Abstract
Background
QRS narrowing after CRT is a predictor of patient outcome. Further narrowing can be obtained by interventricular pacing delay (VVd) optimization, raising interest to inter and intraobserver variation in manual measurements of QRS duration.
Methods
(a) Variation in intrinsic rhythm QRS duration in CRT patients with LBBB: In 40 intrinsic 12‐lead ECGs, six observers measured QRS duration defined as widest QRS in any lead. In 20 of these ECGs, two observers repeated the measurements. (b) Variation in paced QRS duration at different VVd settings and agreement in selecting the narrowest QRS: In 20 CRT patients, five paced ECGs were recorded at different VVds. The most frequently selected VVd(s) estimated to cause the narrowest QRS in each patient defined the optimal VVd. Two observers repeated the measurements and VVd selections.
Results
Absolute interobserver difference in measured QRS duration in intrinsic rhythm ECGs was mean 2 ms, range (−40; 40 ms), mean limits of agreement (LoA): −21; 25 ms. Absolute interobserver difference in measured QRS duration in paced ECGs was mean 3 ms, range (−50; 60 ms), mean LoA: −20; 27 ms. There was no difference in LoA between intrinsic and paced QRS duration (lower limit p = 0.68; upper limit p = 0.44). The optimal VVd was included in 17/20 (85%) of the VVd selections by six observers. Interobserver variation was comparable with the intraobserver variation.
Conclusions
Interobserver variation and intraobserver variation in manually measured paced and intrinsic rhythm QRS duration are clinically acceptable and comparable in a cohort of CRT patients. Inter and intraobserver reproducibility for selecting the optimal VVd is good and warrants manual VVd optimization for QRS narrowing in CRT.
Keywords: cardiac resynchronization therapy, ECG‐guided, interventricular pacing delay, observer variation, optimization, QRS duration
1. INTRODUCTION
Cardiac resynchronization therapy (CRT) improves symptoms and reduces mortality in patients with chronic left ventricular heart failure and a wide QRS complex (European Society of Cardiology (ESC) et al., 2013). An electrocardiogram (ECG) with QRS duration (QRSd) exceeding 130 ms is a main criterion for receiving CRT (Ruschitzka et al., 2013) and there is growing evidence that patients exhibiting left bundle branch block (LBBB) have a higher response rate to CRT (Gervais et al., 2009; Risum et al., 2013; Zareba et al., 2011). Even after careful selection of CRT candidates, up to 30%–40% of patients turn out as non‐responders (Daubert, Behar, Martins, Mabo, & Leclercq, 2017). Besides pre‐implant QRSd and morphology, QRS narrowing after CRT is an important predictor of outcome (Hsing et al., 2011). Further narrowing of paced QRS after CRT can be obtained by individual optimization of the interventricular pacing delay (VVd) and has been proposed to increase the acute hemodynamic response (Tamborero et al., 2009). ECG‐guided VVd optimization appears more reproducible than echocardiographic optimization based on velocity time integrals, which are susceptible to angle errors during image recording and beat‐to‐beat variation (Francis, 2013).
The QRSd is often defined as manual measurement of maximal QRSd in any 12‐lead ECG leads, despite a greater variability has been shown with this definition as compared with measurement of mean QRSd and automatically calculated QRSd (Tomlinson, Bashir, Betts, & Rajappan, 2009). However, manual QRSd is the most clinically applicable definition.
Only few studies have evaluated the reproducibility of measuring QRSd and the implications for selection of CRT candidates (De Guillebon et al., 2010; De Pooter, El Haddad, Stroobandt, De Buyzere, & Timmermans, 2017; Tomlinson et al., 2009), and none have tested the reproducibility of manually measured paced QRSd and the implications for the selection of VVd settings after CRT. Therefore, the aims of this study were twofold: (a) to assess variation in manual measurement of QRSd (between and within observers) in intrinsic rhythm and paced ECGs from patients with LBBB receiving CRT and (b) to asses variations (between and within observers) in manual measurement of paced QRSd at different VVd settings to select the narrowest QRS complex for CRT optimization.
2. METHODS
We included randomly selected patients with LBBB who participated in the Imaging‐CRT trial (empiric vs. imaging‐guided left ventricular lead placement in CRT) (Sommer et al., 2016). Each patient had a 12‐lead ECG recorded before implantation and five 12‐lead ECGs at different VVds after implantation. The stated QRSd was the QRSd in the lead exhibiting the widest QRS complex. The QRSd was reported with a temporal resolution of 10 ms. Onset of the QRS complex was defined as the first positive or negative deflection from the isoelectric line, and offset was defined as being the J‐point: The point where the steep slopes of the RS waves are replaced by the more gradual slopes which precede the first limb of the T wave (Lepeschkin & Surawicz, 1952). For analysis of intrinsic QRSd, baseline ECGs from 40 patients were included, and for the paced QRSd, five ECGs from 20 patients were included. ECGs were recorded using a Schiller Cardiovit AT‐102 plus (Schiller AG, Baar, Switzerland).
