Abstract
Intravenous adenosine is a short‐acting blocker of the atrioventricular node that has been used to unmask subtle or latent preexcitation, and also to enable catheter ablation in selected patients with absent or intermittent preexcitation. Depending on the accessory pathway characteristics, intravenous adenosine may produce specific electrocardiographic changes highly suggestive of the preexcitation variant. Herein, we view different ECG responses to this pharmacological test in various preexcitation patterns that were confirmed by electrophysiological studies. Careful analysis of electrocardiographic changes during adenosine test, with emphasis on P‐delta interval, preexcitation degree, and atrioventricular block, can be helpful to diagnose the preexcitation variant/pattern.
Keywords: adenosine, preexcitation variants, accessory pathway
Intravenous adenosine is a well‐known, short‐acting, blocker of atrioventricular (AV) nodal conduction that is the drug of choice for the acute treatment of regular supraventricular tachycardias.1 Moreover, it has been used in patients with subtle or latent preexcitation to confirm the presence of an accessory pathway (AP) and also to predict its location more precisely by increasing the preexcitation degree.2, 3 This pharmacological test may enable mapping and ablation in selected patients with absent or intermittent preexcitation at the time of electrophysiological study (EPS).4
Nevertheless, depending on the AP characteristics, intravenous adenosine may produce specific electrocardiographic changes highly suggestive of the preexcitation variant/pattern. In this brief article, we view different ECG responses to this pharmacologic test in various preexcitation patterns that were confirmed by a comprehensive EPS.
CASE 1
A 26‐year‐old female patient with Wolff‐Parkinson‐White syndrome underwent an EPS because of recurrent episodes of paroxysmal palpitations. Her basal 12‐lead ECG showed minimal preexcitation with negative delta waves in I and aVL suggesting a left‐sided AP. Intravenous adenosine (Fig. 1) showed a typical response with increased preexcitation (the second half of Fig. 1) and shortening of the His‐Ventricle (HV) interval (Fig. 1B). Importantly, there were no changes in the P‐delta interval or a transient phase of AV block during the test. The EPS confirmed the presence of a Kent‐type AP that was successfully ablated at the lateral mitral annulus using a transaortic approach.
Figure 1.
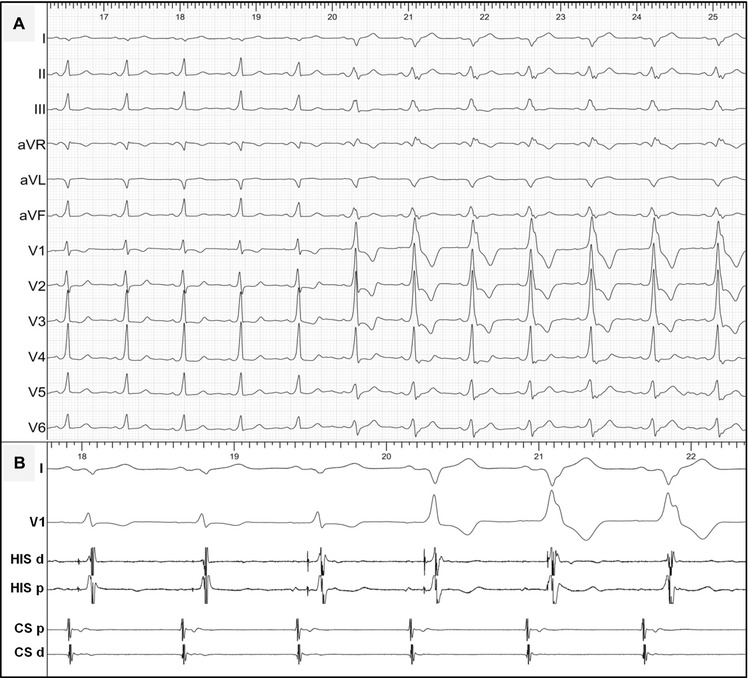
(A) Twelve‐lead ECG and (B) intracardiac recordings during intravenous adenosine test in a patient with a left‐sided, Kent‐type AP (see text for discussion). CS = coronary sinus; d = distal; His = His bundle recording; p = proximal.
CASE 2
A 16‐year‐old male athlete with a minimal preexcitation pattern, persistent at a maximal exercise test, underwent an EPS. Intravenous adenosine (Fig. 2) induced a transient phase of advanced AV block. Interestingly, a few preexcited QRS complexes (the asterisks in Fig. 2) were conducted with significant prolongation of the P‐delta interval, but without any change in QRS morphology or the preexcitation degree/HV interval (Fig. 2B). These ECG findings are highly indicative of an infranodal AP. Indeed, a detailed EPS established the diagnosis of a fasciculoventricular AP showing a fixed and short HV interval (preexcitation degree) during multisite atrial pacing and at different pacing rates. No ablation was attempted as no tachycardia was inducible and because of the innocent nature of this preexcitation variant.
