Abstract
Background
Peripartum cardiomyopathy (PPCM) is an uncommon complication of pregnancy. Clinical courses of PPCM are markedly heterogeneous. Positive T waves in lead aVR (TaVR) are shown to be associated with adverse cardiac events in several cardiovascular diseases. We aimed to investigate the prevalence and prognostic role of positive TaVR in patients with PPCM.
Methods
A total of 82 patients (mean age 29.1 ± 6.3 years) with the diagnosis of PPCM were enrolled. Presentation electrocardiogram (ECG) was investigated for presence of a positive TaVR. The median follow‐up duration was 67.0 months. The primary endpoint was defined as composite cardiac events, including cardiac death, arrhythmic events, or persistent left ventricular systolic dysfunction.
Results
Patients with positive T wave in lead aVR showed higher rates for persistent left ventricular systolic dysfunction, arrhythmic events, and cardiac death compared to patients without it. In multivariate logistic regression analysis, after adjusting for other confounding factors, the presence of positive TaVR was found to be as an independent and strong predictor of primary composite endpoint (odds ratio 6.21, 95% CI 1.45–26.51; p = 0.014). In Kaplan–Meier survival analysis, both primary and secondary endpoints occurred more frequently in the positive TaVR group. Using the cut‐off level of 0.25 mV, T‐wave amplitude in lead aVR predicted primary endpoint with a sensitivity of 100% and specificity of 100%.
Conclusion
Positive T wave in lead aVR, as a simple and feasible electrocardiographic marker, seems to be a novel predictor of adverse cardiovascular outcomes in patients with PPCM.
Keywords: lead aVR, peripartum cardiomyopathy, prognosis
1. INTRODUCTION
Peripartum cardiomyopathy (PPCM) is defined as an unique form of heart failure with reduced ejection fraction (EF), usually <45%, presenting toward the end of pregnancy or in the first months postpartum in previously healthy women, where no other reason of heart failure is found (Sliwa et al., 2010). The exact pathophysiological mechanism of the disease is unknown but various risk factors such as, genetic and hormonal mechanisms, abnormal immune or hemodynamic response to pregnancy, increased oxidative stress and inflammation, have been identified (Biteker, Kayatas, Duman, Turkmen, & Bozkurt, 2014; Dalzell, Jackson, & Gardner, 2011; Fett & McTiernan, 2011). Clinical course range from complete recovery of left ventricular (LV) function to progressive heart failure, thromboembolic complications, life‐threatening arrhythmias, and even cardiac death (Goland et al., 2015; Karaye & Henein, 2013; Nishimoto et al., 2012). Therefore, the ability to determine early predictors of prognosis in women diagnosed with PPCM is of utmost importance in risk stratification, using reasonable management strategies, preventing complications and improving outcome. Predictors of adverse outcomes are inconsistently defined in several studies and include lower baseline LV EF, higher baseline LV end‐diastolic diameter, older age, and black race (Fett, Christie, Carraway, & Murphy, 2005; Goland et al., 2009).
Upright T wave in lead aVR (TaVR) on a routine 12‐lead electrocardiogram (ECG), as a marker of repolarization abnormality, suggested to be a powerful, independent prognostic predictor of cardiovascular (CV) mortality in the general population (Anttila et al., 2011; Badheka et al., 2013; Tan, Engel, Myers, Sandri, & Froelicher, 2008) as well as in some cardiovascular diseases (Ayhan et al., 2013; Okuda et al., 2011; Torigoe et al., 2012). However, evidence on the prognostic role of positive TaVR in PPCM patients has been lacking. Therefore, we aimed to evaluate the prognostic role of upright TaVR in predicting arrhythmic events, persistent LV systolic dysfunction, and cardiovascular mortality in patients with PPCM.
2. MATERIAL AND METHODS
2.1. Study population
We conducted a retrospective analysis of 82 consecutive patients (mean age 29.1 ± 6.3 years) diagnosed with PPCM in our tertiary reference center from April 2009 to May 2017. Data regarding demographic, electrocardiographic, and echocardiographic features of all patients were collected from patients’ files, clinical follow‐up visits, device interrogation, and the electronic database. The study protocol was approved by the local ethics committee.
Peripartum cardiomyopathy was accepted as an occurrence of unexplained cardiomyopathy with LVEF <45% presenting toward the end of pregnancy or soon after delivery in a previously healthy woman (Sliwa et al., 2010). All patients were at least 18 years of age. Women with any previous congenital or significant organic valvular heart disease, coronary heart disease (≥50% luminal stenosis in at least one major coronary arteries and their branches), a history of cardiomyopathy, complete left bundle branch block, right ventricular pacing, or without interpretable admission ECG were excluded from this study.
