated R‐ and Q waves with MI size. Significant correlations appeared between the sum of R‐wave amplitudes and the extent of LV dyssynergy (r = −0.42), EF (r = 0.51), and enzymatic MI size (r = −0.57) after anterior MI; no correlation appeared, however, between the sum of R‐wave amplitudes and EF in inferior MI. The number of Q waves showed a significant negative correlation with EF in anterior (r = −0.50) as well as inferior (r = −0.63) MI, and the sum of Q‐wave amplitudes had a significant positive correlation with enzymatic MI size (r = 0.61). Q waves did not, however, correlate with extent of LV dyssynergy.18, 19, 20 The number of Q waves was recently shown to correlate with MI size by DE‐CMR; however, anterior MIs caused fewer Q waves confined to the precordium, whereas inferior MIs were associated with a larger body surface area of Q waves involving the inferior front and back chest.15
R and S waves, together with Q waves, are considered in the Selvester QRS score, the best validated ECG method for quantification of myocardial infarct.21 This score has been validated in computer models, in postmortem studies, and in clinical studies using enzyme assessment, thallium perfusion imaging, and CMR. In the subacute and chronic phases of infarction, it correlates well with MI size in all the main coronary artery perfusion regions.22, 23, 24
Repolarization Variables
Repolarization variables were clearly inferior to depolarization variables in the acute phase, but in the chronic phase showed comparable, strong correlations. The T‐wave variables (Tamp, T80int, TPEint) performed the best, already showing strong correlations during recovery. The STT and QRSSTT integrals showed strong correlations in the chronic phase, but slightly lower than did the T‐wave variables. At all time points, these repolarization variables, all including a T wave had their best recording site on the precordial area, close to standard leads V5 and V6, where they showed a negative correlation with MI size. The ST‐segment variables showed only weak correlations with MI size, and the best recording locations varied at all stages.
After reperfusion for STEMI, the significance of T‐wave polarity is dependent on time relative to the acute event. In the acute phase, negative T waves in infarction‐related leads are associated with smaller enzymatically determined MI size, better LV function, and long‐term outcome if these initially negative T waves later turn positive; in patients with persistent negative T waves the clinical outcome is worse.25, 26 Positive T waves in the acute phase without early inversion predict, however, larger MI size and more early complications.27, 28 That dynamic T‐wave changes are accentuated in the acute phase can explain why our correlation between T‐wave variables and MI size improved with time, as the T wave‐changes stabilized. One study has found a moderate, negative correlation between DE‐CMR‐defined MI size and T‐wave amplitudes in chronic MI, but only for inferior MI. In anterior MI and in the overall population, such a correlation was not significant.29 That study measured the T‐wave amplitudes in leads showing the greatest ST elevation, and what cannot be ruled out is whether T‐wave amplitudes in another location would have performed better.
Previously, residual ST‐segment elevation after reperfusion therapy has been shown to correlate with MI size by CMR and perfusion imaging in STEMI patients.30, 31 In MI patients presenting without ST elevations, ST depression in the acute phase is associated with adverse long‐term prognosis.32 With time, the ST segment returns to normal, irrespective of reperfusion therapy,33 making it less than surprising that correlations between ST segment and MI size in the chronic phase should be weak. The reason that our ST‐segment variables acutely showed poor correlation with CMR‐defined MI size may be attributed to the pooled cohort of infarcts in different regions of the LV.
BSPM Variables versus CK‐MBm
LV infarct percentage by DE‐CMR correlated well with CK‐MBm, indicating that peak CK‐MBm measured in the acute phase reflects final MI size. Studies show peak CK‐MBm to correlate with MI size determined by imaging and histology in patients with and without reperfusion.34, 35
The same depolarization variables showing the best correlations with LV infarct percentage, except for the 1st QRSint, also showed strong correlations with CK‐MBm, and at the same lead locations. However, the best recording site for the R‐wave variables was also on the upper back, as was true for the width of the reciprocal Q wave. Here, these best performing variables showed strong, positive correlations with CK‐MBm at all time points.
