Abstract
Background
J wave, or early repolarization has recently been associated with an increased risk of lethal arrhythmia and sudden death, both in idiopathic ventricular fibrillation and in the general population. Hypercalcemia is one of the causes of J point and ST segment elevation, but the relationship has not been well studied. The aim of this study was to examine the effects of hypercalcemia on J point elevation.
Methods
Electrocardiographic findings were compared in 89 patients with hypercalcemia and 267 age‐ and sex‐matched healthy controls with normocalcemia. The association of J point elevation with arrhythmia events in patients with hypercalcemia was also studied.
Results
The PR interval and the QRS duration were longer in patients with hypercalcemia than in normocalcemic controls. Both the QT and the corrected QT intervals were shorter in patients with hypercalcemia compared with normocalcemic controls. Conduction disorders, ST‐T abnormalities, and J point elevation were more common in patients with hypercalcemia than normocalcemic controls. Following the resolution of hypercalcemia, the frequency of J point elevation decreased to a level similar to that noted in controls. During hospitalization, no arrhythmia event occurred in patients with hypercalcemia.
Conclusion
Hypercalcemia was associated with J point elevation.
Keywords: hypercalcemia, early repolarization, Brugada syndrome, J wave, J point elevation
J wave, or early repolarization, is characterized by an elevation at the junction between the end of the QRS complex and the beginning of the ST segment (J point) in a 12‐lead electrocardiogram (ECG). The presence of J wave has been considered a benign phenomenon for decades.1 However, there is increasing evidence that early repolarization is associated with an increased risk of ventricular fibrillation and sudden cardiac death.2, 3, 4
J wave has been linked to various biological conditions, such as low body temperature and ischemia.5, 6 Furthermore, hypercalcemia, which may alter cardiac electrophysiology, is another cause of J wave.7, 8, 9 However, the relationship among hypercalcemia and J point and ST segment elevation has been described primarily in case reports, with the exception of one case series7, 10, 11, 12, 13, 14, 15, 16, 17, 18 but has not been studied in detail. Furthermore, the association of hypercalcemia with fatal arrhythmias has been described in only a few case reports and is not well understood.13, 16 Therefore, this study aimed to investigate the role of hypercalcemia in both J point elevation and arrhythmia.
METHODS
Study Population
We conducted a retrospective chart review of the electronic records of patients in our hospitals between 2005 and 2013 to identify patients with hypercalcemia, which was defined as a serum Ca2+ level, corrected for serum albumin, >12 mg/dL. Patients with sinus rhythm as determined in ECGs recorded at the time of their hypercalcemia who did not have structural heart disease were eligible for the analysis. Patients with other causes of J point elevation, including hypercalcemia (K > 5.5 mEq/L), acidosis (pH < 7.35), and hypothermia, were excluded.
Control subjects with sinus rhythm and normocalcemia, defined as a corrected serum Ca2+ level from 8.0 to 10.0 mg/dL, were selected from 86,068 consecutive ECGs stored within the ECG database of Niigata University Medical and Dental Hospital. The subjects were matched to patients with hypercalcemia based on both age and sex (patient: control ratio, 1:3). Subjects with either renal dysfunction or structural heart disease were excluded.
Electrocardiographic Measurements
ECG findings were compared between patients with hypercalcemia and normocalcemic controls. The corrected QT (QTc) interval was calculated using Bazett's formula.19 “J point elevation” was defined as an elevation of the J point, either as a QRS slurring or a notching ≥0.1 mV in ≥2 consecutive leads of a 12‐lead electrocardiogram.2
Statistical Analysis
The data are presented as the medians (25th and 75th percentiles), or numbers (%). The continuous variables were compared using either the unpaired Student's t‐test or the nonparametric Mann‐Whitney's U‐test between groups, or via either the paired t‐test or the nonparametric Wilcoxon rank‐sum test between different serum Ca2+ levels, as appropriate. The categorical variables were compared using the chi‐square test in between groups, or McNemar test between different serum Ca2+ levels. The effects of covariates on J point elevation among hypercalcemic patients were analyzed using multivariate logistic regression models. A two‐sided P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 21.0 (IBM Inc., Armonk, NY, USA). The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agreed with the manuscript as written.
RESULTS
Among the 532 patients with hypercalcemia who were treated at our institutions, we identified 89 patients with 12‐lead ECGs (34 male [38%]; median age, 64 years) with findings consistent with hypercalcemia (Table 1). Common causes of hypercalcemia included malignancy (n = 35 [39%]), primary hyperparathyroidism (n = 25 [28%]), and renal dysfunction (n = 18 [20%]). Among the ECG findings, both the PR interval and the QRS duration were longer in patients with hypercalcemia compared with age‐ and sex‐matched normocalcemic controls (Table 2). Both the QT and the corrected QT intervals were shorter in patients with hypercalcemia than in normocalcemic controls. Conduction disorder and ST‐T abnormalities were each more common in patients with hypercalcemia compared with normocalcemic controls. J point elevation was more common in patients with hypercalcemia (n = 27 [30%]) than in normocalcemic controls (n = 23, [9%]).
Table 1.
