Abstract
Background
Although left ventricular hypertrophy (LVH) detected by electrocardiography (ECG‐LVH) and echocardiography (echo‐LVH) independently predict cardiovascular disease events, it is unclear if ECG‐LVH and echo‐LVH independently predict atrial fibrillation (AF).
Methods
This analysis included 4,904 participants (40% male; 85% white) from the Cardiovascular Health Study who were free of baseline AF and major intraventricular conduction delays. ECG‐LVH was defined by Minnesota Code Classification from baseline ECG data. Echo‐LVH was defined by sex‐specific left ventricular mass values >95th sex‐specific percentiles. Incident AF events were identified during the annual study ECGs and from hospitalization discharge data. Cox regression was used to compute hazard ratios (HR) and 95% confidence intervals (CI) for the association of ECG‐LVH and echo‐LVH with incident AF, separately.
Results
ECG‐LVH was detected in 224 (4.6%) participants and echo‐LVH was present in 231 (4.7%) participants. Over a median follow‐up of 11.9 years, a total of 1,430 AF events were detected. In a multivariable Cox model adjusted for age, sex, race, education, income, smoking, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive medications, and cardiovascular disease, ECG‐LVH (HR = 1.50; 95% CI = 1.18, 1.90) and echo‐LVH (HR = 1.39; 95% CI = 1.09, 1.78) were independently associated with AF. When ECG‐LVH (HR = 1.47, 95% CI = 1.16, 1.87) and echo‐LVH (HR = 1.36, 1.07, 1.75) were included in the same model, both were predictive of incident AF.
Conclusion
The association of ECG‐LVH with AF is not dependent on left ventricular mass detected by echocardiography, suggesting that abnormalities in cardiac electrophysiology provide a distinct profile in the prediction of AF.
Keywords: atrial fibrillation, epidemiology, left ventricle
1. Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice (Go et al., 2001). The prevalence of AF is expected to double by the year 2050, and this increase is largely related to the expected growth in the elderly population of the United States (Go et al., 2001; Lakshminarayan et al., 2006). Due to the increased burden that AF will place on the health care system, it is of paramount importance to identify AF risk factors that will aid in arrhythmia detection before complications, such as stroke, are apparent (Gladstone et al., 2014; Kim et al., 2011).
Data from several population‐based cohorts have shown that left ventricular hypertrophy (LVH) on the ECG (ECG‐LVH) is predictive of future AF events (Benjamin et al., 1994; Chrispin et al., 2014; Watanabe et al., 2006). Similarly, echocardiographic left ventricular enlargement is associated with an increased risk for AF (Vaziri et al., 1994; Verdecchia et al., 2003). However, it is unclear if distinct prognostic information regarding AF development is obtained with ECG‐LVH independent of LVH detected on the echocardiogram (echo‐LVH). It is possible that ECG‐LVH has a separate risk profile for AF development, independent of left ventricular mass detected by echocardiography. Therefore, the purpose of this analysis was to compare the AF risk associated with ECG‐LVH and echo‐LVH, and to determine if ECG‐LVH retains its predictive properties after accounting for echo‐LVH.
2. Methods
2.1. Study population
Details of the Cardiovascular Health Study (CHS) have been previously described (Fried et al., 1991). Briefly, CHS is a prospective cohort study of risk factors for coronary heart disease and stroke in individuals 65 years and older. A total of 5,888 participants with Medicare eligibility were recruited from four field centers in the United States in the following locations: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA. Subjects were followed with semiannual contacts, alternating between telephone calls and surveillance clinic visits. CHS clinic exams ended in June of 1999 and since that time two yearly phone calls to participants have been used to identify events and collect data. The institutional review board at each site approved the study and written informed consent was obtained from participants at enrollment. Participants were excluded if any of the following criteria were met: baseline AF was present, baseline covariate data were missing, QRS duration ≥120 ms, or follow‐up data were missing.