Repolarization variables were inferior to depolarization variables at all time points. In the acute phase, the ST60 and ST80 amplitudes showed strong, positive correlations with CK‐MBm on the precordial area, although their correlations with DE‐CMR were weak. This could be due to the fact that peak CK‐MBm not only reflects final MI size, but is also affected by washout after reperfusion therapy in STEMI.36 In the chronic phase, only the QRSSTTint showed a strong, positive correlation with CK‐MBm on the upper back; during recovery no repolarization variables showed such strong correlations.
Limitations
We pooled STEMIs and non‐STEMIs caused by different culprit‐artery lesions. Despite our patient‐population heterogeneity, we found strong correlations with various ECG variables and MI size, and at all stages of infarction. The explanation could be that in a majority of the patients, infarct scar distribution was multifocal, being present in one or both perfusion beds other than the culprit‐artery related bed. The reliability of our results is strengthened by the fact that the best performing variables showed their best recording locations at the same sites all throughout the infarction process.
Our acute‐phase results cannot be applied early during the acute phase, because at the time of the acute measurement, patients had been successfully reperfused or had stabilized spontaneously.
Because we could not time the CK‐MBm measurement exactly, timing of measurement of the peak CK‐MBm value varied among patients, being between 12 and 24 hours from onset of chest pain in most. Despite this, the peak CK‐MBm value correlated strongly with LV infarct percentage by DE‐CMR, indicating that peak CK‐MBm value reflected final MI size.
CONCLUSION
Automatic, BSPM‐derived single‐lead ECG variables succeeded in assessing the size of permanent myocardial injury at all stages of infarction, from the acute phase to complete healing, irrespective of the acute ischemic event's location. Of all variables, the R width on the upper back—there representing the reciprocal Q wave—showed the strongest correlation with MI size. R‐wave variables and the QRS integral performed well on the precordial area. T‐wave variables showed strong correlations during recovery and in the chronic phase, but no repolarization variables performed well acutely.
In summary, computed single‐lead ECG variables correlate with MI size, and differentiate large infarcts from small. Automatic quantification of MI size by the use of only a few ECG leads may have the potential to be applied in clinical practice.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Average Values of Chronic‐Phase ECG Variables Grouped According to LV Infarct Percentage by DE‐CMR.
Table S2. ECG Variables and their Best Correlations with CK‐MBm at Different Stages of MI.
Acknowledgments
We wish to thank Suvi Heikkilä and Hanna Ranne for help with BSPM measurements, Timo Päivärinta and Aki Syrjälä for performing the CMR measurements, and Carol Norris for language editing.
This study was supported by grants from Helsinki University Central Hospital Research Funds (EVO grant), the Finnish Foundation for Cardiovascular Research, Finska Läkaresällskapet, the Waldemar von Frenckell Foundation, the Instrumentarium Foundation, the Aarne Koskelo Foundation, the Medicine Fund of Helsinki University, the Wilhelm and Else Stockmann Foundation, and the Aarne and Aili Turunen Foundation.