The follow‐up period was commenced with the first admission and ended with the occurrence of death, arrhythmic event or device therapy or the last visit. The duration of follow‐up was at least 12 months after diagnosis for all participants. All patients had undergone echocardiographic measurements at the time of diagnosis and the last follow‐up visit. Recovery of LV systolic function was defined as the presence of LVEF ˃45%. The implantable cardioverter defibrillator (ICD) devices were routinely interrogated whenever symptomatic events relevant to ventricular tachycardia or ICD shock delivery happened and also at 6‐month intervals. Monitorized intracardiac ECG recordings were evaluated regarding cardiac arrhythmias. All patients were given standard treatment for heart failure including beta blockers, angiotensin‐converting enzyme inhibitors (or angiotensin‐receptor blocker), digitalis. None of the women received bromocriptine.
2.2. Study endpoints
The primary endpoint was defined as composite cardiac events that included cardiac death, arrhythmic event (a malignant cardiac arrhythmia [sustained VT and/or VF and/or appropriate ICD shock]), or persistent LV systolic dysfunction. Cardiac mortality, arrhythmic event, persistent LV systolic dysfunction, and a combination of death and arrhythmic event were evaluated, respectively, as secondary endpoints.
2.3. Electrocardiographic evaluation
A standard 12‐lead surface electrocardiogram (25 mm/s and 10 mm = 1 mV) in the supine position was obtained from all patients on first admission. Conventional ECG parameters including heart rate, PR interval, QRS duration, QT duration, bundle branch block, QRS axis, abnormal Q waves, T‐wave inversion, left ventricular hypertrophy (Sokolow & Lyon voltage amplitude criteria SV1 + RV5 or V6) were calculated. The QTc was calculated using Bazett’s formula. Presence of abnormal Q wave was defined as a Q wave with more than 25% of the QRS complex depth in at least two contiguous leads. In addition to these conventional parameters, the T‐wave amplitude in lead aVR was analyzed. The T‐wave amplitude was described as the first deflection after the QRS complex and/or the maximum deviation from the PR isoelectric line. A positive T wave was described as a wave with a positive deflection ˃0 mV. Negative TaVR was defined as TaVR ≤0 mm. The T‐wave amplitudes in lead aVR were measured manually. The amplitude of positive T wave when present was determined in each ECG. The 12‐lead electrocardiogram of each patient was assessed by two independent cardiologists blinded to the patients’ clinical outcomes.
2.4. Echocardiographic evaluation
Standard 2‐dimensional and Doppler echocardiographic measurements were performed in all women by experienced echocardiographers using 2.5 to 4‐MHz transducers (Vivid 7; GE Medical System, Milwaukee, WI, USA). LV EF was measured using the modified Simpson rule.
2.5. Statistical analysis
Statistical analysis was performed using the SPSS 20.0 Statistical Package Program for Windows (SPSS, Inc., IL, USA). Continuous variables were presented as mean ± SD and median with interquartile ranges as appropriate and categorical variables as frequency and percentage. To test normality of distribution, Shapiro–Wilk test was used. Differences between groups were evaluated by using Student’s t test for normally distributed variables and Mann‐Whitney U test for variables without normal distribution. The chi‐square or Fisher’s Exact test was used to compare categorical variables as appropriate. Intraobserver and interobserver reliability analyses using the Kappa statistic were applied to assess the consistency in determination of positive TaVR. We first used a univariate logistic regression analysis to evaluate the association of each variable with the occurrence of adverse cardiac events. To assess the effects of parameters that were found significant in univariate analysis (p < 0.05), we used a multivariate logistic regression analysis. Kaplan–Meier curve analysis was used for freedom from adverse events in patients with or without positive TaVR. Receiver operating characteristic curve (ROC) analysis was used to determine the optimum cut‐off levels of the amplitude of TaVR to predict the arrhythmic events, persistent LV dysfunction and mortality. A p‐value <0.05 (using a two‐sided test) was considered significant. In addition, the sample size and the statistical power of the study was evaluated. For an 80% statistical power, a sample size of 60 is required to demonstrate a 12.5% T‐wave abnormality in the normalized LV function and a 45.0% T‐wave abnormality in the persistent LV dysfunction group (two‐tailed α of 0.05; Tibazarwa et al., 2012). Expected values for positive T wave in lead aVR were 8.6% and 57.4% in the normalized LV function and persistent LV dysfunction groups, respectively. The statistical powers of our study were 99.9% for two‐tailed α of 0.05 and 99.2% for two‐tailed α of 0.01.