2.1. Inter and intraobserver variation in measurement of QRSd in intrinsic rhythm ECGs with LBBB
A 12‐lead intrinsic rhythm ECG was recorded at a paper speed of 25 mm/s with 10 mm/mV gain in patients with LBBB as defined according to the Strauss criteria (Strauss, Selvester, & Wagner, 2011). For assessment of interobserver variation, six observers independently measured QRSd in all 12 leads in each of the 40 ECGs and noted the maximal QRSd for each ECG (Figure 1a, Figure 2 left panel). For intraobserver variation, two observers independently performed two repeated measurements of QRSd in 20 of the ECGs.
Figure 1.
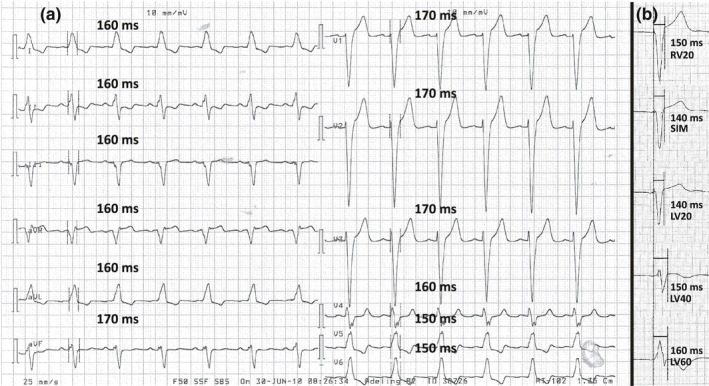
Measurement of QRS duration in paced and intrinsic rhythm electrocardiograms. (a) Measurement of QRS duration in an intrinsic rhythm electrocardiogram with left bundle branch block, paper speed 25 mm/s, and gain 10 mm/mV. QRS duration was measured in each of the 12 leads. In this case, maximal QRS duration was 170 ms. (b) Measurement of biventricular paced QRS duration at five different interventricular pacing delays in patient no. 9, paper speed 50 mm/s, and gain 10 mm/mV; for simplicity, only lead V2 from the 12‐lead electrocardiogram is shown. In this case, SIM and LV20 would be the optimal interventricular pacing delays. LV20/40/60: pacing of left ventricle 20/40/60 ms prior to right ventricle; RV20: pacing of right ventricle 20 ms prior to left ventricle; SIM: simultaneous pacing of both ventricles
Figure 2.
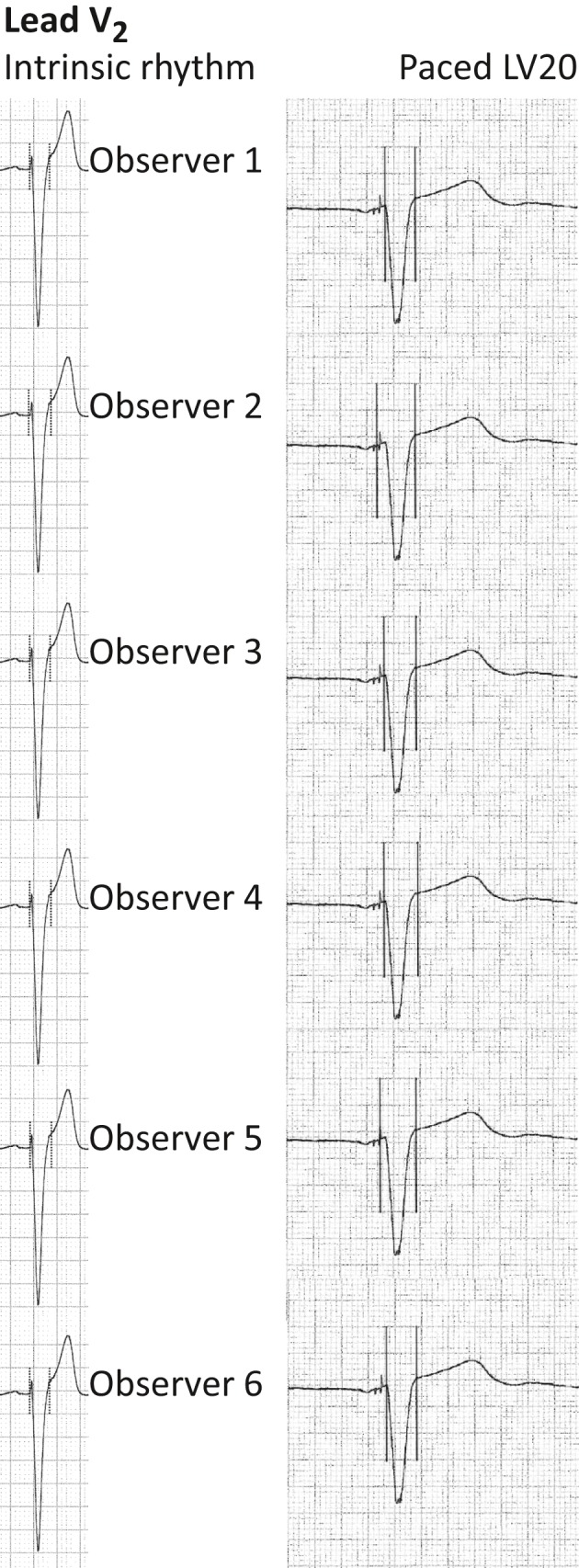
Interobserver variation in measurement of QRS duration illustrated in lead V2 in paced and intrinsic rhythm electrocardiograms. Illustration of interobserver variation in determining the onset and offset of the same QRS complex in lead V2 in an intrinsic rhythm electrocardiogram (left panel) and a LV20 paced electrocardiogram (right panel). The QRS duration measurements were performed by the same six observers who did the QRS duration measurements in current study. Left panel: paper speed 25 mm/s, gain 10 mm/mV. Exact same QRS complex as lead V2 in Figure 1a. Right panel: paper speed 50 mm/s, gain 10 mm/mV. Exact same QRS complex as LV20 in Figure 1b. LV20: pacing of left ventricle 20 prior to right ventricle
2.2. Inter and intraobserver variation in measurement of biventricular paced QRSd and selection of VVd
The day after CRT implantation, five 12‐lead ECGs were recorded at a paper speed of 50 mm/s with 10 mm/mV gain. The ECGs were recorded with the device programmed to each of the following VVds: right ventricular pacing 20 ms prior to left ventricular pacing (RV20), simultaneous biventricular pacing (SIM), left ventricular pacing 20 (LV20), 40 (LV40), and 60 (LV60) ms prior to right ventricular pacing. For each patient, one or more VVds resulting in the narrowest QRS complex was registered. For interobserver variation, six observers independently measured QRSd at each VVd in all 20 patients to identify the VVd, resulting in the shortest QRSd (Figure 1b, Figure 2 right panel). The consensus optimal VVd in each patient was defined as the VVd selected most frequently by the six observers to result in the narrowest biventricular paced QRS complex. For intraobserver variation, two observers independently performed two repeated measurements of QRSd in the 20 patients to define the VVd that resulted in the shortest QRSd.