REFERENCES
- 1. Flowers NC, Horan LG. Body surface potential mapping In Zipes DP, Jamal F. (eds.): Cardiac Electrophysiology: From Cell to Bedsice, 3rd Edition Philadelphia, W.B. Saunders Company, 2000, pp. 737–746. [Google Scholar]
- 2. Kornreich F, Montague TJ, Rautaharju PM. Body surface potential mapping of ST segment changes in acute myocardial infarction. Implications for ECG enrollment criteria for thrombolytic therapy. Circulation 1993;87:773–782. [DOI] [PubMed] [Google Scholar]
- 3. Mirvis DM. Current status of body surface electrocardiographic mapping. Circulation 1987;75:684–688. [DOI] [PubMed] [Google Scholar]
- 4. Vesterinen P, Vaananen H, Stenroos M, et al. Localization of prior myocardial infarction by repolarization variables. Int J Cardiol 2008;124:100–106. [DOI] [PubMed] [Google Scholar]
- 5. Kylmälä MM, Konttila T, Vesterinen P, et al. Predicting recovery of myocardial function by electrocardiography after acute infarction. Ann Noninvasive Electrocardiol 2013;18:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vaananen H, Korhonen P, Montonen J, et al. Non‐invasive arrhythmia risk evaluation in clinical environment. Herzschr Elektrophys 2000;11:229–234. [DOI] [PubMed] [Google Scholar]
- 7. Oostendorp TF, van Oosterom A, Huiskamp G. Interpolation on a triangulated 3D surface. J Comput Phys 1989;80:331–343. [Google Scholar]
- 8. Simson MB. Use of signals in the terminal QRS complex to identify patients with ventricular tachycardia after myocardial infarction. Circulation 1981;64:235–242. [DOI] [PubMed] [Google Scholar]
- 9. Väänänen H. Analysis of electro‐ and magnetocardiographic signals. Helsinki, Finland, Helsinki University of Technology, 2005. [Google Scholar]
- 10. Oikarinen L, Paavola M, Montonen J, et al. Magnetocardiographic QT interval dispersion in postmyocardial infarction patients with sustained ventricular tachycardia: Validation of automated QT measurements. Pacing Clin Electrophysiol 1998;21:1934–1942. [DOI] [PubMed] [Google Scholar]
- 11. Fieno DS, Kim RJ, Chen EL, et al. Contrast‐enhanced magnetic resonance imaging of myocardium at risk: Distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol 2000;36:1985–1991. [DOI] [PubMed] [Google Scholar]
- 12. Wu E, Judd RM, Vargas JD, et al. Visualisation of presence, location, and transmural extent of healed Q‐wave and non‐Q‐wave myocardial infarction. Lancet 2001;357:21–28. [DOI] [PubMed] [Google Scholar]
- 13. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 14. Moon JC, De Arenaza DP, Elkington AG, et al. The pathologic basis of Q‐wave and non‐Q‐wave myocardial infarction: A cardiovascular magnetic resonance study. [see comment]. J Am Coll Cardiol 2004;44:554–560. [DOI] [PubMed] [Google Scholar]
- 15. Bodi V, Sanchis J, Guillem MS, et al. Analysis of the extension of Q‐waves after infarction with body surface map: Relationship with infarct size. Int J Cardiol 2006;111:399–404. [DOI] [PubMed] [Google Scholar]
- 16. Engblom H, Carlsson MB, Hedstrom E, et al. The endocardial extent of reperfused first‐time myocardial infarction is more predictive of pathologic Q waves than is infarct transmurality: A magnetic resonance imaging study. Clin Physiol Funct Imaging 2007;27:101–108. [DOI] [PubMed] [Google Scholar]
- 17. Nijveldt R, van der Vleuten PA, Hirsch A, et al. Early electrocardiographic findings and MR imaging‐verified microvascular injury and myocardial infarct size. J Am Coll Cardiol Cardiovasc Imaging 2009;2:1187–1194. [DOI] [PubMed] [Google Scholar]
- 18. De Ambroggi L, Landolina M, Galdangelo F, et al. Limits of precordial electrocardiographic mapping in assessing anterior myocardial infarction size. G Ital Cardiol 1982;12:317–323. [PubMed] [Google Scholar]
- 19. Hayashi H, Watanabe Y, Ishikawa T, et al. Diagnostic value of body surface map in myocardial infarction: Assessment of location, size and ejection fraction as compared with coronary cineangiography and 201Tl myocardial scintigraphy. Jpn Circ J 1980;44:197–208. [DOI] [PubMed] [Google Scholar]
- 20. Herlitz J, Hjalmarson A, Waldenstrom J. Relationship between electrocardiographically and enzymatically estimated size in anterior myocardial infarction. J Electrocardiol 1984;17:361–370. [DOI] [PubMed] [Google Scholar]