3. RESULTS
From April 2009 to May 2017, 94 patients were identified with the diagnosis of PPCM. A total of 82 patients with diagnosis of PPCM were included in the analysis after excluding 12 patients due to complete left bundle branch block in seven patients, ventricular pacing in two patients, or missing clinical and follow‐up data in three patients. Baseline clinical, demographical, ECG, and echocardiographic characteristics of the study population comparing patients with positive T waves in aVR and patients with negative T waves were described in Table 1. The mean age of the study population at diagnosis was 29.1 ± 6.3 years. Positive T wave in lead aVR was present in 30 patients (36.6%). Negative T wave and flat T wave were present in 47 patients (57.3%) and five patients (6.1%), respectively. The prevalence of smoking, hypertension, diabetes mellitus, hyperlipidemia, positive family history of dilated cardiomyopathy, chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), stroke, and atrial fibrillation were similar in two groups. NYHA functional class 3–4 on admission was significantly higher in patients with positive TaVR (70.0% vs. 19.2%, p < 0.001; Table 1). With regard to echocardiographic parameters, the LV EDD and LV ESD were greater in patients with positive T wave in lead aVR, whereas the LVEF was smaller in patients with positive TaVR. With regard to ECG parameters, the mean heart rate, PR, QRS, QTc interval, T‐wave inversion in inferolateral leads and complete RBBB were similar in two groups. The prevalences of abnormal Q waves were significantly higher in patients with a positive T wave in lead aVR than in those without it. Follow‐up data and clinical outcomes were presented in Table 2. During a follow‐up period of median 67.0 (12.0–192.0) months, primary endpoint developed in 48 of 82 subjects (58.5%). Among these subjects, cardiac death was observed as a secondary endpoint in six subjects (7.3%; sudden death in four, death for progressive heart failure in two), arrhythmic event was observed as a secondary endpoint in eight subjects (9.8%), and persistent LV systolic dysfunction was observed as a secondary endpoint in 47 subjects (57.3%). During the study period, one left ventricular assist device implantation, one heart transplantation, and six embolic events (four among nonrecovery group and two among recovery group) occurred in patients. In addition, electrocardiographic parameters regarding occurrence of death and presence of death or arrhythmic events in patients with positive T wave in lead aVR were compared in Table 3.
Table 1.
Baseline clinical, demographical, ECG, and echocardiographic characteristics of the study population according to the presence of positive T wave in lead aVR (n = 82)
| Total, n = 82 | T wave (+), n = 30 | T wave (−), n = 52 | p value | |
|---|---|---|---|---|
| Age at diagnosis | 29.1 ± 6.3 | 30.3 ± 6.8 | 28.5 ± 6.08 | 0.229 |
| Hypertension | 13 (15.9%) | 4 (13.3%) | 9 (17.3%) | 0.635 |
| Hyperlipidemia | 12 (14.6%) | 6 (20%) | 6 (11.5%) | 0.296 |
| Diabetes mellitus | 3 (3.7%) | 2 (6.7%) | 1 (1.9%) | 0.270 |
| Coronary artery disease | 2 (2.4%) | 1 (3.3%) | 1 (1.9%) | 0.690 |
| NYHA class III–IV | 31 (37.8%) | 21 (70%) | 10 (19.2%) | <0.001 |
| Family history of cardiomyopathy | 12 (14.6%) | 6 (20%) | 6 (11.5%) | 0.296 |
| Atrial fibrillation | 2 (2.4%) | 1 (3.3%) | 1 (1.9%) | 0.690 |
| Stroke | 2 (2.4%) | 0 (0.0) | 2 (3.8%) | 0.277 |
| Embolic events | 7 (8.5%) | 2 (6.7%) | 5 (9.6%) | 0.645 |
| ICD | 25 (30.5%) | 9 (30%) | 16 (30.8%) | 0.942 |
| Echocardiographic parameters | ||||
| LVEDD (mm) | 57.0 ± 5.1 | 59.1 ± 4.4 | 55.7 ± 5.3 | 0.005 |
| LVESD (mm) | 48.4 ± 9.5 | 53.3 ± 8.1 | 42.3 ± 7.6 | 0.010 |
| LA diameter (mm) | 39.1 ± 7.4 | 42.4 ± 6.1 | 37.4 ± 7.6 | 0.070 |
| LVEF (%) | 30.1 ± 7.5 | 26.5 ± 7.2 | 32.3 ± 6.9 | 0.001 |
| LVEF <35 (%) | 60 (73.2%) | 28 (93.3%) | 32 (61.5%) | 0.002 |
| SPAP | 37.0 ± 10.8 | 40.2 ± 12.6 | 35.2 ± 9.6 | 0.188 |
| Electrocardiographic parameters | ||||
| Heart rate | 84.1 ± 19.1 | 86.7 ± 25.4 | 82.7 ± 14.4 | 0.365 |
| Duration of PR interval | 181.8 ± 37.1 | 176.3 ± 37.7 | 184.8 ± 36.8 | 0.335 |
| Duration of QRS interval | 106.2 ± 14.3 | 104.7 ± 17.9 | 107.1 ± 11.8 | 0.461 |
| Duration of QTc interval | 469.1 ± 32.6 | 474.2 ± 23.3 | 466.2 ± 36.9 | 0.289 |
| Abnormal Q wave | 11 (13.4%) | 9 (30.0%) | 2 (3.8%) | 0.001 |
| T‐wave inversion | 28 (34.1%) | 10 (33.3%) | 18 (34.6%) | 0.906 |
| T‐wave inversion in anterior leads | 14 (17.1%) | 4 (13.3%) | 10 (19.2%) | 0.494 |
| T‐wave inversion in inferior leads | 3 (3.7%) | 3 (10.0%) | 0 (0.0%) | 0.020 |
| T‐wave inversion in lateral leads | 16 (19.5%) | 8 (26.7%) | 8 (15.4%) | 0.214 |
| RBBB | 5 (6.1%) | 1 (3.3%) | 4 (7.7%) | 0.427 |
| Left axis deviation | 38 (46.3%) | 11 (36.7%) | 27 (51.9%) | 0.182 |
| Left ventricular hypertrophy | 9 (11.0%) | 0 (0%) | 9 (17.3%) | 0.016 |
ICD: implantable cardioverter defibrillator; LVEDD: left ventricular end‐diastolic diameter; LVEF: left ventricular ejection fraction; LVEDD: left ventricular end‐diastolic diameter; LVESD: left ventricular end‐systolic diameter; NYHA: New York Heart Association; RBBB: right bundle branch block; SPAP: systolic pulmonary artery pressure.