2.3. Statistics
Interobserver variation and intraobserver variation of QRSd measurements between and within observers are presented using the Bland–Altman method with 95% limits of agreement (LoA), defined as the mean difference ±2 × standard deviation (SD) (Bland & Altman, 1986). A 95% confidence interval (CI) is computed for the lower (LL) and upper (UL) LoA, respectively (Mantha, Roizen, Fleisher, Thisted, & Foss, 2000).
Limits of agreement for QRSd ±20 ms are considered clinically acceptable, meaning that the two measurements of QRSd performed by two observers or twice by the same observer can be used interchangeably.
Normality of data is tested using Q–Q plots. Continuous data are presented as means (range) and categorical data as numbers (%) and medians. Paired t‐tests are used for comparisons of continuous data and chi‐squared tests are used for comparison of categorical data. Commercially available software (Stata 14.2; Stata‐Corp, College Station, TX, USA) is used for analysis.
3. RESULTS
3.1. Interobserver variation in paced and intrinsic rhythm ECG QRSd
Pairwise comparisons of six observers measuring QRSd in intrinsic rhythm ECGs with LBBB from 40 patients showed absolute difference with a mean of 2 ms (−40; 40 ms), mean LoA: −21; 25 ms. Average span between LL and UL LoA was 46 ms, ranging from 28 to 62 ms between observers (Figure 3, top).
Figure 3.

Interobserver variation in QRS duration measured in paced and intrinsic rhythm electrocardiograms. Top: Bland–Altman plots illustrating interobserver variation in QRS duration measured by independent observers in 40 intrinsic rhythm electrocardiograms with left bundle branch block. Top left: comparison of the two observers with largest span in limits of agreement (LoA) (Observer 2 vs. Observer 6: 62 ms). Top right: comparison of the two observers with the smallest span in LoA (Observer 4 vs. Observer 6: 28 ms). Bottom: Bland–Altman plots illustrating interobserver variation in QRS duration measured by independent observers in 100 electrocardiograms with biventricular paced QRS complexes. Bottom left: comparison of the two observers with largest span in LoA (Observer 3 vs. Observer 6:56 ms). Bottom right: comparison of the two observers with smallest span in LoA (Observer 4 vs. Observer 6:36 ms). Mean difference in QRS duration and LoA with 95% confidence intervals are shown in all four plots (see Table 2). ECG: electrocardiogram; Obs.: observer; QRSd: QRS duration
When comparing QRSd measurements from the six observers measuring 100 biventricular paced ECGs (from 20 patients with five different VVds), absolute difference in QRSd had a mean of 3 ms (−50; 60 ms), mean LoA: −20; 27 ms. Average span between LL and UL LoA was 47 ms, ranging from 36 to 56 ms between observers (Figure 3, bottom). All LoA with 95% CI for the pairwise comparisons of observers are shown in Table 1.