Clinical Characteristics of Hyercalcemic Patients
| Characteristic | Patients, n = 89 |
|---|---|
| Male sex, no. (%) | 34 (38) |
| Age (years)a | 64 (54‐72) |
| Etiologies of hypercalcemia | |
| Hyperparathyroid (primary and secondary) | 36 (40) |
| Cancer | 34 (38) |
| Overdose of vitamin D | 10 (11) |
| Saricoidosis | 4 (4) |
| Others | 5 (6) |
| Hypertension, no. (%) | 25 (28) |
| Diabetes mellitus, no. (%) | 15 (17) |
| Heart diseases, no. (%) | 5 (6) |
| Medication, no. (%) | 26 (29) |
| β‐Blocker, no. (%) | 4 (4) |
| Antiarrhythmic drugs,b no. (%) | 2 (2) |
| Ca2+ channel blocker, no. (%) | 20 (22) |
aMedian (interquartile range); aOne patient had pilsicainide and another had amiodarone.
Table 2.
Comparison of Electrocardiographic Characteristics in Hypercalcemia Patients and Normocalcemia Controls
| Hypercalcemia, n = 89 | Normocalcemia Control, n = 267 | P Value | |
|---|---|---|---|
| Heart rate (bpm)a | 76 (66–89) | 68 (61–76) | <0.001 |
| PQ interval (ms)a | 160 (145–185) | 148 (137–164) | <0.001 |
| QRS duration (ms)a | 99 (92–108) | 92 (86–99) | <0.001 |
| QT interval (ms)a | 354 (332–372) | 378 (361–397) | <0.001 |
| Corrected QT interval (ms)a | 396 (378–417) | 407 (391–419) | 0.01 |
| Conduction disorder, no. (%) | 22 (25) | 10 (4) | <0.001 |
| Prolonged PR interval, no. (%) | 12 (13) | 8 (3) | <0.001 |
| Right bundle branch block, no. (%) | 4 (4) | 2 (0.7) | 0.04 |
| Left anterior hemiblock, no. (%) | 10 (11) | 4(1) | <0.001 |
| Left bundle branch block, no. (%) | 1 (1) | 0 (0) | 0.25 |
| ST segment/T wave abnormality, no. (%) | 29 (33) | 12 (4) | <0.001 |
| J point elevation, no. (%) | 27 (30) | 23 (9) | <0.001 |
| Location of J point elevation | |||
| Inferior leads, no. (%) | 9 (10) | 10 (4) | 0.03 |
| Lateral leads, no. (%) | 4 (4) | 9 (3) | 0.74 |
| Right precordial leads, no. (%) | 7 (8) | 1 (0.4) | <0.001 |
| Multiple locations, no. (%) | 7 (8) | 3 (1) | 0.003 |
Median (interquartile range). CI, confidence interval.
After exclusion of the patients who had abnormal serum electrolyte levels or antiarrhythmic drugs, both of which may affect ECG findings analysis was repeated in 50 patients with hypercalcemia and 150 controls (Table S1). We replicated similar results that hypercalcemia was associated with conduction disorder, ST segment/T wave abnormality, and J wave.
Although the causes of hypercalcemia were various, majority of patients had hyperparathyroidism or cancer. Therefore, we compered ECG findings between patients with hyperparathyroidism and those with cancer (Table S2). The frequency of conduction disorder, ST segment/T wave abnormality, and J wave were similar between two groups.
The clinical and ECG characteristics of the 27 patients with hypercalcemia who had early repolarization, and the representative ECGs characterized by J point elevation, are included in Table 3 and Figure 1, respectively. J point elevation was noted in the inferior leads in 9 patients (10%), in the lateral leads in 4 patients (4%), and the right precordial leads in 7 patients (8%). J point elevation was noted at multiple locations in 7 patients (8%). There were three morphologies of J point elevation, including a “scooped appearance” J point and ST segment elevation without a distinct T wave (n = 5),18 Brugada‐type ECG findings (n = 5),20 and an early repolarization pattern without ST segment elevation (n = 17).
Table 3.