- 21. Pahlm US, Chaitman BR, Rautaharju PM, et al. Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle. Am J Cardiol 1998;81:809–815. [DOI] [PubMed] [Google Scholar]
- 22. Selvester RH, Wagner GS, Hindman NB. The Selvester QRS scoring system for estimating myocardial infarct size. The development and application of the system. Arch Intern Med 1985;145:1877–1881. [PubMed] [Google Scholar]
- 23. Juergens CP, Fernandes C, Hasche ET, et al. Electrocardiographic measurement of infarct size after thrombolytic therapy. J Am Coll Cardiol 1996;27:617–624. [DOI] [PubMed] [Google Scholar]
- 24. Engblom H, Hedstrom E, Heiberg E, et al. Size and transmural extent of first‐time reperfused myocardial infarction assessed by cardiac magnetic resonance can be estimated by 12‐lead electrocardiogram. Am Heart J 2005;150:920e1–920e9. [DOI] [PubMed] [Google Scholar]
- 25. Sakata K, Yoshino H, Houshaku H, et al. Myocardial damage and left ventricular dysfunction in patients with and without persistent negative T waves after Q‐wave anterior myocardial infarction. Am J Cardiol 2001;87:510–515. [DOI] [PubMed] [Google Scholar]
- 26. Lancellotti P, Gerard PL, Kulbertus HE, et al. Persistent negative T waves in the infarct‐related leads as an independent predictor of poor long‐term prognosis after acute myocardial infarction. Am J Cardiol 2002;90:833–837. [DOI] [PubMed] [Google Scholar]
- 27. Matetzky S, Barabash GI, Shahar A, et al. Early T wave inversion after thrombolytic therapy predicts better coronary perfusion: Clinical and angiographic study. J Am Coll Cardiol 1994;24:378–383. [DOI] [PubMed] [Google Scholar]
- 28. Sorensen JT, Murinson MA, Kaltoft AK, et al. Significance of T‐wave amplitude and dynamics at the time of reperfusion in patients with acute ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Electrocardiol 2009;42:677–683. [DOI] [PubMed] [Google Scholar]
- 29. Meijs LP, Gorgels AP, Bekkers SC, et al. The relationship between serial postinfarction T wave changes and infarct size and ventricular function as determined by cardiac magnetic resonance imaging. J Electrocardiol 2011;44:555–560. [DOI] [PubMed] [Google Scholar]
- 30. Sciagra R, Parodi G, Migliorini A, et al. ST‐segment analysis to predict infarct size and functional outcome in acute myocardial infarction treated with primary coronary intervention and adjunctive abciximab therapy. Am J Cardiol 2006;97:48–54. [DOI] [PubMed] [Google Scholar]
- 31. Hallen J, Sejersten M, Johanson P, et al. Influence of ST‐segment recovery on infarct size and ejection fraction in patients with ST‐segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Am J Cardiol 2010;105:1223–1228. [DOI] [PubMed] [Google Scholar]
- 32. Yan RT, Yan AT, Mahaffey KW, et al. Prognostic utility of quantifying evolutionary ST‐segment depression on early follow‐up electrocardiogram in patients with non‐ST‐segment elevation acute coronary syndromes. Eur Heart J 2010;31:958–966. [DOI] [PubMed] [Google Scholar]
- 33. Montague TJ, Smith ER, Johnstone DE, et al. Temporal evolution of body surface map patterns following acute inferior myocardial infarction. J Electrocardiol 1984;17:319–327. [DOI] [PubMed] [Google Scholar]
- 34. Hackel DB, Reimer KA, Ideker RE, et al. Comparison of enzymatic and anatomic estimates of myocardial infarct size in man. Circulation 1984;70:824–835. [DOI] [PubMed] [Google Scholar]
- 35. Choi KM, Kim RJ, Gubernikoff G, et al. Transmural extent of acute myocardial infarction predicts long‐term improvement in contractile function. Circulation 2001;104:1101–1107. [DOI] [PubMed] [Google Scholar]
- 36. Tamaki S, Murakami T, Kadota K, et al. Effects of coronary artery reperfusion on relation between creatine kinase‐MB release and infarct size estimated by myocardial emission tomography with thallium‐201 in man. J Am Coll Cardiol 1983;2:1031–1038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Average Values of Chronic‐Phase ECG Variables Grouped According to LV Infarct Percentage by DE‐CMR.
Table S2. ECG Variables and their Best Correlations with CK‐MBm at Different Stages of MI.