2.2. Left ventricular hypertrophy
LVH was determined from the baseline ECG or echocardiogram. Identical electrocardiographs (MAC PC, Marquette Electronics Inc., Milwaukee, WI, USA) were used at all clinic sites, and resting, 10‐s standard simultaneous 12‐lead ECGs were recorded in all participants (Furberg et al., 1992). ECG‐LVH was defined by Minnesota Code Classification (Prineas, Crow, & Blackburn, 1982). A baseline echocardiogram was obtained for each study participant according to previously described techniques (Gardin et al., 1992). Measurements were made from digitized images using an off‐line image‐analysis system equipped with customized computer algorithms. Left ventricular mass was derived from standard formulas described by Devereux et al. (1986). Echo‐LVH was defined by sex‐specific left ventricular mass values >95th sex‐specific percentiles (male: >213 g; female: >175 g).
2.3. Atrial fibrillation
In this analysis, AF events included paroxysmal, persistent, and permanent cases. Baseline AF cases were identified from the initial study ECG or by self‐reported history of a physician diagnosis. Incident AF cases were identified during the annual study ECGs that were performed annually until 1999. Additionally, hospitalization discharge data were used to identify incident AF events using International Classification of Diseases codes 427.31 and 427.32. Hospital diagnosis codes for AF ascertainment have been shown to have a positive predictive value of 98.6% (Alonso et al., 2009).
2.4. Covariates
Participant characteristics were collected during the initial CHS interview and questionnaire. Age, sex, race, income, education, and smoking status were self‐reported. Annual income was dichotomized at $25,000 and education was dichotomized at “high school or less.” Smoking was defined as current or ever smoker. Participants' blood samples were obtained after a 12‐hr fast at a local field center. Measurements of total cholesterol, high‐density lipoprotein cholesterol, and plasma glucose were used in this analysis. Diabetes was defined as self‐reported history of a physician diagnosis, a fasting glucose value ≥126 mg/dl, or by the current use of insulin or oral hypoglycemic medications. Blood pressure was measured for each participant in the seated position and systolic measurements were used in this analysis. The use of aspirin and antihypertensive medications were self‐reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Baseline cardiovascular disease was determined by a self‐reported history or by medical record adjudication of the following diagnoses: myocardial infarction, angina pectoris without myocardial infarction, coronary revascularization procedures (angioplasty and coronary artery bypass graft surgery), congestive heart failure, and claudication (Psaty et al., 1995).
2.5. Statistical analysis
Categorical variables were reported as frequency and percentage while continuous variables were recorded as mean ± standard deviation. Statistical significance for categorical variables was tested using the chi‐square method and the student's t‐test procedure for continuous variables. Comparisons were examined between participants with and without ECG‐LVH and echo‐LVH, separately. We examined the association between ECG‐LVH and echo‐LVH at baseline with incident AF. Follow‐up time was defined as the time from the initial study exam until one of the following: AF, death, loss to follow‐up, or end of follow‐up. Unadjusted Kaplan–Meier estimates were used to compute the cumulative incidence of AF by ECG‐LVH and echo‐LVH, and the differences in estimates were compared using the log‐rank procedure (Gray & Tsiatis, 1989). Cox regression was used to compute hazard ratios (HR) and 95% confidence intervals (CI) for the association between LVH and incident AF. We examined the association of ECG‐LVH and echo‐LVH with AF in isolation, and also in combination to determine if ECG‐LVH was predictive of AF independent of echo‐LVH. Multivariable models were constructed as follows: Model 1 adjusted for age, sex, race, education, and income; Model 2 adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive medications, and cardiovascular disease; Model 3 adjusted for Model 2 covariates and included both ECG‐LVH and echo‐LVH. The proportional hazards assumption was not violated in our analyses. Statistical significance was defined as p < .05. SAS Version 9.4 (Cary, NC, USA) was used for all analyses.
3. Results
ECG‐LVH was detected in 224 (4.6%) participants and echo‐LVH was present in 231 (4.7%) participants. Baseline characteristics stratified by ECG‐LVH and echo‐LVH are shown in Table 1. Over a median follow‐up of 11.9 years, a total of 1,430 AF events were detected. The unadjusted cumulative incidence curves of AF by ECG‐LVH and echo‐LVH are shown in Figures 1 and 2, respectively.
Table 1.