Clinical and Electrocardiographic Characteristics of Hypercalcemia Patients with J Point Elevation
| No. | Sex | Age (year)a | Etiology of Hypercalcemia | Ca2+ Levels (mg/dL)a | Heart Rate (bpm)a | PR Interval (ms)a | QRS Duration, (ms)a | QT Interval (ms)a | QTc Interval (ms)a | Conduction Disorder | ST Segment/T Wave Abnormality | Location of J Point Elevation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 47 | Primary hyperparathyroidism | 12.1 | 70 | 160 | 112 | 350 | 379 | – | ST elevation in I II V6 | I II V6 |
| 2 | F | 35 | Over dose of Vitamin D | 14.2 | 71 | 153 | 81 | 354 | 385 | – | – | V3‐6,I II |
| 3 | F | 48 | Primary hyperparathyroidism | 13.0 | 67 | 143 | 87 | 350 | 370 | – | – | IaVL V5‐6 |
| 4 | F | 78 | Hepatocellular cancer | 13.9 | 48 | 227 | 94 | 402 | 363 | Prolonged PR | – | I II aVL V5‐6 |
| 5 | M | 50 | Squamous cell cancer | 15.0 | 117 | 157 | 93 | 273 | 382 | – | – | II aVF V6 |
| 6 | M | 64 | Esophageal cancer | 13.2 | 80 | 155 | 106 | 391 | 450 | – | ST elevation in II III aVF | II III aVF |
| 7 | M | 67 | Laryngeal cancer | 12.2 | 60 | 212 | 88 | 392 | 302 | Prolonged PR | – | II III aVF |
| 8 | F | 59 | Primary hyperparathyroidism | 12.0 | 62 | 187 | 113 | 362 | 370 | – | – | II III aVF |
| 9 | F | 60 | Secondary hyperparathyroidism | 13.7 | 63 | 193 | 90 | 387 | 397 | – | – | III aVF |
| 10 | F | 65 | Sarcoidosis | 12.9 | 69 | 131 | 106 | 368 | 397 | – | – | II III aVF |
| 11 | F | 65 | Ovarian cancer | 15.6 | 89 | 175 | 86 | 320 | 391 | – | ST elevation in V1‐2 | II III aVF |
| 12 | F | 68 | Primary hyperparathyroidism | 12.9 | 91 | 173 | 100 | 320 | 395 | – | – | II III aVF |
| 13 | M | 35 | Primary hyperparathyroidism | 12.0 | 62 | 147 | 112 | 340 | 348 | – | ST elevation in I II V3‐6 | II III aVF6 |
| 14 | M | 72 | Primary hyperparathyroidism | 12.7 | 68 | 141 | 100 | 357 | 380 | – | – | I II aVF V4‐6 |
| 15 | M | 62 | Oropharyngeal cancer | 14.4 | 89 | 128 | 88 | 372 | 452 | – | – | I II aVF V6 |
| 16 | F | 55 | Primary hyperparathyroidism | 13.4 | 58 | 168 | 100 | 365 | 361 | – | – | I,II aVF,V6 |
| 17 | M | 27 | Primary hyperparathyroidism | 15.0 | 61 | 178 | 102 | 386 | 388 | LAH | ST elevation in V1‐3 | V1‐3 |
| 18 | M | 57 | Non‐Hodgkin's lymphoma | 16.9 | 66 | 167 | 107 | 362 | 381 | – | ST elevation and T wave invertion in V1‐2 | V1‐3 |
| 19 | M | 59 | Non‐Hodgkin's lymphoma | 17.3 | 87 | 210 | 114 | 337 | 408 | Prolonged PR | ST elevation in V1‐2 | V2‐3 |
| 20 | M | 69 | Unknown | 12.6 | 75 | 196 | 110 | 335 | 375 | – | ST elevation in V1‐3 | V1‐3 |
| 21 | F | 64 | Squamous cell cancer | 13.8 | 100 | 208 | 96 | 354 | 456 | – | ST elevation in V1‐2 | V1‐2 |
| 22 | F | 73 | Buccal mucosa cancer | 15.8 | 78 | 145 | 98 | 336 | 384 | – | ST elevation in V1‐2 | V1‐2 |
| 23 | F | 80 | Overdose of vitamin D | 13.2 | 71 | 325 | 120 | 338 | 369 | Prolonged PR | ST elevation in V1‐3 | V1‐2 |
| 24 | M | 58 | Primary hyperparathyroidism | 12.5 | 55 | 183 | 115 | 362 | 348 | – | ST elevation in I II V1‐6 | I II V4‐6, V1 |
| 25 | F | 84 | Tongue cancer | 15.2 | 89 | 174 | 165 | 370 | 452 | LBBB | ST elevation in I aVL V2‐5 | I II aVL V1‐5 |
| 26 | M | 64 | Overdose of vitamin D | 15.5 | 79 | 216 | 90 | 352 | 403 | Prolonged PR | ST elevation in V2‐3, ST depression in V5‐6 | II III aVf V2‐3 |
| 27 | M | 64 | Overdose of vitamin D | 15.2 | 86 | 157 | 108 | 329 | 395 | – | ST elevation in V1‐3 | II III aVF V1‐3 |
| Total | M:14 | 64 (55–68) | 13.7 (12.7–15.2) | 71 (62–87) | 173 (153–196) | 100 (90–112) | 354 (337–370) | 384 (370–397) | Yes: 7 | Yes: 15 | inferior: 9, lateral: 4, right precordial: 7, multiple : 7 |
Median (interquartile range). LAH, left anterior hemiblock; LBBB, left bundle branch block.
Figure 1.
