Abstract
Background
Interatrial block (IAB) is a strong predictor of recurrence of atrial fibrillation (AF). IAB is a conduction delay through the Bachman region, which is located in the upper region of the interatrial space. During IAB, the impulse travels from the right atrium to the interatrial septum (IAS) and coronary sinus to finally reach the left atrium in a caudocranial direction. No relation between the presence of IAB and IAS thickness has been established yet.
Objective
To determine whether a correlation exists between the degree of IAB and the thickness of the IAS and to determine whether IAS thickness predicts AF recurrence.
Methods
Sixty‐two patients with diagnosis of paroxysmal AF undergoing catheter ablation were enrolled. IAB was defined as P‐wave duration ≥120 ms. IAS thickness was measured by cardiac computed tomography.
Results
Among 62 patients with paroxysmal AF, 45 patients (72%) were diagnosed with IAB. Advanced IAB was diagnosed in 24 patients (39%). Forty‐seven patients were male. During a mean follow‐up period of 49.8 ± 22 months (range 12–60 months), 32 patients (51%) developed AF recurrence. IAS thickness was similar in patients with and without IAB (4.5 ± 2.0 mm vs. 4.0 ± 1.4 mm; p = .45) and did not predict AF. Left atrial size was significantly enlarged in patients with IAB (40.9 ± 5.7 mm vs. 37.2 ± 4.0 mm; p = .03). Advanced IAB predicted AF recurrence after the ablation (OR: 3.34, CI: 1.12–9.93; p = .03).
Conclusions
IAS thickness was not significantly correlated to IAB and did not predict AF recurrence. IAB as previously demonstrated was an independent predictor of AF recurrence.
Keywords: interatrial bock, interatrial septal thickness, atrial fibrillation, recurrence
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice in the general population and is associated with increased cardiovascular morbidity and mortality (Wilke et al., 2013). Electrical isolation of the pulmonary veins has been established as a cornerstone treatment for paroxysmal AF (Haissaguerre et al., 1998). Despite progress in catheter ablation techniques, the recurrence rate of catheter ablation is still high and remains around 30%–35% within a year (Calkins et al., 2012).
Electrocardiographic (ECG) indices as predictors of AF recurrence have been previously investigated. Interatrial block (IAB) is one of the most powerful ECG predictors for AF recurrence. IAB is classified into partial and advanced forms, depending on the severity of the conduction delay (Ariyarajah, Asad, Tandar, & Spodick, 2005). The relationship between advanced IAB and atrial arrhythmias is called “Bayes syndrome” (Baranchuk & Bayes‐Genis, 2016; Conde & Baranchuk, 2015). Patients with advanced IAB are at higher risk of AF recurrence after catheter ablation (Caldwell et al., 2014).
Prior reports indicated that interatrial septal (IAS) thickness is increased in patients with AF (Lopez‐Candales, Grewal, & Katz, 2005). IAS thickness measured by cardiac computed tomography (CT) was associated with the extent of fractionated atrial signals within the left atrium (LA), and it was related to acute procedural success of ablation (Park et al., 2015). However, the correlation between IAB and IAS thickness has not been investigated yet nor the ability of IAS thickness to predict postablation AF. The aim of our study was to establish a correlation between IAB and IAS thickness and to determine whether IAS thickness could predict postablation AF recurrence.
2. Material and Methods
2.1. Study participants
This is a retrospective study including 62 consecutive patients with paroxysmal AF who underwent cardiac CT prior to catheter ablation. Paroxysmal AF was defined according to the HRS/EHRA/ECAS 2012 Consensus Statement as an episode of AF that terminates spontaneously or with intervention in less than 7 days (Calkins et al., 2012). Patient demographics, and drugs at the time of initial ablation, were obtained from medical records. Patients with persistent AF were excluded from the study. All patients included in the study provided verbal and written informed consent for participation in the study. This study was approved by the local institutional review board.
2.2. Procedure
Patients were brought to the Electrophysiology lab in fasting, and after venous access and transseptal puncture, AF ablation was performed with a standard wide area circular ablation (WACA) approach using irrigated 4 mm ablation catheters (power of 25–30 W, temperature of 40°C). Pulmonary vein disconnection was considered the outcome. The patients were kept overnight at hospital with close monitoring and discharged the following day.