Baseline characteristics (N = 4,904)
| Characteristic | ECG‐LVH (n = 224) | No ECG‐LVH (n = 4,680) | p Valuea | Echo‐LVH (n = 231) | No Echo‐LVH (n = 4,673) | p Valuea |
|---|---|---|---|---|---|---|
| Age, years | ||||||
| 65–70 (%) | 64 (29) | 2,107 (45) | 115 (50) | 2,056 (44) | ||
| 71–74 (%) | 38 (17) | 1,120 (24) | 52 (22) | 1,106 (24) | ||
| 75–80 (%) | 82 (36) | 1,020 (22) | 44 (19) | 1,058 (23) | ||
| >80 (%) | 40 (18) | 433 (9) | <.001 | 20 (9) | 453 (9) | .35 |
| Male (%) | 92 (41) | 1,888 (40) | .83 | 93 (40) | 1,887 (40) | .97 |
| White (%) | 153 (68) | 4,008 (86) | <.001 | 125 (54) | 4,036 (86) | <.001 |
| High school or less (%) | 136 (61) | 2,689 (57) | .34 | 150 (65) | 2,675 (57) | .021 |
| Diabetes (%) | 41 (18) | 709 (15) | .20 | 78 (34) | 672 (14) | <.001 |
| Income <$25,000 (%) | 167 (75) | 2,964 (63) | <.001 | 173 (75) | 2,958 (63) | <.001 |
| Ever smoker (%) | 112 (50) | 2,505 (54) | .30 | 119 (52) | 2,498 (53) | .56 |
| Body mass index, mean ± SD, kg/m2 | 26 ± 3.8 | 27 ± 4.1 | .37 | 32 ± 3.8 | 26 ± 3.9 | <.001 |
| Systolic blood pressure, mean ± SD, mm Hg | 156 ± 24 | 139 ± 20 | <.001 | 148 ± 23 | 139 ± 20 | <.001 |
| Total cholesterol, mean ± SD, mg/dl | 210 ± 38 | 213 ± 39 | .21 | 207 ± 38 | 213 ± 39 | .027 |
| HDL cholesterol, mean ± SD, mg/dl | 54 ± 14 | 55 ± 16 | .36 | 50 ± 14 | 55 ± 16 | <.001 |
| Antihypertensive medication use (%) | 160 (71) | 2,066 (44) | <.001 | 153 (66) | 2,073 (44) | <.001 |
| Aspirin use (%) | 90 (40) | 1,521 (33) | .017 | 78 (34) | 1,533 (33) | .76 |
| Cardiovascular disease (%) | 81 (36) | 824 (18) | <.001 | 78 (34) | 827 (18) | <.001 |
ECG‐LVH, electrocardiographic left ventricular hypertrophy; echo‐LVH, echocardiographic left ventricular hypertrophy; HDL, high‐density lipoprotein; SD, standard deviation.
Statistical significance for continuous data was tested using the student's t‐test procedure and categorical data were tested using the chi‐square test.
Figure 1.
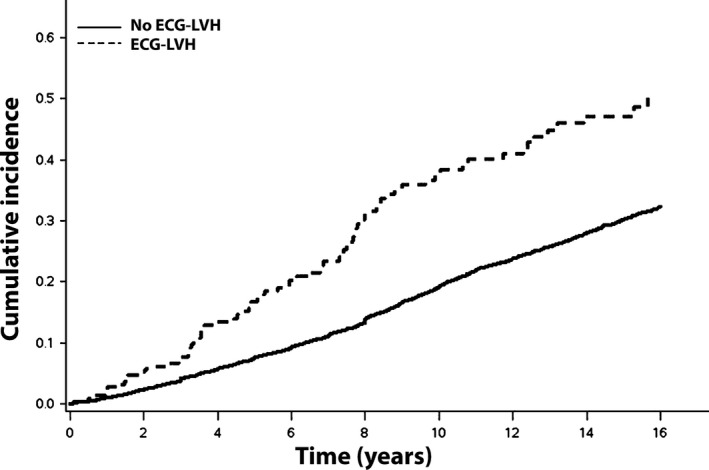
Unadjusted cumulative incidence of AF by ECG‐LVH*. *The unadjusted cumulative incidence curves are statistically different (log‐rank p < .001). AF = atrial fibrillation; ECG‐LVH = electrocardiographic left ventricular hypertrophy
Figure 2.