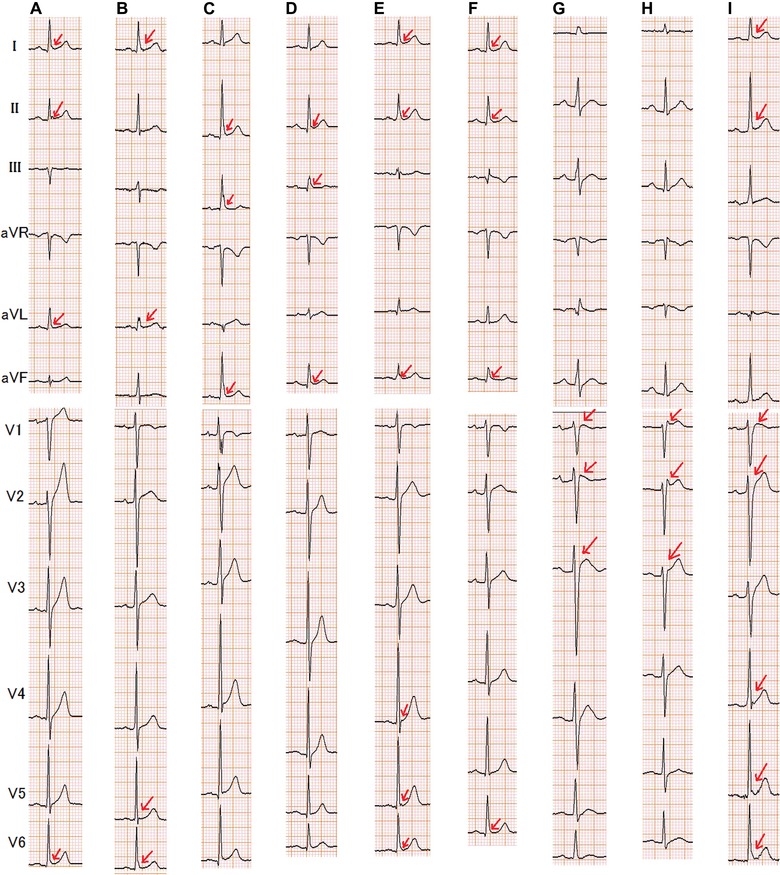
Representative 12‐lead ECGs recorded in hypercalcemic patients showing early repolarizations. (A) Patient 1; (B) Patient 3; (C) Patient 13; (D) Patient 12; (E) Patient 14; (F) Patient 16; (G) Patient 19; (H) Patient 20; (I) Patient 24. Patient numbers correspond to those listed in Table 3. The arrows indicate J point elevation.
Among the 89 patients with ECGs recorded in the setting of hypercalcemia, there were no differences in the ages, sex, serum electrolyte levels, and causes of hypercalcemia between patients with J point elevation and those without J point elevation (Table 4). Both the QT interval and the corrected QT interval were shorter in patients with J point elevation compared with those without J point elevation, whereas the heart rate, PQ interval, and QRS duration were similar between the two groups. The frequency of conduction disorder including prolonged PR interval, right bundle branch block, left anterior hemiblock, and left bundle branch block was similar between two groups. ST segment/T wave abnormality was more common in patients with J point elevation compared with those without J point elevation.
Table 4.
Comparison of Characteristics in Hypercalcemia Patients with J Point Elevation and Those without J Point Elevation
| J Point Elevation (+) | J Point Elevation (–) | ||
|---|---|---|---|
| Characteristic | n = 27 | n = 62 | P Value |
| Age (years)a | 64 (55–68) | 64 (54–73) | 0.46 |
| Male sex, no. (%) | 14 (52) | 20 (32) | 0.08 |
| Etiology of hypercalcemia | |||
| Hyperparathyroidism, no. (%) | 10 (37) | 26 (42) | 0.67 |
| Cancer, no. (%) | 11 (41) | 23 (37) | 0.75 |
| Overdose of vitamin D, no. (%) | 4 (15) | 6 (10) | 0.34 |
| Sarcoidosis, no. (%) | 1 (4) | 3 (5) | 0.65 |
| Others, no. (%) | 1 (4) | 4 (6) | 0.52 |
| Diabetes mellitus, no. (%) | 5 (16) | 10 (16) | 0.50 |
| Hypertension, no. (%) | 6 (22) | 19 (31) | 0.42 |
| Serum Ca2+ (mg/dL)a | 13.7 (12.7–15.2) | 13.1 (12.4–14.0) | 0.13 |
| Serum K+ (mEq/L)a | 3.9 (3.5‐4.4) | 4.3 (3.7‐4.5) | 0.53 |
| Serum creatinine (mg/dL)a | 1.1 (0.7‐2.2) | 1.1 (0.7‐2.2) | 0.69 |
| Ca2+ channel blocker, no. (%)a | 5 (19) | 15 (24) | 0.56 |
| Heart rate (bpm)a | 71 (62–87) | 77 (66–91) | 0.29 |
| PQ interval (ms)a | 173 (153–196) | 156 (143–181) | 0.08 |
| QRS duration (ms)a | 100 (90–112) | 98 (92–107) | 0.56 |
| QT interval (ms)a | 354 (337–370) | 353 (329–374) | 0.94 |
| Corrected QT interval (ms)a | 384 (370–397) | 400 (382–420) | 0.02 |
| Conduction disorder, no. (%) | 7 (26) | 15 (24) | 0.86 |
| Prolonged PR interval, no. (%) | 5 (19) | 7 (11) | 0.27 |
| Right bundle branch block, no. (%) | 0 (0) | 4 (6) | 0.23 |
| Left anterior hemiblock, no. (%) | 1 (4) | 9 (15) | 0.13 |
| Left bundle branch block, no. (%) | 1 (4) | 0 (0) | 0.30 |
| ST segment/T wave abnormality, no. (%) | 15 (56) | 14 (23) | 0.002 |
Median (interquartile range).