Figure 2.
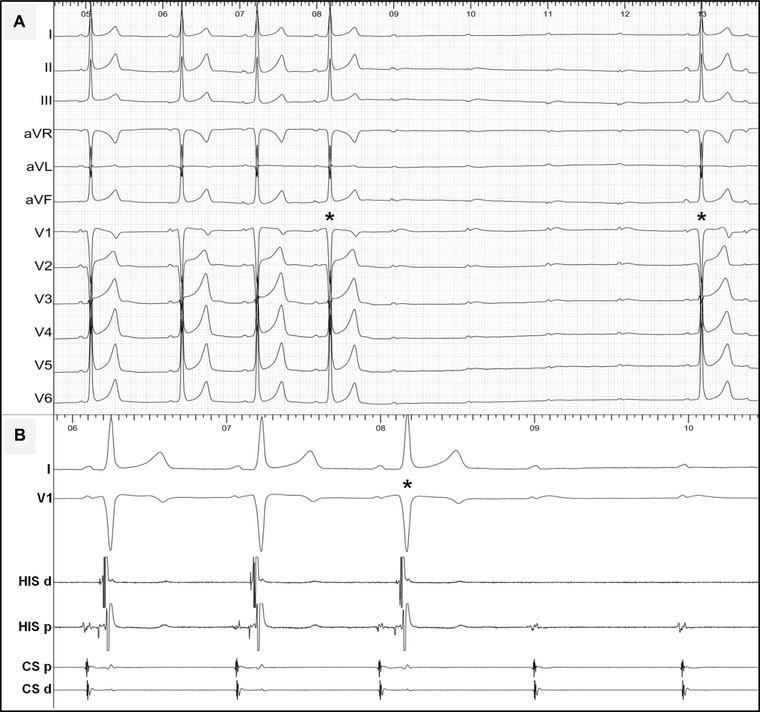
(A) Twelve‐lead ECG and (B) intracardiac recordings during intravenous adenosine test in a patient with a fasciculoventricular pathway (see text for discussion). Abbreviations are as in Figure 1.
CASE 3
A 33‐year‐old male athlete with sporadic episodes of palpitations was referred to our center for evaluation. His basal 12‐lead ECG showed a normal PR interval with minimal delta waves. Intravenous adenosine (Fig. 3) led to a transient phase of 2:1 AV block. The conducted QRS complexes (the asterisks) during this phase showed a longer P‐delta interval and, notably, an increased preexcitation degree (a slightly shorter HV interval, Fig. 3B). This phenomenon is incompatible with a lone fasciculoventricular pathway due to the variable preexcitation degree. The EPS in this patient confirmed the presence of a nodoventricular AP with unchanged preexcitation morphology during multisite atrial pacing. No tachycardia was inducible and no ablation was attempted. Unlike fasciculoventricular pathways, nodoventricular pathways might participate in reentrant tachycardias and cryoablation may offer a viable therapeutic option when indicated.5 However, the latter response to intravenous adenosine could also be seen in a Mahaim‐like AV AP and a detailed EPS is essential to differentiate between these preexcitation variants. Differential atrial pacing (e.g., at the lateral tricuspid annulus) should modify the preexcitation degree over an atriofascicular/ventricular AP, while the preexcitation pattern remains unchanged in the case of a nodoventricular "true Mahaim" AP independently of the atrial pacing site.6
Figure 3.
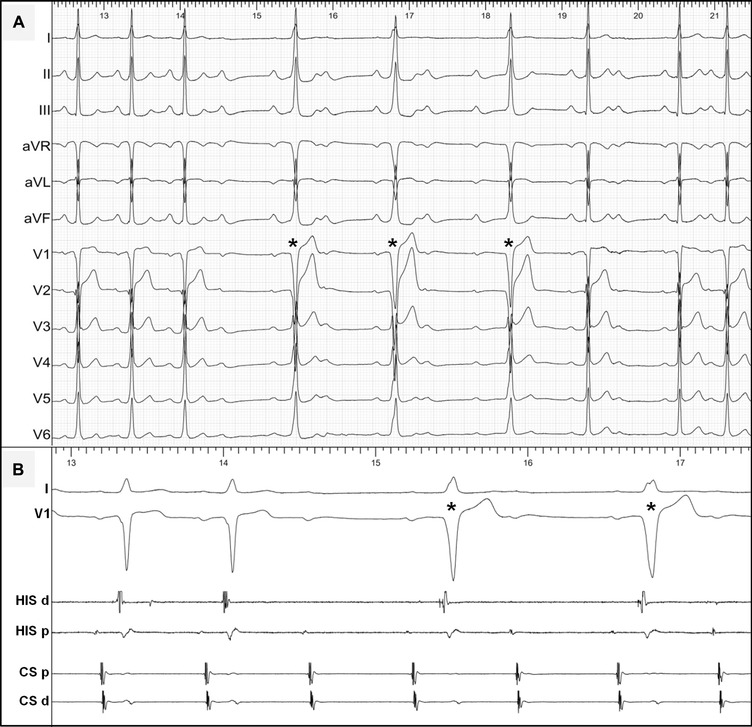
(A) Twelve‐lead ECG and (B) intracardiac recordings during intravenous adenosine test in a patient with a nodoventricular pathway (see text for discussion). Abbreviations are as in Figure 1.