Data are presented mean ± SD or n (%).
Bolded values indicate statistical significance (p < 0.05).
Table 2.
Comparison of clinical outcomes according to the presence of positive T wave in lead aVR
| Parameter | Total, n = 82 | T wave (+), n = 30 | T wave (−), n = 52 | p |
|---|---|---|---|---|
| Cardiac death | 6 (7.3%) | 6 (20%) | 0 (0%) | 0.001 |
| Arrhythmic event | 8 (9.8%) | 6 (20%) | 2 (3.8%) | 0.018 |
| Persistent LV dysfunction | 47 (57.3%) | 27 (90%) | 20 (38.5%) | 0.000 |
| Death or arrhythmic event | 13 (15.9%) | 11 (36.7%) | 2 (3.8%) | 0.000 |
| Primary composite endpoint | 48 (58.5%) | 27 (90.0%) | 21 (40.4%) | 0.000 |
LV: left ventricular.
Bolded values indicate statistical significance (p < 0.05).
Table 3.
Comparison of electrocardiographic parameters regarding occurrence of death and presence of death or arrhythmic events in patients with positive T wave in lead aVR (n = 30)
| Death | Death or arrhythmic events | |||||
|---|---|---|---|---|---|---|
| Yes | No | p value | Yes | No | p value | |
| n = 6 | n = 24 | n = 11 | n = 19 | |||
| Electrocardiographic parameters | ||||||
| Heart rate | 84.6 ± 34.8 | 87.2 ± 23.4 | 0.828 | 79.9 ± 22.5 | 90.6 ± 26.7 | 0.271 |
| Duration of PR interval | 182.6 ± 36.2 | 174.6 ± 38.7 | 0.652 | 183.2 ± 39.3 | 171.8 ± 37.0 | 0.445 |
| Duration of QRS interval | 118.3 ± 5.1 | 101.3 ± 18.3 | 0.035 | 107.7 ± 15.8 | 103.01 ± 19.1 | 0.496 |
| Duration of QTc interval | 474.1 ± 32.6 | 474.2 ± 21.2 | 0.991 | 466.1 ± 21.9 | 478.9 ± 23.3 | 0.151 |
| Abnormal Q wave | 2 (33.3%) | 7 (29.2%) | 0.842 | 3 (27.3%) | 6 (31.6%) | 0.804 |
| T‐wave inversion | 3 (50.0%) | 7 (29.2%) | 0.333 | 4 (36.4%) | 6 (31.6%) | 0.789 |
| T‐wave inversion in anterior leads | 2 (33.3%) | 0 (0.0%) | 0.003 | 2 (18.2%) | 0 (0.0%) | 0.054 |
| T‐wave inversion in inferior leads | 0 (0.0%) | 3 (12.5%) | 0.361 | 0 (0.0%) | 3 (15.8%) | 0.165 |
| T‐wave inversion in lateral leads | 1 (16.7%) | 7 (29.2%) | 0.536 | 3 (27.3%) | 5 (26.3%) | 0.954 |
| RBBB | 1 (16.7%) | 0 (0.0%) | 0.042 | 1 (9.1%) | 0 (0.0%) | 0.181 |
| Left axis deviation | 2 (33.3%) | 9 (37.5%) | 0.850 | 4 (36.4%) | 7 (36.8%) | 0.979 |
| Left ventricular hypertrophy | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
RBBB: right bundle branch block.