In univariate models, among ECG findings, shortening of QT interval (odds ratio, 0.986; 95% confidence interval, 0.972–1.000; P = 0.046) and ST segment/T wave abnormality (odds ratio, 4.286; 95% confidence interval, 1.633–11.246; P = 0.003) were associated with the presence of early repolarization in patients with hypercalcemia. Multivariate models adjusted for sex, age, and all covariates revealed that shortening of corrected QT interval (odds ratio, 0.985; 95% confidence interval, 0.971–1.000; P = 0.045) and ST segment/T wave abnormality (odds ratio, 4.091; 95% confidence interval, 1.486–11.261; P = 0.01) were associated with the presence of early repolarization in patients with hypercalcemia (Table 5).
Table 5.
Association of ECG Findings with J Point Elevation in Hypercalcemia
| Effect | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Male sex | 0.481 | 0.172–1.348 | 0.16 |
| Age | 0.998 | 0.964–1.034 | 0.93 |
| Corrected QT interval | 0.985 | 0.971–1.000 | 0.045 |
| ST segment/T wave abnormality | 4.091 | 1.486–11.261 | 0.01 |
Models were adjusted for all covariates.
Abbreviations as in test.
ECGs were recorded in both a hypercalcemic state (serum Ca2+ levels, 14.3 ± 1.4 mg/dL) and a normocalcemic state (serum Ca2+ levels, 9.4 ± 0.5 mg/dL) in 28 patients. In these patients, both the PQ interval and the QRS duration were longer, and the QT interval was shorter in the setting of hypercalcemia compared with normocalcemia, whereas the heart rate was not different between the two states (Table 6). Among the 11 patients with J point elevation in the setting of hypercalcemia who had repeated ECG recording after hypercalcemia was resolved, J point elevation was disappeared after hypercalcemia was resolved in eight patients (73%), and the frequency of J point elevation became similar to that in controls (Fig. 2; P = 0.30). During hospitalization, no arrhythmia event occurred in patients with hypercalcemia.
Table 6.
Comparision of Electrocardiographic Characteristics between Hypercalcemic State and Normocarcemic State in 28 Patients
| Hypercalcemia | Normocalcemia | P Value | |
|---|---|---|---|
| Heart rate, (bpm)a | 73 (66–85) | 70 (65–80) | 0.21 |
| PQ interval (ms)a | 176 (154–206) | 155 (142–173) | <0.001 |
| QRS duration (ms)a | 106 (94–116) | 99 (89–107) | 0.004 |
| QT interval (ms)a | 354 (337–391) | 392 (376–414) | 0.004 |
| Corrected QT interval (ms)a | 396 (381–415) | 429 (399–446) | 0.002 |
| Conduction disorder, no. (%) | 12 (43) | 10 (36) | 0.50 |
| Prolonged PR interval, no. (%) | 7 (25) | 3 (11) | 0.13 |
| Right bundle branch block, no. (%) | 3 (11) | 3 (11) | 1.00 |
| Left anterior hemiblock, no. (%) | 6 (21) | 4 (14) | 0.50 |
| Left bundle branch block, no. (%) | 1 (4) | 1 (4) | 1.00 |
| ST segment/T wave abnormality, no. (%) | 13 (46) | 5 (18) | 0.04 |
| J point elevation, no. (%) | 11 (39) | 4 (14) | 0.04 |
| Location of J point elevation | |||
| Inferior leads, no. (%) | 2 (7) | 1 (4) | 1.00 |
| Lateral leads, no. (%) | 1 (4) | 2 (7) | 1.00 |
| Right precordial leads, no. (%) | 6 (21) | 1 (4) | 0.06 |
| Multiple locations, no. (%) | 2 (7) | 0 (0) | – |
Median (interquartile range).
Abbreviations as in test.
Figure 2.