Table 1.
Limits of agreement for pairwise interobserver variations in measuring QRS duration
| Observers | Mean difference, ms | LL LoA, ms | 95% CI | UL LoA, ms | 95% CI |
|---|---|---|---|---|---|
| 40 intrinsic rhythm ECGs | |||||
| 1 vs. 2 | −10 | −36 | −43; −29 | 15 | 8; 23 |
| 1 vs. 3 | −11 | −27 | −32; −22 | 6 | 1; 11 |
| 1 vs. 4 | −1 | −21 | −27; −15 | 20 | 14; 26 |
| 1 vs. 5 | −12 | −29 | −34; −24 | 6 | 1; 11 |
| 1 vs. 6 | 5 | −14 | −19; −9 | 24 | 18; 29 |
| 2 vs. 3 | 0 | −30 | −38; −21 | 29 | 21; 37 |
| 2 vs. 4 | 10 | −20 | −29; −12 | 40 | 31; 48 |
| 2 vs. 5 | −1 | −32 | −41; −23 | 30 | 21; 38 |
| 2 vs. 6 | 15 | −16 | −25; −8 | 46 | 38; 55 |
| 3 vs. 4 | 10 | −12 | −18; −6 | 32 | 26; 38 |
| 3 vs. 5 | −1 | −19 | −24; −14 | 17 | 12; 22 |
| 3 vs. 6 | 15 | −6 | −12; 0 | 37 | 31; 43 |
| 4 vs. 5 | −11 | −36 | −43; −29 | 14 | 7; 21 |
| 4 vs. 6 | 5 | −9 | −13; −5 | 19 | 15; 23 |
| 5 vs. 6 | 16 | −5 | −11; 1 | 37 | 31; 43 |
| Mean | 2 | −21 | −27; −14 | 25 | 18; 31 |
| 100 biventricular paced ECGs | |||||
| 1 vs. 2 | −7 | −30 | −34; −26 | 16 | 12; 20 |
| 1 vs. 3 | −15 | −33 | −36; −30 | 4 | 0; 7 |
| 1 vs. 4 | −3 | −29 | −33; −24 | 22 | 18; 27 |
| 1 vs. 5 | −4 | −24 | −27; −20 | 15 | 12; 18 |
| 1 vs. 6 | 6 | −15 | −18; −11 | 26 | 23; 30 |
| 2 vs. 3 | −8 | −29 | −33; −25 | 14 | 10; 18 |
| 2 vs. 4 | 4 | −22 | −26; −17 | 30 | 25; 34 |
| 2 vs. 5 | 3 | −21 | −26; −17 | 27 | 23; 31 |
| 2 vs. 6 | 13 | −14 | −19; −10 | 40 | 35; 45 |
| 3 vs. 4 | 12 | −15 | −20; −10 | 38 | 33; 43 |
| 3 vs. 5 | 10 | −13 | −17; −9 | 34 | 29; 38 |
| 3 vs. 6 | 20 | −7 | −12; −3 | 48 | 43; 53 |
| 4 vs. 5 | −1 | −28 | −32; −23 | 25 | 21; 30 |
| 4 vs. 6 | 9 | −9 | −12; −6 | 27 | 24; 30 |
| 5 vs. 6 | 10 | −13 | −18; −9 | 34 | 30; 38 |
| Mean | 3 | −20 | −24; −16 | 27 | 22; 31 |
CI: confidence intervals; ECG: electrocardiogram; LL: lower limit; LoA: limits of agreement; UL: upper limit.
Limits of agreement with 95% confidence intervals for pairwise interobserver variations between the six observers measuring QRS duration in 40 intrinsic rhythm electrocardiograms with left bundle branch block (upper part of table) and 100 biventricular paced electrocardiograms (bottom part of table).
The variation in LoA between intrinsic rhythm and paced ECGs did not differ significantly (difference in LL intrinsic and LL paced QRSd, p = 0.68; difference in UL intrinsic and UL paced QRSd, p = 0.44).
3.2. Intraobserver variation in intrinsic rhythm and paced ECG QRSd
For Observer a, intraobserver variation of QRSd in 20 intrinsic rhythm ECGs showed variation with a mean of −2.5 ms (−20; 20 ms), LoA: −24; 19 ms. For Observer b, QRSd varied with a mean of 4.5 ms (−10; 20 ms), LoA: −13; 22 ms (Figure 4, top). For Observer a, intra‐observer variation of QRSd in 100 paced ECGs was −6.3 ms in mean (−30; 10 ms) with LoA: −27; 14 ms. For Observer b, QRSd varied with a mean of −1.3 ms (−20; 20 ms) with LoA: −17; 14 ms (Figure 4, bottom).