Unadjusted cumulative incidence of AF by echo‐LVH*. *The unadjusted cumulative incidence curves are statistically different (log‐rank p < .001). AF = atrial fibrillation; echo‐LVH = echocardiographic left ventricular hypertrophy
In a multivariable Cox regression analysis, ECG‐LVH (HR = 1.50, 95% CI = 1.18, 1.90) and echo‐LVH (HR = 1.39, 95% CI = 1.09, 1.78) were independently associated with AF (Table 2). When ECG‐LVH (HR = 1.47, 95% CI = 1.16, 1.87) and echo‐LVH (HR = 1.36, 1.07, 1.75) were included in the same model (Model 3), both were predictive of incident AF (Table 2). Additionally, when LVH was detected by the ECG‐LVH or echo‐LVH, it was predictive of AF (HR = 1.36, 95% CI = 1.12, 1.64; Table 2).
Table 2.
Risk of atrial fibrillation
| Events/No. at risk | Incidence Rate per 1,000 person‐years (95% CI) | Model 1a HR (95% CI) | p Value | Model 2b HR (95% CI) | p Value | Model 3c HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|---|---|
| ECG | ||||||||
| No LVH | 1,352/4,680 | 23.5 (22.3, 24.8) | 1.0 | — | 1.0 | — | 1.0 | — |
| LVH | 78/224 | 41.3 (33.1, 51.5) | 1.82 (1.45, 2.30) | <.001 | 1.50 (1.18, 1.90) | <.001 | 1.47 (1.16, 1.87) | .0014 |
| Echocardiogram | ||||||||
| No LVH | 1,353/4,673 | 23.7 (22.5, 25.0) | 1.0 | — | 1.0 | — | 1.0 | — |
| LVH | 77/231 | 33.8 (27.1, 42.3) | 1.73 (1.37, 2.19) | <.001 | 1.39 (1.09, 1.78) | .0090 | 1.36 (1.07, 1.75) | .014 |
| ECG or Echocardiogram | ||||||||
| No LVH | 1,293/4,482 | 23.3 (22.1, 24.6) | 1.0 | — | 1.0 | — | — | — |
| LVH | 137/422 | 34.7 (29.4, 41.0) | 1.68 (1.40, 2.01) | <.001 | 1.36 (1.12, 1.64) | .0016 | — | — |
ECG‐LVH, electrocardiographic left ventricular hypertrophy; echo‐LVH, echocardiographic left ventricular hypertrophy; LVH, left ventricular hypertrophy.
Adjusted for age, sex, race, education, and income.
Adjusted for Model 1 covariates plus smoking, systolic blood pressure, diabetes, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, antihypertensive medications, and cardiovascular disease.
Adjusted for Model 2 covariates and included both ECG‐LVH and echo‐LVH.
4. Discussion
In this analysis from CHS, both ECG‐LVH and echo‐LVH were predictive of future AF events, independent of well‐known AF risk factors. The predictive ability of ECG‐LVH did not depend on echo‐LVH, suggesting that ECG‐LVH is an important electrophysiological marker of cardiac abnormalities independent of left ventricular mass detected by echocardiography.
Data from the Framingham Heart Study (Benjamin et al., 1994), the Niigata Preventive Medicine Study (Watanabe et al., 2006), and the Multi‐Ethnic Study of Atherosclerosis (Chrispin et al., 2014), have demonstrated that ECG‐LVH is associated with an increased risk for AF. Left ventricular mass detected by echocardiography also was associated with AF in the Framingham Heart Study (Vaziri et al., 1994), and in a cohort of hypertensive patients in the Progetto Ipertensione Umbria Monitoraggio Ambulatoriale study (Verdecchia et al., 2003). However, to the best of our knowledge, prior studies have not examined if ECG‐LVH and echo‐LVH have distinct AF risk profiles. In the current analysis, ECG‐LVH was predictive of AF after accounting for echo‐LVH. This suggests that ECG‐LVH detects an abnormal electrophysiological profile that increases one's risk for AF, independent of left ventricular mass detected by echocardiography. Additionally, participants with echo‐LVH were at an increased risk for AF development, and this relationship was not influenced by ECG‐LVH, further supporting that both entities are clinically distinct when assessing AF risk.