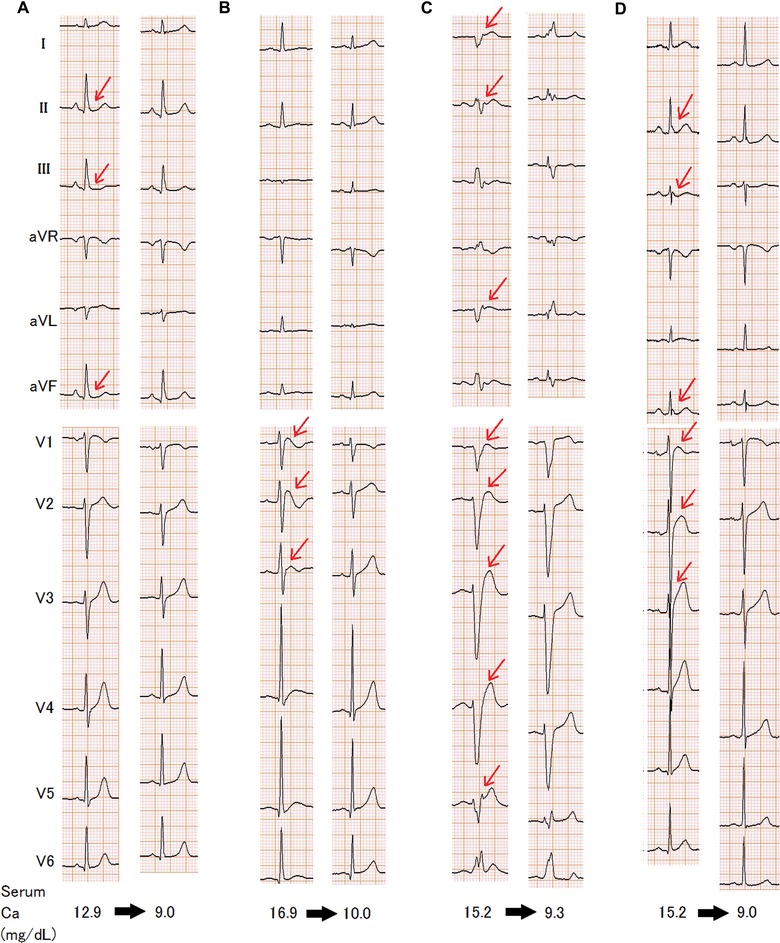
Representative 12‐lead ECGs in both hypercalcemic (left side) and normal calcium states (right side). (A) Patient 10; (B) Patient 18; (C) Patient 25; (D) Patient 27. Patient numbers correspond to those listed in Table 3. The arrows indicate J point elevation. Serum Ca+ level was corrected using the serum albumin level.
DISCUSSION
In this study, we found that the frequency of J point elevation was increased in patients with hypercalcemia. Furthermore, hypercalcemia was associated with the prolongation of both the PR interval and the QRS interval, and with the shortening of the QT interval. In spite of these ECG changes, no arrhythmia event occurred in patients with hypercalcemia.
Hypercalcemia alters ionic current kinetics in cardiomyocytes and may induce ECG abnormalities such as the shortening of QT interval and the prolongation of both the PR interval and the QRS duration.7, 8, 9 Hypercalcemia was associated with QT interval shortening and delayed cardiac conduction; these ECG changes were restored when serum Ca2+ levels returned to the normal range.
Furthermore, hypercalcemia has been linked to J point elevation,7, 10, 11, 12, 13, 14, 15, 16, 17, 18 and we found J point elevations with various morphologies, including a J point with a scooped appearance and ST segment elevation, as well as Brugada‐type ECG findings and early repolarization, which were present in 30% of patients with hypercalcemia. The frequency of J point elevation was not different among causes of hypercalcemia. A previous case series study described 16 patients with hypercalcemia due to various diseases who exhibited a J point with both a characteristic scooped appearance and ST segment elevation.18 In this study, 6% of patients with hypercalcemia exhibited the scooped appearance. Brugada‐type ECG findings have been reported in the right precordial leads in the setting of hypercalcemia, in two case reports,13, 14 and were found in 6% of patients with hypercalcemia in this study. Furthermore, this study demonstrated that hypercalcemia was also associated with early repolarization, as was the case in previous case reports.16, 21 The evidence that the J point elevation resolved following the resolution of hypercalcemia supports our hypothesis.
J point elevations including early repolarization and Brugada‐type ECG are associated with an increased risk of ventricular tachyarrhythmias and sudden cardiac death.2, 22 Furthermore, ventricular tachyarrhythmias have occasionally been reported in patients with hyperparathyroidism who have J point elevation in the setting of hypercalcemia. 7, 10, 11, 12, 13, 14, 15, 16, 17, 18, 23 However, although J point elevation was frequently found in patients with hypercalcemia due to various diseases, no fatal arrhythmia was observed during short periods of hospitalization in this study. The number of patients and observation periods in this study may not be enough to observe the association of J point elevation with the risk of arrhythmia. Ventricular tachyarrhythmias have developed in patients without J point elevation in the setting of hypercalcemia due to hyperparathyroidism,24 and ventricular tachyarrhythmia may be a complication of hyperparathyroidism, and J point elevation in the setting of hypercalcemia may not be directly associated with an increased risk of ventricular tachyarrhythmia.