Figure 4.

Intraobserver variation in QRS duration measured in paced and intrinsic rhythm electrocardiograms. Top: Bland–Altman plots illustrating intraobserver variation in two repeated measurements of QRS duration performed by Observers a and b in 20 intrinsic rhythm electrocardiograms. Observer a: limits of agreement (LoA): lower limit (LL) −24 ms (95% confidence interval [CI] −33; −15 ms); upper limit (UL) 19 ms (95% CI 10; 28 ms), Observer b: LoA: LL −13 ms (95% CI −20; −6 ms); UL 22 ms (95% CI 15; 29 ms). Bottom: intraobserver variation in two repeated measurements of QRS duration performed by Observers a and b in 100 paced electrocardiograms. Observer a: LoA: LL −27 ms (95% CI −30; −23 ms); UL 14 ms (95% CI 10; 17 ms), Observer b: LoA: LL −17 ms (95% CI −19; −14 ms); UL 14 ms (95% CI 11; 17 ms). Mean difference between first and second measurement of QRS duration and LoA with 95% CI are shown in all four plots. ECG: electrocardiogram; Obs.: observer; QRSd: QRS duration
3.3. Interobserver precision in selecting the VVd resulting in the narrowest QRS complex
In the majority of patients, more than one VVd was defined as optimal when aiming for narrowest paced QRS complex (Table 2). In 12/20 (65%) of the patients, the six observers identified >1 VVd (median 2) resulting in the narrowest QRS complex. Analyzing the 20 sets of ECGs with five different VVds, mean change in QRSd between the different VVds within the same patient for the 6 observers was 22 ms (12; 38).
Table 2.
Selections of interventricular pacing delay creating the shortest QRS duration performed by six observers
| Patient | RV20 | SIM | LV20 | LV40 | LV60 | Total number of VVds selected by six observers to result in the shortest QRSd | Mean range in QRSd, ms | Absolute range in QRSd measured by any of the six observers at any of the five VVds, ms | |
| 1 | 2 | 3 | 5 | 3 | 1 | 14 | 18 | (110;140) | |
| 2 | 0 | 4 | 6 | 0 | 0 | 10 | 15 | (110;140) | |
| 3 | 4 | 4 | 3 | 1 | 0 | 12 | 25 | (130;180) | |
| 4 | 2 | 1 | 6 | 1 | 0 | 10 | 20 | (130;160) | |
| 5 | 1 | 3 | 5 | 0 | 1 | 10 | 22 | (110;150) | |
| 6 | 3 | 3 | 3 | 4 | 3 | 16 | 18 | (100;140) | |
| 7 | 1 | 3 | 6 | 2 | 0 | 12 | 27 | (130;180) | |
| 8 | 3 | 3 | 4 | 2 | 0 | 12 | 25 | (140;180) | |
| 9 | 4 | 4 | 2 | 0 | 0 | 10 | 38 | (120;180) | |
| 10 | 1 | 1 | 6 | 4 | 2 | 14 | 12 | (150;170) | |
| 11 | 0 | 0 | 4 | 5 | 0 | 9 | 25 | (100;150) | |
| 12 | 1 | 1 | 3 | 4 | 0 | 9 | 25 | (110;150) | |
| 13 | 2 | 5 | 4 | 3 | 1 | 15 | 18 | (100;140) | |
| 14 | 3 | 5 | 1 | 0 | 0 | 9 | 33 | (120;160) | |
| 15 | 1 | 6 | 4 | 1 | 0 | 12 | 17 | (130;170) | |
| 16 | 1 | 3 | 4 | 5 | 1 | 14 | 20 | (110;150) | |
| 17 | 1 | 0 | 2 | 5 | 1 | 9 | 18 | (120;150) | |
| 18 | 2 | 1 | 5 | 5 | 1 | 14 | 13 | (140;170) | |
| 19 | 4 | 3 | 1 | 1 | 0 | 9 | 25 | (130;170) | |
| 20 | 0 | 0 | 2 | 6 | 1 | 9 | 25 | (100;140) | |
| Sum | 36 | 53 | 76 | 52 | 12 | 229 | Average | 22 | (120;159) |
LV20/40/60: pacing of left ventricle 20/40/60 ms prior to right ventricle, respectively; QRSd: QRS duration; RV20: pacing of right ventricle 20 ms prior to left ventricle; SIM: simultaneous pacing of both ventricles. Left: numbers in RV20‐ LV60 indicate the number out of the six observers, who selected that specific interventricular pacing delay (VVd) as the one, or one of more, resulting in the shortest QRS duration. Highlights: dark blue = the consensus optimal VVd: the VVd selected most frequently by the six observers to result in the shortest QRS duration for each patient; medium blue = the second most frequently selected VVd; light blue = the VVd selected most rarely. Right: mean and absolute range in QRS duration for the six observers when changing VVd from RV20 to LV60.