Similarly to its effect on acutely disconnected pulmonary veins, intravenous adenosine can be used to unmask dormant conduction of APs after catheter ablation.7 In this study, adenosine test revealed AP dormant conduction in 12% of patients and was associated with higher recurrence rates requiring repeat ablation. Hyperpolarization of AP membrane potential was hypothesized to be the underlying mechanism of adenosine‐induced AP conduction after ablation.7
The last figure (Fig. 4) presents a simplified algorithm to interpret the electrocardiographic changes during adenosine test in various patterns of manifested APs. Interestingly, these phenomena may be also observed during vagal maneuvers or spontaneous phases of increased vagal tone (e.g., during Holter monitoring). Nevertheless, a detailed EPS is still the gold standard test for the definitive diagnosis of preexcitation variants and their arrhythmic characteristics.
Figure 4.
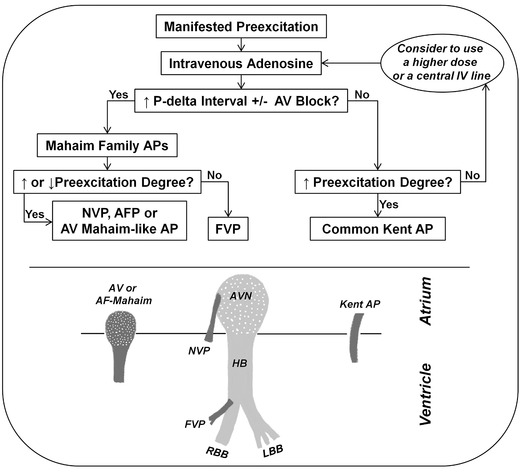
(Top) A simplified algorithm to interpret the electrocardiographic response to intravenous adenosine in various preexcitation patterns. (Bottom) A schematic design of the normal conduction system and various patterns of accessory pathways. The dotted areas indicate adenosine‐sensitive structures as the compact AV node and Mahaim accessory pathways. AF = atriofascicular; AFP = atriofascicular pathway; AV = atrioventricular; AVN = AV node; FVP = fasciculoventricular pathway; HB = His bundle; IV = intravenous; LBB = left bundle branch; NVP = nodoventricular pathway; RBB = right bundle branch.
In conclusion, intravenous adenosine has been used to unmask latent/subtle preexcitation, dormant AP conduction after ablation, and to predict more precisely the AP site. Furthermore, careful analysis of specific electrocardiographic changes during this test, with emphasis on P‐delta interval, preexcitation degree and AV block, can be helpful to diagnose the preexcitation variant/pattern.
Disclosures/Conflicts of interest: None.
REFERENCES
- 1. Link MS. Evaluation and initial treatment of supraventricular tachycardia. N Engl J Med 2012;367:1438–1448. [DOI] [PubMed] [Google Scholar]
- 2. Cohen TJ, Tucker KJ, Abbott JA, et al. Usefulness of adenosine in augmenting ventricular preexcitation for non‐invasive localization of accessory pathways. Am J Cardiol 1992;69:1178–1185. [DOI] [PubMed] [Google Scholar]
- 3. Garratt CJ, Antoniou A, Griffith MJ, et al. Use of intravenous adenosine in sinus rhythm as a diagnostic test for latent preexcitation. Am J Cardiol 1990;65:868–873. [DOI] [PubMed] [Google Scholar]
- 4. Lapage MJ, Walsh MJ, Reed JH, et al. Adenosine mapping for adenosine‐dependent accessory pathway ablation. Pacing Clin Electrophysiol 2014;37:610–615. [DOI] [PubMed] [Google Scholar]
- 5. Papagiannis J, Vachtsevanos L, Rammos S, et al. Cryoablation of a nodoventricular Mahaim fiber. J Interv Card Electrophysiol 2005;14:111–116. [DOI] [PubMed] [Google Scholar]
- 6. Ali H, Sorgente A, Lupo PP, et al. Nodo‐ and fasciculoventricular pathways: Electrophysiologic features and a proposed diagnostic algorithm for pre‐excitation variants. Heart Rhythm 2015;12:1677–1682. [DOI] [PubMed] [Google Scholar]
- 7. Spotnitz MD, Markowitz SM, Liu CF, et al. Mechanisms and clinical significance of adenosine‐induced dormant accessory pathway conduction after catheter ablation. Circ Arrhythm Electrophysiol 2014;7:1136–1143. [DOI] [PubMed] [Google Scholar]