Hypercalcemia is associated with various electrophysiologic changes, including shortening of QT interval, decreased cardiac conduction, and J point elevation, all of which can create a substrate for arrhythmia.2, 7, 8, 9, 25, 26 However, ventricular tachyarrhythmias have occurred only rarely in patients with hypercalcemia, based on the findings of this study and those of previous reports, suggesting that hypercalcemia may also exert proarrhythmic effects.8, 16 In experimental studies, elevated extracellular Ca2+ exerts a stabilizing effect on the membrane and decreases excitability.27, 28 Although dispersion of QT interval, which indicates an arrhythmogenic substrate, is increased in patients with short QT syndrome, hypercalcemia does not increase QT interval dispersion, despite a shortened QT interval.29, 30, 31
The mechanism by which hypercalcemia induces J point elevation is unclear. Data from our study and from other studies suggest that J point elevation likely occurs in the setting of a shortened QT interval.2, 32, 33 The frequency of early repolarization is elevated in patients with short QT syndrome and asymptomatic subjects with a short QT interval.33 The QT interval is mildly short, and abnormally short repolarization periods have been identified in the inferolateral area of the left ventricle in patients with early repolarization syndrome.2, 32 Brugada‐type ECG findings may occur in the settings of various pathophysiologic conditions, and hypercalcemia may unmask J point elevation. However, this can explain our findings only in a fraction of patients because Brugada syndrome is a rare disease.20 Actually, sodium channel blocker challenge, a provocative test for Brugada syndrome, is negative at normocalcemic state in a patient with hypercalcemia‐induced Brugada type ECG,13 and it is positive after parathyroidectomy in another patient with hypercalcemia‐induced Brugada type ECG.34
Hypercalcemia‐induced J point elevation may be explained by transmural differences in the magnitude of the action potential notch, which has been proposed as one of the mechanisms for early repolarization and Brugada type ECG.35 Decreased inward Na+–Ca2+ exchanger current is one of the major changes in ionic currents caused by hypercalcemia.8, 28 Because of the heterogeneous expression of the Na+–Ca2+ exchanger throughout the myocardium, the change in inward Na+–Ca2+ exchanger current may be more pronounced in the epicardium of the ventricles compared with the endocardium, possibly resulting in increased transmural differences in the action potential notch and the appearance of J point elevation.36
This study had several limitations. As discussed above, it is unknown whether hypercalcemia‐induced J point elevation increases the risk of ventricular tachyarrhythmias or not. The number of patients included in this study may have been too small to detect the relationship between J point elevation and the risk of arrhythmia in the setting of hypercalcemia. Only a small number of patients had follow‐up ECG recordings after hypercalcemia was resolved. Holter ECG monitoring was not performed and we could not asses the frequency of nonsustained ventricular tachyarrhythmias and premature complexes. The precise mechanism by which hypercalcemia induces early repolarization or Brugada‐type ECG is unknown. This was a retrospective study; prospective studies are warranted to investigate the role of early repolarization in the prediction of arrhythmia in patients with various diseases that may cause hypercalcemia.
Supporting information
Table S1. Comparison of Electrocardiographic Characteristics in Hypercalcemia Patients (Who Had Normal Na and K Levels without Any Medicine Affecting Ion Channels) and Normocalcemia Controls.
Table S2. Comparison of Characteristics between Hypercalcemia Patients with Hyperparathyroidism and Those with Cancer.
Disclosure: We have no disclosure and financial support.
REFERENCES
- 1. Klatsky AL, Oehm R, Cooper RA, et al. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med 2003;115(3):171–177. [DOI] [PubMed] [Google Scholar]
- 2. Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358(19):2016–2023. [DOI] [PubMed] [Google Scholar]
- 3. Rosso R, Kogan E, Belhassen B, et al. J‐point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J Am Coll Cardiol 2008;52(15):1231–1238. [DOI] [PubMed] [Google Scholar]
- 4. Tikkanen JT, Anttonen O, Junttila MJ, et al. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med 2009;361(26):2529–2537. [DOI] [PubMed] [Google Scholar]
- 5. Shinde R, Shinde S, Makhale C, et al. Occurrence of “J waves” in 12‐lead ECG as a marker of acute ischemia and their cellular basis. Pacing Clin Electrophysiol 2007;30(6):817–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osborn JJ. Experimental hypothermia: Respiratory and blood pH changes in relation to cardiac function. Am J Physiol 1953;175(3):389–398. [DOI] [PubMed] [Google Scholar]
- 7. Sridharan MR, Horan LG. Electrocardiographic J wave of hypercalcemia. Am J Cardiol 1984;54(6):672–673. [DOI] [PubMed] [Google Scholar]
- 8. El‐Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J 2011;18(3):233–245. [PubMed] [Google Scholar]
- 9. Surawicz B. Relationship between electrocardiogram and electrolytes. Am Heart J 1967;73(6):814–834. [DOI] [PubMed] [Google Scholar]