On average, observers included the consensus optimal VVd in their selection of VVds in 17/20 (85%) of the patients. In the 3/20 (15%) cases, where observers did not include the consensus VVd in the selection of optimal VVds, the difference in QRSd from the consensus VVd was 10–20 ms.
In all the VVd settings, selected as optimal by all observers, only 36 of 229 (16%) differed more than one setting (±20 ms) from the consensus VVd.
Interobserver sensitivity was 81% for including consensus VVd in the selection of VVd(s). Interobserver specificity for choosing same VVd was 74% with a positive predictive value of 49% and negative predictive value of 94%.
3.4. Intraobserver precision in selecting the VVd creating the narrowest QRS complex
Observers a and b selected the same VVd when repeating ECG‐guided VVd optimization in 17/20 (85%) and 16/20 (80%) of the patients, respectively. Of these, the same VVd was included in a selection of optimal VVds in 12/17 and 10/16 of the patients in second ECG analysis, respectively. Differences in QRSd measurements led to a different selection of optimal VVd in 3/20 (15%) and 4/20 (20%) of the patients for Observers a and b, respectively. This was based on absolute maximal differences in measured QRSd of 30 and 10 ms, respectively. When selecting VVd second time, the two observers selected a VVd setting more than two settings away (> ± 20 ms) in 7 of the total 42 VVd selections (17%) for Observer a and in 5/37 (14%) for Observer b.
4. DISCUSSION
This study reports clinically acceptable and comparable inter and intraobserver precision in measuring QRSd in both intrinsic rhythm and biventricular paced ECGs from LBBB patients treated with CRT.
The results suggest consistency in reproducibility and repeatability of VVd optimization, with a good likelihood for the observers to select the same VVd setting within 20 ms. Within patients, the biventricular paced QRSd measured at different VVds differed with a mean of 22 ms.
4.1. Measuring QRSd
A narrow biventricular paced QRS complex has been associated with response to CRT (Hsing et al., 2011), and further narrowing of paced QRS width by altering VVd has been shown to increase the acute hemodynamic response to CRT (Tamborero et al., 2009). Thus, a good reproducibility of measurement of QRSd may be important for an optimal ECG‐guided VVd optimization after CRT.
Measurement of maximal QRSd in 12‐lead ECGs is the most commonly used definition of QRSd (Cleland et al., 2005). Even though guidelines use QRSd as criterion for receiving CRT and for ECG‐guided optimization after CRT (European Society of Cardiology (ESC) et al., 2013), no official guidelines on how to measure QRSd before CRT exist. Precise identification of the onset of the QRS complex in a paced ECG can be more difficult than determining the onset in an intrinsic rhythm ECG, since electrical signals that do not necessarily represent the onset of the QRS complex will be recorded in the ECG after the pacing artifact. Also, termination of QRS is often less clearly defined in paced ECGs and ECGs with LBBB, with a more gradual change in the inclination sometimes taking 0.04 s or more. Such a gradual transition could lead to both greater inter and intra‐observer variation in measurement of QRSd in ECGs with LBBB as compared with ECGs without conduction disturbances (Lepeschkin & Surawicz, 1952). In addition, increasing QRS width by increasing the ECG paper speed might not make it easier to determine the onset or termination of the QRS complex as more details on cardiac electrical signals could be revealed. The interobserver variation in identification of the onset and offset of the QRS complex is clearly illustrated in Figure 2. Recommendations from the American Heart Association suggests that global QRSd defined as the interval between first onset of QRS in any lead until the latest offset in any lead from is the most correct way to measure QRSd (Surawicz et al., 2009). However, it has been shown that interobserver difference in measurements of global QRSd increases when measuring QRSd in LBBB and even more when measuring paced QRS complexes as compared with measurement of QRSd in narrow QRS complexes (De Pooter et al., 2017).
In this study, LoA for QRSd were comparable for paced and LBBB intrinsic rhythm ECGs (both inter and intraobserver). The LoA for QRSd were all around ±20 ms, which is judged to be acceptable from a clinical view. Furthermore, the LoA for QRSd were in accordance with the LoA reported in the study by Pooter et al. (De Pooter et al., 2017) reporting on global QRSd. However, the span in interobserver LoA was moderate, when comparing the pairs of observers with the largest and smallest span in LoA (Figure 3).
4.2. Implications of inter and intraobserver variation for the reproducibility of ECG‐guided VVd optimization
Intra and interobserver agreement on VVd selection was comparable with a precision of 80%–85%. A reproducible pattern in the selection of consensus VVd for most patients was seen, with only few selections of optimal VVd not within ±20 ms from the consensus VVd (Table 2). However, from the interobserver results on selection of VVd, it can be seen that potential different selections of VVd settings may vary up to 80 ms. In these cases, it is noticeable that the relative change in QRSd with changing VVd differs between individuals and that often more than one VVd is considered optimal.