Data are presented mean ± SD or n (%).
Bolded values indicate statistical significance (p < 0.05).
A Kaplan–Meier analysis showed a significantly lower primary composite event‐free survival rate in patients with positive TaVR (log‐rank, p = 0.001; Figure 1). Total arrhythmic events occurred more frequently in the positive TaVR group (log‐rank, p = 0.017). Persistent LV systolic dysfunction developed more frequently in the positive TaVR than in those with negative T waves in lead aVR (log‐rank, p = 0.001) Also, patients with positive TaVR had a higher cardiac death rate compared with patients without positive TaVR (log‐rank, p = 0.001; Figure 2).
Figure 1.
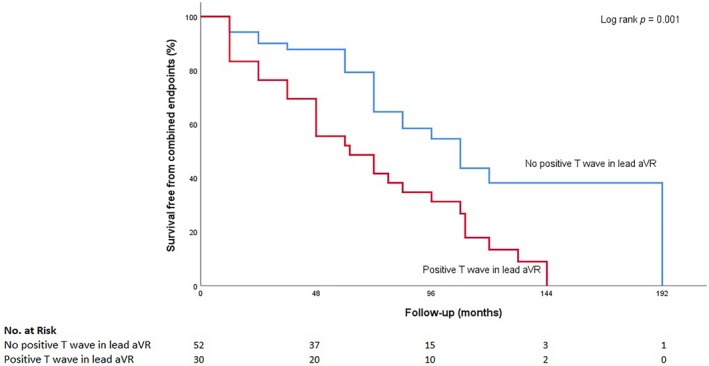
Kaplan–Meier curve analysis of the composite primary endpoint
Figure 2.
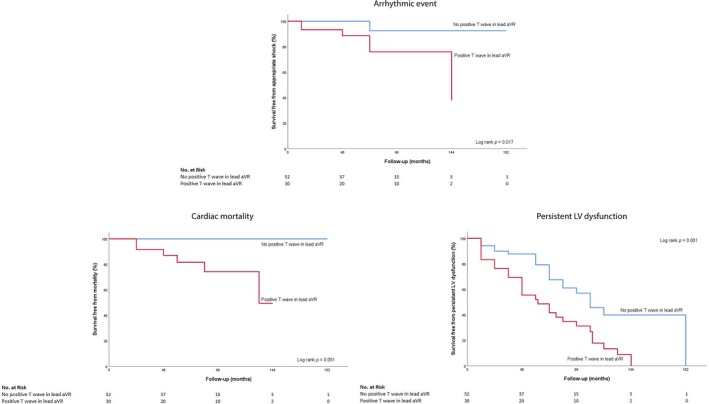
Kaplan–Meier curve analysis of secondary endpoints. The effects of positive TaVR on arrhythmic event, cardiac death persistent LV dysfunction‐free survival were examined. TaVR: T waves in lead aVR
Univariate logistic regression analyses showed that initial LV EF, LV EDD, duration of QTc interval, positive T waves in lead aVR were significantly associated with the primary endpoint (for all, p < 0.05; Table 3). In multivariate logistic regression analysis, the presence of positive TaVR was determined as single independent predictor of composite cardiac events even after adjustment for other confounding factors (odds ratio 6.21; 95% confidence interval 1.45–26.51; p = 0.014; Table 4).
Table 4.
Univariate and multivariate logistic regression analysis for prediction of primary endpoint
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | Adjusted OR | 95% CI | p value | |
| Age at diagnosis | 1.004 | 0.936–1.077 | 0.909 | |||
| Hypertension | 1.160 | 0.344–3.911 | 0.811 | |||
| Hyperlipidemia | 2.385 | 0.594–9.566 | 0.220 | |||
| Diabetes mellitus | 1.221 | 0.956–1.479 | 0.999 | |||
| Coronary artery disease | 0.761 | 0.991–1.020 | 0.298 | |||
| Stroke | 0.510 | 0.155–1.675 | 0.267 | |||
| Thromboembolic events | 1.860 | 0.330–10.209 | 0.475 | |||
| Family history of cardiomyopathy | 9.811 | 1.201–80.131 | 0.033 | 7.320 | 0.630–85.048 | 0.112 |
| Atrial fibrillation | 1.194 | 1.040–1.367 | 0.999 | |||
| ECG parameters | ||||||
| Duration of PR interval | 1.009 | 0.996–1.021 | 0.177 | |||
| Duration of QRS interval | 1.033 | 1.001–1.067 | 0.044 | |||
| Duration of QTc interval | 1.027 | 1.010–1.044 | 0.002 | 1.015 | 0.996–1.034 | 0.115 |
| Abnormal Q wave | 0.978 | 0.320–2.983 | 0.968 | |||
| T‐wave inversion | 1.440 | 0.562–3.290 | 0.448 | |||
| RBBB | 2.014 | 0.564–7.193 | 0.281 | |||
| Left axis deviation | 0.635 | 0.262–1.538 | 0.314 | |||
| Left ventricular hypertrophy | 0.311 | 0.072–1.345 | 0.118 | |||
| Positive T wave in aVR | 13.28 | 3.566–49.493 | 0.000 | 6.212 | 1.456–26.513 | 0.014 |
| Echocardiographic parameters | ||||||
| LVEDD (mm) | 1.179 | 1.065–1.306 | 0.002 | 1.050 | 0.918–1.202 | 0.478 |
| LVESD (mm) | 1.785 | 1.000–3.187 | 0.050 | |||
| LA diameter (mm) | 1.180 | 1.035–1.346 | 0.013 | |||
| LVEF (%) | 0.861 | 0.795–0.932 | 0.000 | 0.919 | 0.822–1.027 | 0.138 |
| SPAP | 1.024 | 0.958–1.096 | 0.485 | |||
CI: confidence interval; ECG: electrocardiography; LA: left atrium; LVEDD: left ventricular end‐diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end‐systolic diameter; NYHA: New York Heart Association; OR: odds ratio; RBBB: right bundle branch block; SPAP: systolic pulmonary artery pressure.