2.3. ECG analysis
Surface 12‐lead standard ECGs were recorded from each participant with a 25 mm/s paper speed at 10 mm/mV amplitude on the day before catheter ablation. Obtained ECGs were scanned at a 600 dpi resolution and analyzed digitally by two observers. Partial IAB was defined as a P‐wave duration ≥120 ms; advanced IAB was defined as a P‐wave duration ≥120 ms along with a biphasic morphology (±) in the inferior leads (Bayes de Luna et al., 2012). Interobserver variability was calculated and disagreements were solved by consensus.
2.4. Cardiac CT—IAS thickness
Cardiac computed tomography (CT) was performed with prospective ECG gating and contrast enhancement with a 320‐slice CT scanner, Toshiba (Aquillon One, Irvine, CA, USA). Patients were pretreated with beta‐blocker aiming for a heart rate less than 100 bpm. CT parameters were set at gantry rotation time of 350 ms and a tube voltage of 100 Kv. Following multiple breath‐hold exercises, an 80‐ml bolus of iodinated contrast media (Omnipaque 350; GE Healthcare, Milwaukee, WI, USA) was injected intravenously at 5 ml/s, followed by a 30 ml flush of normal saline at 5 ml/s. Images were obtained every 2 s at the level of the left atrium to monitor for appearance of contrast. Once contrast was visualized, a breath‐hold instruction was given and the scan was initiated. Image acquisition was completed with a single breath hold with the patient in the supine position. The entire heart was imaged in one heartbeat.
Images were reconstructed at 18–20 cm field of view and 0.5 mm slice thickness at 75% of cardiac cycle (Figure 1). Images were initially oriented in a four‐chamber axial view. Then, a second image plane was oriented to align parallel to the IAS. The IAS could now be viewed in two orthogonal 90 degree views. The midpoint of the IAS in both the superior‐inferior and the anteroposterior (AP) planes was determined. The image was magnified to allow improved visualization and measurement of the IAS. While the Bachmann bundle may be superior to the midpoint of the IAS, the midpoint of the IAS is the standard point of measurement in previous IAS studies (Lim et al., 2015; Park et al., 2015). Measurements were made 1 cm superior to the AV junction in order to allow a consistent site of measurement between patients. All images available were reviewed by a CCT trained, Level III certified, cardiologist (RSP) who was blind to other clinical patient data.
Figure 1.
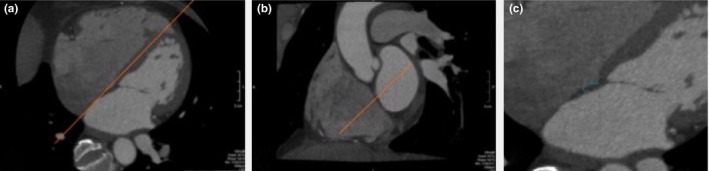
Interatrial septal (IAS) thickness measured by multi‐slice computed tomography (CT). (a) Axial view with red line showing view aligned to IAS. (b) Coronal view with red line showing view aligned to IAS. (c) Axial view, now oriented to IAS in two planes, and magnified for measurement of IAS (3.4 mm)
2.5. Follow‐up
After ablation, all patients received anticoagulation for at least 3 months and were maintained on anticoagulation according to CHA2DS2‐VASc score. Patients were evaluated by 24 hr ECG Holter monitoring at 3 and 6 months and yearly thereafter. Recurrence was defined as an episode of any atrial arrhythmia lasting more than 30 s and occurring at least 3 months after ablation (postblanking period) (Calkins et al., 2012).
2.6. Statistics
Data were collected in an Excel file and imported into IBM SPSS (Version 23 for Windows, Armonk, New York, 2015) for statistical analysis. Data were initially described using means, standard deviations, and medians for continuous data, and frequencies and percentages for categorical data; continuous data were also graphed to assess their underlying distribution. Associations between IAS thickness and the continuous data (P‐wave duration) were assessed using Pearson correlations, with Spearman's rho correlation in the event of nonnormal distributions. The association between presence of IAB and IAS thickness was assessed using independent samples t tests, with the Mann‐Whitney U in the event of nonnormal distributions. Logistic regression analysis was performed to determine predictors of AF recurrence.
3. Results
Baseline clinical and laboratory characteristics of the groups are summarized in Table 1. IAB was present in 45 patients (72%) of which 21 (47%) were partial IAB and 24 (53%) were advanced. The mean IAS thickness was 4.4 ± 1.9 mm. During a follow‐up of 49.8 ± 22 months (range 12 and 60 months) after a single ablation procedure, 30 patients (49%) maintained sinus rhythm.
Table 1.