4.3. Limitations
We used different paper speed for recording ECGs for the paced and intrinsic rhythm ECGs, as 25 mm/s is the paper speed used for intrinsic rhythm ECGs and 50 mm/s is the paper speed used for ECG‐guided VVd optimization at our institution.
The consensus optimal VVd was selected as a circular reference, as the one selected by the majority of investigators since there is no generally accepted definition of the true optimal VVd in CRT patients.
QRS duration interobserver variation and intraobserver variation in the current study were not compared with variation from automated measurements, but as shown in the study by De Pooter et al., the span in LoA in paced ECGs was larger for automated measurements than for manual measurements, whereas LoA for automated and manual measurements in intrinsic rhythm ECGs with LBBB were comparable (De Pooter et al., 2017).
In a routine clinical setting, simultaneous semi‐quantitative examination of all ECGs recorded at different VVd settings is often performed to select the VVd setting that produces the narrowest QRS complex. In this study, observers were instructed to measure the widest QRSd in ECGs obtained at different VVd settings and define the optimal VVd from these measurements. However, this might have resulted in a more optimal and reproducible selection of VVds. Whether a mean change in QRSd of 22 ms when changing VVd from RV20 to LV60 is clinically important is not within the frame of the current study but is of future interest.
5. CONCLUSIONS
Interobserver variation and intraobserver variation in manually measured paced and intrinsic rhythm QRSd are clinically acceptable and comparable in a cohort of patients receiving CRT. Selection of the optimal VVd producing the shortest QRSd is reproducible within ±20 ms both within and between observers. The reproducibility is good and warrants manual VVd optimization for QRS narrowing in CRT. The clinical impact of optimizing VVd based on shortest QRSd needs further investigation in prospective, randomized studies.
CONFLICT OF INTEREST
Charlotte Stephansen, Christoffer Tobias Witt, Jens Kristensen, Christian Gerdes, and Anders Sommer have no conflict of interest to declare. Mads Brix Kronborg has received speaker's fee from Biotronik. Jensen has received speaker's fee from Bracco Imaging. Jens Cosedis Nielsen is supported by a grant from the Novo Nordisk Foundation (NNF16OC0018658).
Stephansen C, Kronborg MB, Witt CT, et al. Reproducibility of measuring QRS duration and implications for optimization of interventricular pacing delay in cardiac resynchronization therapy. Ann Noninvasive Electrocardiol. 2019;24:e12621 10.1111/anec.12621
Funding information
This study is financially supported with unrestricted grants from the Danish Heart Foundation (grant no. 14‐R97‐A5149‐22865 and 15‐R99‐A5878‐22937).
REFERENCES
- Bland, J. M. , & Altman, D. G. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet, 1(8476), 307–310. S0140-6736(86)90837-8 [PubMed] [Google Scholar]
- European Society of Cardiology (ESC) , European Heart Rhythm Association (EHRA) , Brignole, M. , Auricchio, A. , Baron‐Esquivias, G. , Bordachar, P. , … Vardas, P. E. (2013). 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: The task force on cardiac pacing and resynchronization therapy of the european society of cardiology (ESC). developed in collaboration with the european heart rhythm association (EHRA). Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology, 15(8), 1070–1118. 10.1093/europace/eut206; 10.1093/europace/eut206 [DOI] [PubMed] [Google Scholar]
- Cleland, J. G. F. , Daubert, J.‐C. , Erdmann, E. , Freemantle, N. , Gras, D. , Kappenberger, L. , … Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators (2005). The effect of cardiac resynchronization on morbidity and mortality in heart failure. The New England Journal of Medicine, 352(15), 1539–1549. 10.1056/NEJMoa050496 [DOI] [PubMed] [Google Scholar]
- Daubert, C. , Behar, N. , Martins, R. P. , Mabo, P. , & Leclercq, C. (2017). Avoiding non‐responders to cardiac resynchronization therapy: A practical guide. European Heart Journal, 38(19), 1463–1472. 10.1093/eurheartj/ehw270 [DOI] [PubMed] [Google Scholar]
- De Guillebon, M. , Thambo, J. B. , Ploux, S. , Deplagne, A. , Sacher, F. , Jais, P. , … Bordachar, P. (2010). Reliability and reproducibility of QRS duration in the selection of candidates for cardiac resynchronization therapy. Journal of Cardiovascular Electrophysiology, 21(8), 890–892. 10.1111/j.1540-8167.2010.01743.x [DOI] [PubMed] [Google Scholar]