Bolded values indicate statistically significant odds ratio.
The ROC curve analysis explored the discriminatory capability of T‐wave positivity in lead aVR for primary composite endpoint. Area under the curve was 1.000 (p < 0.001). Using a cut‐off level of 0.25 mV, T‐wave amplitude in lead aVR predicted primary endpoint with a sensitivity of 100% and specificity of 100% (Figure 3). In addition, the specificity and positive predictive value to predict primary endpoint were 91.2% and 90%, respectively
Figure 3.
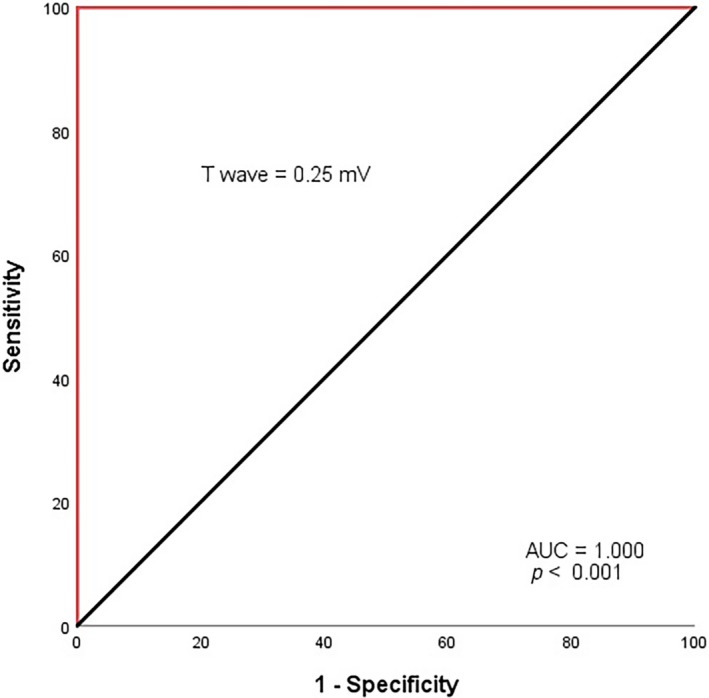
Receiver operating characteristic curve of positive TaVR for predicting primary endpoint. TaVR: T waves in lead aVR
4. DISCUSSION
The main findings of present study were as follows: (a) the presence of positive T wave in lead aVR was demonstrated as an independent and powerful predictor of composite CV events even after adjustment for established risk factors in patients with PPCM, (b) positive TaVR was significantly associated with all secondary endpoints; CV death as well as arrhythmic events and persistent LV systolic dysfunction, and (c) using a cut‐off level of 0.25 mV, T‐wave amplitude in lead aVR predicted primary endpoint with a sensitivity of 100% and specificity of 100%.
Peripartum cardiomyopathy is a unique type of cardiomyopathy with a significant likelihood of myocardial recovery. Previous studies have revealed that many women with PPCM recover LV function partially or fully; however, markedly reduced cardiac function and failure to recover can be associated with adverse cardiac events including lethal ventricular tachyarrhythmias, thromboembolic complications, and even death (Goland et al., 2009; Misumida et al., 2016). The attempts to discover clinical markers for poor prognosis in women with PPCM have resulted in determination of a number of parameters with moderate and inconsistent relations with cardiac outcomes. Several investigators have demonstrated a correlation between a lower LV EF at the time of diagnosis and a worse outcome in these women (Fett et al., 2005; Sliwa et al., 2010). Also, some studies have showed a relation between an increased LV EDD on the initial echocardiogram and persistent LV systolic dysfunction (Chapa et al., 2005). However, other investigators have found no correlation between the admission LVEF‐LV EDD and survival (Amos, Jaber, & Russell, 2006; Forster et al., 2008). The lack of ability to predict outcomes accurately makes clinical decision‐making challenging as a PPCM patient with initially low EF may recover; therefore, the use of advanced therapies such as ICDs, left ventricular assist devices, or heart transplantation, may have been risky if used too late.