It has been suggested that ECG‐LVH and echo‐LVH detect different pathologies. The QRS changes that occur with ECG‐LVH have been shown to represent a combination of anatomical and electrical remodeling (Bacharova et al., 2010, 2012). In contrast, echo‐LVH relies entirely on left ventricular mass (Armstrong et al., 2014). Although differences potentially exist in the cardiac pathology detected, equally important prognostic information regarding AF risk is obtained from both ECG‐LVH and echo‐LVH. The implication for the clinician assessing AF risk associated with LVH is that the combination of clinically relevant risk factors and data from the standard 12‐lead ECG are able to provide valuable prognostic information regarding future AF risk, in lieu of more expensive imaging modalities. Nonetheless, we acknowledge that both modalities examined in this analysis provide important information for the prediction of AF.
The national incremental cost of AF is estimated to range from $6.0 billion for AF‐related costs alone to $26.0 billion when accounting for cardiovascular‐related events (Kim et al., 2011). Accordingly, it is critical to identify at‐risk individuals to implement preventive measures to reduce the high costs attributed to this arrhythmia and its associated complications. Because of its low cost and widespread availability, the ECG possibly is a cost‐effective tool for identifying individuals who are at risk for AF, and the findings in this report support this claim. Additionally, ECG‐LVH possibly identifies patients who will benefit from targeted therapies to reduce AF risk. Treatments that are known to induce ECG‐LVH regression, such as angiotensin‐converting‐enzyme inhibitors, are such therapies that potentially reduce the occurrence of AF (Okin et al., 2003).
The results of the current analysis should be interpreted in the context of several limitations. ECG‐LVH was defined according to Minnesota Code Classification, the most widely utilized system for population‐based ECG categorization, and it is possible that our results vary with other criteria. Similarly, echo‐LVH was defined by left ventricular mass values greater than the 95th sex‐specific percentiles, and the results possibly vary with other definitions. Finally, several potential confounders were included in our multivariable models that likely influenced the development of incident AF, but we acknowledge the possibility of residual confounding.
In this study, ECG‐LVH was shown to predict AF independent of echo‐LVH, suggesting that a distinct electrophysiological profile exists in which AF development is likely independent of left ventricular mass. Further research is needed to explore the potential for ECG‐LVH regression as a marker of reduced AF risk, and if these changes are dependent on left ventricular mass.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
This manuscript was prepared using Cardiovascular Health Study Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the Cardiovascular Health Study or the National Heart, Lung, and Blood Institute.
Patel N, O'Neal WT, Whalen SP, Soliman EZ. Electrocardiographic left ventricular hypertrophy predicts atrial fibrillation independent of left ventricular mass. Ann Noninvasive Electrocardiol. 2017;22:e12419. 10.1111/anec.12419
References
- Alonso, A. , Agarwal, S. K. , Soliman, E. Z. , Ambrose, M. , Chamberlain, A. M. , Prineas, R. J. , … Folsom, A. R. (2009). Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. American Heart Journal, 158, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, A. C. , Gjesdal, O. , Almeida, A. , Nacif, M. , Wu, C. , Bluemke, D. A. , … Lima, J. A. C. (2014). Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: The multi‐ethnic study of atherosclerosis. Echocardiography‐A Journal of Cardiovascular Ultrasound and Allied Techniques, 31, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharova, L. , Szathmary, V. , Kovalcik, M. , & Mateasik, A. (2010). Effect of changes in left ventricular anatomy and conduction velocity on the QRS voltage and morphology in left ventricular hypertrophy: A model study. Journal of Electrocardiology, 43, 200–208. [DOI] [PubMed] [Google Scholar]
- Bacharova, L. , Szathmary, V. , Potse, M. , & Mateasik, A. (2012). Computer simulation of ECG manifestations of left ventricular electrical remodeling. Journal of Electrocardiology, 45, 630–634. [DOI] [PubMed] [Google Scholar]