- De Pooter, J. , El Haddad, M. , Stroobandt, R. , De Buyzere, M. , & Timmermans, F. (2017). Accuracy of computer‐calculated and manual QRS duration assessments: Clinical implications to select candidates for cardiac resynchronization therapy. International Journal of Cardiology, 236, 276–282. S0167-5273(16)34305-4 [DOI] [PubMed] [Google Scholar]
- Francis, D. P. (2013). How to reliably deliver narrow individual‐patient error bars for optimization of pacemaker AV or VV delay using a "pick‐the‐highest" strategy with haemodynamic measurements. International Journal of Cardiology, 163(3), 221–225. 10.1016/j.ijcard.2012.03.128 [DOI] [PubMed] [Google Scholar]
- Gervais, R. , Leclercq, C. , Shankar, A. , Jacobs, S. , Eiskjaer, H. , Johannessen, A. , … CARE‐HF Investigators (2009). Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: A sub‐analysis of the CARE‐HF trial. European Journal of Heart Failure, 11(7), 699–705. 10.1093/eurjhf/hfp074 [DOI] [PubMed] [Google Scholar]
- Hsing, J. M. , Selzman, K. A. , Leclercq, C. , Pires, L. A. , McLaughlin, M. G. , McRae, S. E. , … Zimetbaum, P. J. (2011). Paced left ventricular QRS width and ECG parameters predict outcomes after cardiac resynchronization therapy: PROSPECT‐ECG substudy. Circulation: Arrhythmia and Electrophysiology, 4(6), 851–857. 10.1161/CIRCEP.111.962605 [DOI] [PubMed] [Google Scholar]
- Lepeschkin, E. , & Surawicz, B. (1952). The measurement of the duration of the QRS interval. American Heart Journal, 44(1), 80–88. 0002-8703(52)90174-9 [DOI] [PubMed] [Google Scholar]
- Mantha, S. , Roizen, M. F. , Fleisher, L. A. , Thisted, R. , & Foss, J. (2000). Comparing methods of clinical measurement: Reporting standards for bland and altman analysis. Anesthesia and Analgesia, 90(3), 593–602. 10.1097/00000539-200003000-00018 [DOI] [PubMed] [Google Scholar]
- Risum, N. , Strauss, D. , Sogaard, P. , Loring, Z. , Hansen, T. F. , Bruun, N. E. , … Kisslo, J. (2013). Left bundle‐branch block: The relationship between electrocardiogram electrical activation and echocardiography mechanical contraction. American Heart Journal, 166(2), 340–348. 10.1016/j.ahj.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Ruschitzka, F. , Abraham, W. T. , Singh, J. P. , Bax, J. J. , Borer, J. S. , Brugada, J. … EchoCRT Study Group (2013). Cardiac‐resynchronization therapy in heart failure with a narrow QRS complex. The New England Journal of Medicine, 369(15), 1395–1405. 10.1056/NEJMoa1306687;10.1056/NEJMoa1306687 [DOI] [PubMed] [Google Scholar]
- Sommer, A. , Kronborg, M. B. , Norgaard, B. L. , Poulsen, S. H. , Bouchelouche, K. , Bottcher, M. , … Nielsen, J. C. (2016). Multimodality imaging‐guided left ventricular lead placement in cardiac resynchronization therapy: A randomized controlled trial. European Journal of Heart Failure, 18(11), 1365–1374. 10.1002/ejhf.530 [DOI] [PubMed] [Google Scholar]
- Strauss, D. G. , Selvester, R. H. , & Wagner, G. S. (2011). Defining left bundle branch block in the era of cardiac resynchronization therapy. The American Journal of Cardiology, 107(6), 927–934. 10.1016/j.amjcard.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Surawicz, B. , Childers, R. , Deal, B. J. , Gettes, L. S. , Bailey, J. J. , Gorgels, A. , … Heart Rhythm Society (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part III: Intraventricular conduction disturbances: A scientific statement from the american heart association electrocardiography and arrhythmias committee, council on clinical cardiology; the american college of cardiology foundation; and the heart rhythm society. endorsed by the international society for computerized electrocardiology. [AHA definition af LBBB]. Journal of the American College of Cardiology, 53(11), 976–981. 10.1016/j.jacc.2008.12.013 [DOI] [PubMed] [Google Scholar]
- Tamborero, D. , Mont, L. , Sitges, M. , Silva, E. , Berruezo, A. , Vidal, B. , … Brugada, J. (2009). Optimization of the interventricular delay in cardiac resynchronization therapy using the QRS width. The American Journal of Cardiology, 104(10), 1407–1412. 10.1016/j.amjcard.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Tomlinson, D. R. , Bashir, Y. , Betts, T. R. , & Rajappan, K. (2009). Accuracy of manual QRS duration assessment: Its importance in patient selection for cardiac resynchronization and implantable cardioverter defibrillator therapy. Europace: European Pacing, Arrhythmias, and Cardiac Electrophysiology, 11(5), 638–642. 10.1093/europace/eup001 [DOI] [PubMed] [Google Scholar]
- Zareba, W. , Klein, H. , Cygankiewicz, I. , Hall, W. J. , McNitt, S. , Brown, M. , … Moss, A. J. (2011). Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy (MADIT‐CRT). Circulation, 123(10), 1061–1072. 10.1161/CIRCULATIONAHA.110.960898 [DOI] [PubMed] [Google Scholar]