- 10. Ashizawa N, Arakawa S, Koide Y, et al. Hypercalcemia due to vitamin D intoxication with clinical features mimicking acute myocardial infarction. Intern Med (Tokyo, Jpn) 2003;42(4):340–344. [DOI] [PubMed] [Google Scholar]
- 11. Otero J, Lenihan DJ. The “normothermic” Osborn wave induced by severe hypercalcemia. Texas Heart Instit J 2000;27(3):316–317. [PMC free article] [PubMed] [Google Scholar]
- 12. Chhabra L, Spodick DH. Milk Alkali syndrome: An electrocardiographic masquerader for non‐hypothermic Osborn phenomenon. Heart (Br Cardiac Soc) 2013;99(17):1302–1303. [DOI] [PubMed] [Google Scholar]
- 13. Zeb M, McKenzie DB, Naheed B, et al. Hypercalcaemia and a Brugada‐like ECG: An independent risk factor for fatal arrhythmias. Resuscitation 2010;81(8):1048–1050. [DOI] [PubMed] [Google Scholar]
- 14. Mehta S, Parameswaran AC, Greenspan A, et al. Hypercalcemia due to rhabdomyolysis mimicking Brugada syndrome. Pacing Clin Electrophysiol 2009;32(11):e14–e15. [DOI] [PubMed] [Google Scholar]
- 15. Nishi SP, Barbagelata NA, Atar S, et al. Hypercalcemia‐induced ST‐segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006;39(3):298–300. [DOI] [PubMed] [Google Scholar]
- 16. Kiewiet RM, Ponssen HH, Janssens EN, et al. Ventricular fibrillation in hypercalcaemic crisis due to primary hyperparathyroidism. Netherlands J Med 2004;62(3):94–96. [PubMed] [Google Scholar]
- 17. Topsakal R, Saglam H, Arinc H, et al. Electrocardiographic J wave as a result of hypercalcemia aggravated by thiazide diuretics in a case of primary hyperparathyroidism. Jpn Heart J 2003;44(6):1033–1037. [DOI] [PubMed] [Google Scholar]
- 18. Littmann L, 3rd Taylor L, , et al. ST‐segment elevation: A common finding in severe hypercalcemia. J Electrocardiol 2007;40(1):60–62. [DOI] [PubMed] [Google Scholar]
- 19. Bazett HC. The time relations of the blood‐pressure changes after excision of the adrenal glands, with some observations on blood volume changes. J Physiol 1920;53(5):320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111(5):659–670. [DOI] [PubMed] [Google Scholar]
- 21. Namboodiri N, Bohora S, Dora SK, et al. Electrocardiographical case. J wave and presyncope in a middle‐aged woman. Singapore Med J 2008;49(2):160–163; quiz 164. [PubMed] [Google Scholar]
- 22. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992;20(6):1391–1396. [DOI] [PubMed] [Google Scholar]
- 23. Chadli MC, Chaieb L, Jemni L, et al. Bigeminal arrhythmia associated with hyperparathyroid crisis. Can Med Assoc J 1988;138(12):1115–1116. [PMC free article] [PubMed] [Google Scholar]
- 24. Chang CJ, Chen SA, Tai CT, et al. Ventricular tachycardia in a patient with primary hyperparathyroidism. PACE 2000;23(4 Pt 1):534–537. [DOI] [PubMed] [Google Scholar]
- 25. Iribarren C, Round AD, Peng JA, et al. Short QT in a cohort of 1.7 million persons: Prevalence, correlates, and prognosis. Ann Noninvasive Electrocardiol 2014;19(5):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Antzelevitch C. Basic mechanisms of reentrant arrhythmias. Curr Opin Cardiol 2001;16(1):1–7. [DOI] [PubMed] [Google Scholar]
- 27. Weidmann S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol 1955;129(3):568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leitch SP, Brown HF. Effect of raised extracellular calcium on characteristics of the guinea‐pig ventricular action potential. J Mol Cell Cardiol 1996;28(3):541–551. [DOI] [PubMed] [Google Scholar]
- 29. Yelamanchi VP, Molnar J, Ranade V, et al. Influence of electrolyte abnormalities on interlead variability of ventricular repolarization times in 12‐lead electrocardiography. Am J Therapeut 2001;8(2):117–122. [DOI] [PubMed] [Google Scholar]
- 30. Anttonen O, Vaananen H, Junttila J, et al. Electrocardiographic transmural dispersion of repolarization in patients with inherited short QT syndrome. Ann Noninvasive Electrocardiol 2008;13(3):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milberg P, Tegelkamp R, Osada N, et al. Reduction of dispersion of repolarization and prolongation of postrepolarization refractoriness explain the antiarrhythmic effects of quinidine in a model of short QT syndrome. J Cardiovasc Electrophysiol 2007;18(6):658–664. [DOI] [PubMed] [Google Scholar]
- 32. Ghosh S, Cooper DH, Vijayakumar R, et al. Early repolarization associated with sudden death: Insights from noninvasive electrocardiographic imaging. Heart Rhythm. 2010;7(4):534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe H, Makiyama T, Koyama T, et al. High prevalence of early repolarization in short QT syndrome. Heart Rhythm. 2010;7(5):647–652. [DOI] [PubMed] [Google Scholar]
- 34. Wu LS, Wu CT, Hsu LA, et al. Brugada‐like electrocardiographic pattern and ventricular fibrillation in a patient with primary hyperparathyroidism. Europace: Eur Pacing, Arrhythmias Cardiac Electrophysiol 2007;9(3):172–174. [DOI] [PubMed] [Google Scholar]
- 35. Antzelevitch C. Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J 2012;76(5):1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaborit N, Le Bouter S, Szuts V, et al. Regional and tissue specific transcript signatures of ion channel genes in the non‐diseased human heart. J Physiol 2007;582(Pt 2):675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of Electrocardiographic Characteristics in Hypercalcemia Patients (Who Had Normal Na and K Levels without Any Medicine Affecting Ion Channels) and Normocalcemia Controls.
Table S2. Comparison of Characteristics between Hypercalcemia Patients with Hyperparathyroidism and Those with Cancer.