Baseline characteristics of patients with paroxysmal AF
| Number of patients | 62 |
| Age, years | 58.4 ± 11.3 |
| Male, n (%) | 47 (76) |
| DM, n (%) | 8 (13) |
| IHD, n (%) | 9 (14) |
| SA, n (%) | 16 (26) |
| LVEF, % | 62.2 ± 8.7 |
| LA, mm | 40.1 ± 5.6 |
| LAVI, mm/m2 | 36.4 ± 12.2 |
| IAB, n (%) | 45 (72) |
| pIAB, n (%) | 21 (47) |
| aIAB, n (%) | 24 (53) |
| FU, months | 49.8 ± 22.0 |
| Recurrence of AF, n (%) | 32 (51) |
DM, diabetes mellitus; IHD, ischemic heart disease; SA, sleep apnea; LVEF, left ventricular ejection fraction; LA, left atrium; LAVI, left atrial volume index; IAB, interatrial block; pIAB, partial interatrial block; aIAB, advanced interatrial block; FU, follow‐up.
Baseline characteristics were comparable in patients with and without IAB (Table 2). Only LA size was significantly higher in patients with IAB (40.9 ± 5.7 mm vs. 37.2 ± 4.0 mm; p = .03, respectively). IAS thickness was comparable in both groups (Table 2). The presence of IAB did not show any significant correlation with IAS thickness (r .06, p = .64).
Table 2.
Characteristics of patients with and without interatrial block (IAB)
| IAB (+) n = 45 | IAB (−) n = 17 | p Value | |
|---|---|---|---|
| Age, years | 59.2 ± 10.9 | 56.2 ± 12.2 | .36 |
| Male, n (%) | 35 (78) | 12 (70) | .74 |
| DM, n (%) | 5 (11) | 3 (18) | .67 |
| IHD, n (%) | 5 (11) | 4 (23) | .24 |
| SA, n (%) | 12 (27) | 4 (23) | .80 |
| LVEF, % | 63.6 ± 7.5 | 58.5 ± 10.9 | .05 |
| LA, mm | 40.9 ± 5.7 | 37.2 ± 4.0 | .03 |
| LAVI, mm/m2 | 36.7 ± 12.3 | 35.9 ± 12.5 | .86 |
| FU, months | 48.9 ± 22.6 | 47.0 ± 20.2 | .76 |
| IAS, mm | 4.5 ± 2.0 | 4.0 ± 1.4 | .45 |
| Recurrence of AF, n (%) | 25 (55) | 7 (41) | .37 |
As shown in Table 3, age, comorbidities, LVEF, LA size, and LAVI were comparable in patients with AF recurrence compared to those without. IAS thickness and the prevalence of IAB (partial and advanced) were also comparable in patients with and without AF recurrence (4.3 ± 1.9 mm vs. 4.3 ± 1.8 mm, p = .94 and 78% vs. 67%; p = .55, respectively). However, the prevalence of advanced IAB was significantly higher in patients with AF recurrence compared with those without AF recurrence (50% vs. 26%, p = .027) (Table 3). In addition, logistic regression analysis showed that advanced IAB predicted AF recurrence after the ablation (OR 3.34, CI 1.12–9.93; p = .03).
Table 3.
Comparison of patients with and without recurrence of AF
| AF (+) n = 32 | AF (−) n = 30 | p Value | |
|---|---|---|---|
| Age, years | 55.7 ± 11.9 | 61.4 ± 9.9 | .05 |
| Male, n (%) | 28 (87) | 19 (63) | .07 |
| DM, n (%) | 3 (9) | 5 (16) | .28 |
| IHD, n (%) | 5 (16) | 4 (13) | .58 |
| SA, n (%) | 10 (31) | 6 (20) | .28 |
| LVEF, % | 60.8 ± 8.6 | 63.9 ± 8.7 | .18 |
| LA, mm | 40.9 ± 6.3 | 39.1 ± 4.6 | .23 |
| LAVI, mm/m2 | 38.7 ± 12.9 | 33.7 ± 10.9 | .19 |
| FU, months | 52.3 ± 23.4 | 43.9 ± 19.2 | .13 |
| IAS, mm | 4.3 ± 1.9 | 4.3 ± 1.8 | .94 |
| IAB, n (%) | 25 (78) | 20 (67) | .55 |
| aIAB, n (%) | 16 (50) | 8 (26) | .027 |
aIAB, advanced interatrial block.