From this perspective, novel approaches to risk stratification require establishments of new, widely available risk markers such as those identified from the surface 12‐lead electrocardiogram. To our knowledge, there is a paucity of ECG data in PPCM, and scarce data on its use in the risk stratification of PPCM. In recent years, the presence of positive T wave in lead aVR on a routine 12‐lead ECG has become a marker of repolarization abnormality. It has been shown that positive TaVR is associated with increased mortality and arrhythmic events both in the general population (Anttila et al., 2011; Badheka et al., 2013) as well as in some clinical settings such as renal failure on hemodialysis, acute coronary syndromes, or myocardial infarction and ischemic or nonischemic cardiomyopathies (Sato, Hayashi, Joki, & Fujimoto, 2017; Separham et al., 2018; Tanaka et al., 2017). In a study with 7,928 participants enrolled in the National Health and Nutrition Examination Survey (NHANES) III, Badheka et al. (2013) showed that the amplitude of T wave in lead aVR was a significant and independent predictor of cardiovascular adverse outcomes. In addition, adding this factor to Framingham risk score could improve model’s discriminator capability on intermediate‐risk subjects. Ayhan et al. (2013) examined 169 patients with anterior wall STEMI undergoing primary PCI and found that a positive T wave in lead aVR was strongly associated with increased in‐hospital cardiovascular mortality.
Although there are no studies concerning the prognostic role of positive T wave in lead aVR in PPCM patients, there are few studies in which T wave in lead aVR has been examined in patients with heart failure. In a recent study, Tanaka et al. (2017) investigated 93 ischemic and nonischemic patients with ICD and found a more positive T wave in lead aVR as an independent prognostic factor for risk stratification for cardiac events in heart failure patients. In another study, Shinozaki, Tamura, and Kadota (2011) studied 122 patients with anterior wall old MI who underwent diagnostic or follow‐up cardiac catheterization including left ventriculography, and found patients with upright T waves in lead aVR had lower LV ejection fractions, higher pulmonary arterial, pulmonary capillary wedge pressures, and greater LV end‐diastolic and end‐systolic volumes than those without it. In compatible with previous studies, in our study patients with a positive T wave in lead aVR had more severely reduced cardiac function, lower LV EF and greater LV end‐diastolic and end‐systolic dimensions. Thus, one may hypothesize that positive TaVR may be associated independently with pathological LV remodeling in PPCM.
The underlying mechanism for development of positive T wave in lead aVR in patients with PPCM is unclear. Lead aVR is the augmented unipolar right limb lead and may be considered as looking into the cavity of the heart and opposes the direction of the main cardiac vector. Under normal circumstances, all upright deflections on the ECG will be negative in this lead (Rautaharju et al., 2009a). When repolarization of injured myocardial cells is delayed compared with that of normal regions, the direction of the T‐wave vector alters toward the injured myocardial regions. Given the position of the aVR lead, the presence of injured myocardium in the apical, inferior, and lower lateral regions of the left ventricle would lead to a normally negative T wave inverted and manifested as a positive T wave in lead aVR (Rautaharju et al., 2009b). It has been assumed that any myocardial disease process that would cause T‐wave inversions in the inferolateral leads would be accompanied by a positive T wave in lead aVR (George, Arumugham, & Figueredo, 2010). However, according to our study results, only aVR revealed an independent and significant association with both primary and secondary endpoints in terms of logistic regression analysis. Therefore, we hypothesized that positive T waves in lead aVR were not just mirror images of negative T waves in inferolateral leads, but also a more sensitive marker of myocardial injury and presumably widespread pathological remodeling in cases of PPCM. These findings should encourage prospective outcome studies accompanied by imaging studies to explain the underlying pathophysiology.
We also studied other ECG findings; however, only positive T wave in lead aVR showed a strong and significant association with primary endpoint by multivariate logistic regression analysis even after adjustment for LV EF and LV EDD, which are well‐known but inconsistent traditional predictors of CV outcomes of PPCM patients. The Kaplan–Meier curves began to separate early and then continued to stay separate until the end of follow‐up for CV death, arrhythmic events, and persistent LV dysfunction. Our findings supported the findings of previous studies and extended the literatural knowledge about the association of positive T wave in lead aVR with adverse cardiac outcomes. Positive TaVR, as a simple, widely available, and unique ECG marker, seems to be a new predictor of worsening heart failure, arrhythmic events, and cardiovascular mortality in patients with PPCM. This parameter may be used to identify patients at high risk for adverse events and guiding selection for aggressive therapy in patients with PPCM.