- Benjamin, E. J. , Levy, D. , Vaziri, S. M. , D'Agostino, R. B. , Belanger, A. J. , & Wolf, P. A. (1994). Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. Journal of the American Medical Association, 271, 840–844. [PubMed] [Google Scholar]
- Chrispin, J. , Jain, A. , Soliman, E. Z. , Guallar, E. , Alonso, A. , Heckbert, S. R. , … Nazarian, S. (2014). Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi‐Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology, 63, 2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux, R. B. , Alonso, D. R. , Lutas, E. M. , Gottlieb, G. J. , Campo, E. , Sachs, I. , & Reichek, N. (1986). Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. American Journal of Cardiology, 57, 450–458. [DOI] [PubMed] [Google Scholar]
- Fried, L. P. , Borhani, N. O. , Enright, P. , Furberg, C. D. , Gardin, J. M. , Kronmal, R. A. , … Weiler, P. G. (1991). The cardiovascular health study: Design and rationale. Annals of Epidemiology, 1, 263–276. [DOI] [PubMed] [Google Scholar]
- Furberg, C. D. , Manolio, T. A. , Psaty, B. M. , Bild, D. E. , Borhani, N. O. , Newman, A. , … Rautaharju, P. M. (1992). Major electrocardiographic abnormalities in persons aged 65 years and older (the Cardiovascular Health Study). Cardiovascular Health Study Collaborative Research Group. American Journal of Cardiology, 69, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Gardin, J. M. , Wong, N. D. , Bommer, W. , Klopfenstein, H. S. , Smith, V. E. , Tabatznik, B. , … Manolio, T. A. (1992). Echocardiographic design of a multicenter investigation of free‐living elderly subjects: The Cardiovascular Health Study. Journal of the American Society of Echocardiography, 5, 63–72. [DOI] [PubMed] [Google Scholar]
- Gladstone, D. J. , Spring, M. , Dorian, P. , Panzov, V. , Thorpe, K. E. , Hall, J. , … Coordinators, E. (2014). Atrial fibrillation in patients with cryptogenic stroke. New England Journal of Medicine, 370, 2467–2477. [DOI] [PubMed] [Google Scholar]
- Go, A. S. , Hylek, E. M. , Phillips, K. A. , Chang, Y. C. , Henault, L. E. , Selby, J. V. , & Singer, D. E. (2001). Prevalence of diagnosed atrial fibrillation in adults ‐ National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Journal of the American Medical Association, 285, 2370–2375. [DOI] [PubMed] [Google Scholar]
- Gray, R. J. , & Tsiatis, A. A. (1989). A linear rank test for use when the main interest is in differences in cure rates. Biometrics, 45, 899–904. [PubMed] [Google Scholar]
- Kim, M. H. , Johnston, S. S. , Chu, B. C. , Dalal, M. R. , & Schulman, K. L. (2011). Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circulation: Cardiovascular Quality and Outcomes, 4, 313–320. [DOI] [PubMed] [Google Scholar]
- Lakshminarayan, K. , Solid, C. A. , Collins, A. J. , Anderson, D. C. , & Herzog, C. A. (2006). Atrial fibrillation and stroke in the general medicare population ‐ A 10‐year perspective (1992 to 2002). Stroke, 37, 1969–1974. [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Devereux, R. B. , Jern, S. , Kjeldsen, S. E. , Julius, S. , Nieminen, M. S. , … Dahlöf, B. (2003). Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: The Losartan Intervention for Endpoint reduction in Hypertension (LIFE) Study. Circulation, 108, 684–690. [DOI] [PubMed] [Google Scholar]
- Prineas, R. J. , Crow, R. S. , & Blackburn, H. W. (1982). The Minnesota code manual of electrocardiographic findings: Standards and procedures for measurement and classification. Boston, MA: J. Wright. [Google Scholar]
- Psaty, B. M. , Kuller, L. H. , Bild, D. , Burke, G. L. , Kittner, S. J. , Mittelmark, M. , … Robbins, J. (1995). Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Annals of Epidemiology, 5, 270–277. [DOI] [PubMed] [Google Scholar]
- Vaziri, S. M. , Larson, M. G. , Benjamin, E. J. , & Levy, D. (1994). Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation, 89, 724–730. [DOI] [PubMed] [Google Scholar]
- Verdecchia, P. , Reboldi, G. , Gattobigio, R. , Bentivoglio, M. , Borgioni, C. , Angeli, F. , … Porcellati, C. (2003). Atrial fibrillation in hypertension: Predictors and outcome. Hypertension, 41, 218–223. [DOI] [PubMed] [Google Scholar]
- Watanabe, H. , Tanabe, N. , Makiyama, Y. , Chopra, S. S. , Okura, Y. , Suzuki, H. , & … Aizawa, Y. (2006). ST‐segment abnormalities and premature complexes are predictors of new‐onset atrial fibrillation: The Niigata preventive medicine study. American Heart Journal, 152, 731–735. [DOI] [PubMed] [Google Scholar]