4. Discussion
IAB was not correlated with IAS thickness in patients with paroxysmal AF undergoing catheter ablation. IAS thickness was similar in patients with and without AF recurrence.
The relationship between advanced IAB and atrial arrhythmias is now called “Bayés’ syndrome” (Baranchuk & Bayes‐Genis, 2016; Conde & Baranchuk, 2015). Several studies have demonstrated that IAB is associated with the development of AF and predict AF recurrence in many different clinical scenarios like postelectrical cardioversion, after cavotricuspid isthmus ablation and AF ablation (Agarwal, Aronow, Levy, & Spodick, 2003; Baranchuk & Bayes‐Genis, 2016; Conde & Baranchuk, 2014; Enriquez et al., 2014, 2015; Sadiq‐Ali et al., 2015). Advanced IAB reflects underlying atrial disease manifested as atrial fibrosis involving the Bachmann's region. Atrial fibrosis is associated with increased recurrence of AF after catheter ablation (Akoum et al., 2011; den Uijl et al., 2011). Caldwell et al. (2014) have demonstrated that prolonged P‐wave duration is associated with a higher risk of AF recurrence postablation for paroxysmal AF. They found that max P‐wave duration >140 ms identifies a subgroup of paroxysmal AF patients where significant atrial remodeling has already occurred. Most recently, Wu et al. (2015) showed that patients with advanced IAB are at an increased risk of AF recurrence after circumferential pulmonary vein ablation (CPVA).
IAB as conduction delay through the high septum (Bachmann's region) plays an important role in the prognosis of AF. As part of the interatrial conduction may occur through the Fossa Ovalis, the role of IAS thickness as a possible predictor of AF recurrence needed to be investigated in a clinical setting. It has been demonstrated that IAS has a more complex anatomy than previously assumed (Sweeney & Rosenquist, 1979). Few studies have shown that atrial arrhythmias are related to IAS thickness (Hutter & Page, 1971; Kluge, 1969). Changes in atrial wall thickness might correspond to changes in the atrial extracellular matrix components, which may trigger atrial remodeling. Atrial arrhythmias may be related to fibrosis or fatty infiltration of the IAS (Hutter & Page, 1971). Previous studies demonstrated that localized IAS fat, known as lipomatous septal hypertrophy, was associated with a significantly higher prevalence of atrial arrhythmias, including AF (Heyer, Kagel, Lemburg, Bauer, & Nicolas, 2003). There have been reports that IAS thickness is increased in patients with AF (Lopez‐Candales et al., 2005). IAS thickness was significantly correlated with adipose tissue and independently associated with structural remodeling of the LA in patients with persistent AF (Shin et al., 2011). IAS thickness measured by cardiac CT is associated with the extent of fractionated areas within the LA and is related to acute procedural success of ablation (Park et al., 2015). Very recently, Lim et al. (2015) have demonstrated that IAS thickness is correlated with parameters for LA structural and functional remodeling; however, IAS thickness alone did not independently predict recurrence of AF after catheter ablation. A nonsignificant correlation between IAS and AF has been previously reported. Platonov, Mitrofanova, Chireikin, and Olsson (2002) demonstrated in a series of postmortem studies a huge variability in the interatrial connections leading to the inability of the IAS thickness to associate with history of AF. Lopez‐Candales et al. (2005) did not find any correlation between IAS thickness and the duration of AF. In a very elegant study, Platonov et al. described the muscular structure of IAS providing a detailed account for the IAS conduction substrate and its relationships with IAS anatomy. They found that interatrial bundles are not limited only to the Bachmann bundle, but they may be also located anteriorly, posteriorly between the right pulmonary veins, and inferiorly between the coronary sinus and the right inferior pulmonary vein. The inferior connections can be more prominent than the Bachmann bundle (Platonov, Mitrofanova, Ivanov, & Ho, 2008). Our results were in line with the results of previous studies about the inability of the IAS thickness to predict AF recurrence following catheter ablation. IAS thickness did not correlate with IAB in the surface ECG possibly indicating that fibrosis associated to IAB occurs in the higher portion of the interatrial septum rather than at the level of the Fossa Ovalis. This may explain, at least in part, that despite the thickness of the IAS, caudocranial conduction to the left atrium still occurs during IAB. The results of our study suggest the predominantly role of the Bachmann interatrial connection and its fibrosis, rather than extended fibrosis to the IAS, as the anatomical substrate for AF.
5. Limitations
This is a single‐center, retrospective study and bias is inherent to this type of design. Large‐scale, prospective studies are required to better define the pathophysiological role of IAS thickness in atrial remodeling in patients with AF undergoing catheter ablation. Estimation of sample size calculation was not done as no prior available publications in this domain (clinical setting) were available.
6. Conclusion
In our series, no significant correlation between IAS thickness and IAB was found. In addition, IAS thickness did not predict AF recurrence after catheter ablation. IAB as previously demonstrated was an independent predictor of AF recurrence.
Conflict of interest and disclosure of funding
All authors declare that the manuscript, as submitted or its essence in another version, is not under consideration for publication elsewhere, and it will not be submitted elsewhere until a final decision is made by the editors of Annals of Noninvasive Electrocardiology. The authors have no commercial associations or sources of support that might pose a conflict of interest. All authors have made substantive contributions to the study, and all authors endorse the data and conclusions. Nevertheless, confirmation of informed patient consent for publication was obtained.
Gul EE, Pal R, Caldwell J, et al. Interatrial block and interatrial septal thickness in patients with paroxysmal atrial fibrillation undergoing catheter ablation: Long‐term follow‐up study. Ann Noninvasive Electrocardiol. 2017;22:e12428 10.1111/anec.12428
References
- Agarwal, Y. K. , Aronow, W. S. , Levy, J. A. , & Spodick, D. H. (2003). Association of interatrial block with development of atrial fibrillation. American Journal of Cardiology, 91(7), 882. [DOI] [PubMed] [Google Scholar]
- Akoum, N. , Daccarett, M. , McGann, C. , Segerson, N. , Vergara, G. , Kuppahally, S. , … Marrouche, N. (2011). Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: A DE‐MRI guided approach. Journal of Cardiovascular Electrophysiology, 22(1), 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyarajah, V. , Asad, N. , Tandar, A. , & Spodick, D. H. (2005). Interatrial block: Pandemic prevalence, significance, and diagnosis. Chest, 128(2), 970–975. [DOI] [PubMed] [Google Scholar]
- Baranchuk, A. , & Bayes‐Genis, A. (2016). Bayes’ Syndrome. Revista espanola de cardiologia, 69(4), 439. [DOI] [PubMed] [Google Scholar]
- Bayes de Luna, A. , Platonov, P. , Cosio, F. G. , Cygankiewicz, I. , Pastore, C. , Baranowski, R. , … Spodick, D. (2012). Interatrial blocks. A separate entity from left atrial enlargement: A consensus report. Journal of Electrocardiology, 45(5), 445–451. [DOI] [PubMed] [Google Scholar]
- Caldwell, J. , Koppikar, S. , Barake, W. , Redfearn, D. , Michael, K. , Simpson, C. , … Baranchuk, A. (2014). Prolonged P‐wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 39(2), 131–138. [DOI] [PubMed] [Google Scholar]
- Calkins, H. , Kuck, K. H. , Cappato, R. , Brugada, J. , Camm, A. J. , Chen, S. A. , … Wilber, D. (2012). 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace, 14(4), 528–606. [DOI] [PubMed] [Google Scholar]
- Conde, D. , & Baranchuk, A. (2014). Interatrial block as anatomical‐electrical substrate for supraventricular arrhythmias: Bayes syndrome. Archivos de cardiologia de Mexico, 84(1), 32–40. [DOI] [PubMed] [Google Scholar]
- Conde, D. , Baranchuk, A. , & Bayes de Luna, A. (2015). Advanced interatrial block as a substrate of supraventricular tachyarrhythmias: A well recognized syndrome. Journal of Electrocardiology, 48(2), 135–140. [DOI] [PubMed] [Google Scholar]
- Enriquez, A. , Conde, D. , Hopman, W. , Mondragon, I. , Chiale, P. A. , de Luna, A. B. , Baranchuk, A. (2014). Advanced interatrial block is associated with recurrence of atrial fibrillation post pharmacological cardioversion. Cardiovascular Therapeutics, 32(2), 52–56. [DOI] [PubMed] [Google Scholar]
- Enriquez, A. , Sarrias, A. , Villuendas, R. , Ali, F. S. , Conde, D. , Hopman, W. M. , … Baranchuk, A. (2015). New‐onset atrial fibrillation after cavotricuspid isthmus ablation: Identification of advanced interatrial block is key. Europace, 17(8), 1289–1293. [DOI] [PubMed] [Google Scholar]
- Haissaguerre, M. , Jais, P. , Shah, D. C. , Takahashi, A. , Hocini, M. , Quiniou, G. , … Clementy, J. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England Journal of Medicine, 339(10), 659–666. [DOI] [PubMed] [Google Scholar]
- Heyer, C. M. , Kagel, T. , Lemburg, S. P. , Bauer, T. T. , & Nicolas, V. (2003). Lipomatous hypertrophy of the interatrial septum: A prospective study of incidence, imaging findings, and clinical symptoms. Chest, 124(6), 2068–2073. [DOI] [PubMed] [Google Scholar]
- Hutter, A. M. Jr , & Page, D. L. (1971). Atrial arrhythmias and lipomatous hypertrophy of the cardiac interatrial septum. American Heart Journal, 82(1), 16–21. [DOI] [PubMed] [Google Scholar]
- Kluge, W. F. (1969). Lipomatous hypertrophy of the interatrial septum. Northwest Medicine, 68(1), 25–30. [PubMed] [Google Scholar]
- Lim, H. E. , Na, J. O. , Im, S. I. , Choi, C. U. , Kim, S. H. , Kim, J. W. , … Hwang, C. (2015). Interatrial septal thickness as a marker of structural and functional remodeling of the left atrium in patients with atrial fibrillation. Korean Journal of Internal Medicine, 30(6), 808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Candales, A. , Grewal, H. , & Katz, W. (2005). The importance of increased interatrial septal thickness in patients with atrial fibrillation: A transesophageal echocardiographic study. Echocardiography, 22(5), 408–414. [DOI] [PubMed] [Google Scholar]
- Park, Y. M. , Park, H. C. , Ban, J. E. , Choi, J. I. , Lim, H. E. , Park, S. W. , Kim, Y. J. (2015). Interatrial septal thickness is associated with the extent of left atrial complex fractionated atrial electrograms and acute procedural outcome in patients with persistent atrial fibrillation. Europace, 17(11), 1700–1707. [DOI] [PubMed] [Google Scholar]
- Platonov, P. G. , Mitrofanova, L. B. , Chireikin, L. V. , & Olsson, S. B. (2002). Morphology of inter‐atrial conduction routes in patients with atrial fibrillation. Europace, 4(2), 183–192. [DOI] [PubMed] [Google Scholar]
- Platonov, P. G. , Mitrofanova, L. , Ivanov, V. , & Ho, S. Y. (2008). Substrates for intra‐atrial and interatrial conduction in the atrial septum: Anatomical study on 84 human hearts. Heart Rhythm, 5(8), 1189–1195. [DOI] [PubMed] [Google Scholar]
- Sadiq‐Ali, F. , Enriquez, A. , Conde, D. , Redfearn, D. , Michael, K. , Simpson, C. , … Baranchuk, A. (2015). Advanced interatrial block predicts new onset atrial fibrillation in patients with severe heart failure and cardiac resynchronization therapy. Annals of Noninvasive Electrocardiology, 20(6), 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, S. Y. , Yong, H. S. , Lim, H. E. , Na, J. O. , Choi, C. U. , Choi, J. I. , … Kim, Y. J. (2011). Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology, 22(6), 647–655. [DOI] [PubMed] [Google Scholar]
- Sweeney, L. J. , & Rosenquist, G. C. (1979). The normal anatomy of the atrial septum in the human heart. American Heart Journal, 98(2), 194–199. [DOI] [PubMed] [Google Scholar]
- den Uijl, D. W. , Delgado, V. , Bertini, M. , Tops, L. F. , Trines, S. A. , van de Veire, N. R. , … Bax, J. J. (2011). Impact of left atrial fibrosis and left atrial size on the outcome of catheter ablation for atrial fibrillation. Heart, 97(22), 1847–1851. [DOI] [PubMed] [Google Scholar]
- Wilke, T. , Groth, A. , Mueller, S. , Pfannkuche, M. , Verheyen, F. , Linder, R. , … Breithardt, G. (2013). Incidence and prevalence of atrial fibrillation: An analysis based on 8.3 million patients. Europace, 15(4), 486–493. [DOI] [PubMed] [Google Scholar]
- Wu, J. T. , Long, D. Y. , Dong, J. Z. , Wang, S. L. , Fan, X. W. , Yang, H. T. , … Yang, C. K. (2015). Advanced interatrial block predicts clinical recurrence of atrial fibrillation after catheter ablation. Journal of Cardiology, 68(4), 352–356. [DOI] [PubMed] [Google Scholar]