5. LIMITATIONS
The present study should be interpreted with certain limitations. First, this was a retrospective single‐center study. Although a relatively large series of patients with PPCM were investigated, the study population was limited in size due to the paucity of PPCM. Prospectively designed studies on larger cohorts are necessary to validate our findings, to clarify the underlying mechanism, and to elucidate the prognostic utility of positive TaVR more accurately. Rather than a causal relation, we only demonstrated an association between upright T waves in lead aVR and adverse cardiovascular outcomes. Finally, we evaluated only initial presenting electrocardiograms, potential temporal changes in T wave were not examined in this study. It is probable that sequential measurements of T‐wave amplitudes may have altered the results, effecting predictive value of TaVR either positively or negatively in PPCM patients.
6. CONCLUSION
Our findings revealed that positive T wave in lead aVR is significantly and independently associated with persistent LV systolic dysfunction, arrhythmic events as well as cardiac death in PPCM patients. This unique ECG parameter in the often ignored lead gives additional prognostic information beyond what is available with other known conventional risk factors and allows the recognition of patients at high risk of adverse CV outcomes. We recommend that a special attention should be paid to T‐wave positivity in lead aVR whenever evaluating a woman with PPCM at initial evaluation given its high specificity and positive predictive value for predicting adverse cardiac events.
CONFLICT OF INTEREST
The author declares that he/she has no competing interests.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
This study was submitted to and approved by the Ethics Commission of Turkiye Yuksek Ihtisas Training and Research Hospital. This is a retrospective study, so the content to participate is not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets supporting the conclusions of the study are included in the article. Any additional data will be available on request.
ACKNOWLEDGMENTS
None.
Ekizler FA, Cay S, Kafes H, et al. The prognostic value of positive T wave in lead aVR: A novel marker of adverse cardiac outcomes in peripartum cardiomyopathy. Ann Noninvasive Electrocardiol. 2019;24:e12631 10.1111/anec.12631
REFERENCES
- Amos, A. M. , Jaber, W. A. , & Russell, S. D. (2006). Improved outcomes in peripartum cardiomyopathy with contemporary. American Heart Journal, 152(3), 509–513. 10.1016/j.ahj.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Anttila, I. , Nikus, K. , Nieminen, T. , Jula, A. , Salomaa, V. , Reunanen, A. , … Kahonen, M. (2011). Relation of positive T wave in lead aVR to risk of cardiovascular mortality. American Journal of Cardiology, 108(12), 1735–1740. 10.1016/j.amjcard.2011.07.042 [DOI] [PubMed] [Google Scholar]
- Ayhan, E. , Isik, T. , Uyarel, H. , Ergelen, M. , Cicek, G. , Ghannadian, B. , & Eren, M. (2013). Prognostic significance of T‐wave amplitude in lead aVR on the admission electrocardiography in patients with anterior wall ST‐elevation myocardial infarction treated by primary percutaneous intervention. Annals of Noninvasive Electrocardiology, 18(1), 51–57. 10.1111/j.1542-474X.2012.00530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badheka, A. O. , Patel, N. J. , Grover, P. M. , Shah, N. , Singh, V. , Deshmukh, A. , … Cohen, M. G. (2013). ST‐T wave abnormality in lead aVR and reclassification of cardiovascular risk (from the National Health and Nutrition Examination Survey‐III). American Journal of Cardiology, 112(6), 805–810. 10.1016/j.amjcard.2013.04.058 [DOI] [PubMed] [Google Scholar]
- Biteker, M. , Kayatas, K. , Duman, D. , Turkmen, M. , & Bozkurt, B. (2014). Peripartum cardiomyopathy: Current state of knowledge, new developments and future directions. Current Cardiology Reviews, 10(4), 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapa, J. B. , Heiberger, H. B. , Weinert, L. , Decara, J. , Lang, R. M. , & Hibbard, J. U. (2005). Prognostic value of echocardiography in peripartum cardiomyopathy. Obstetrics and Gynecology, 105(6), 1303–1308. 10.1097/01.AOG.0000161382.30233.ba [DOI] [PubMed] [Google Scholar]
- Dalzell, J. R. , Jackson, C. E. , & Gardner, R. S. (2011). An update on peripartum cardiomyopathy. Expert Review of Cardiovascular Therapy, 9(9), 1155–1160. 10.1586/erc.11.121 [DOI] [PubMed] [Google Scholar]
- Fett, J. D. , Christie, L. G. , Carraway, R. D. , & Murphy, J. G. (2005). Five‐year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clinic Proceedings, 80(12), 1602–1606. 10.4065/80.12.1602 [DOI] [PubMed] [Google Scholar]
- Fett, J. D. , & McTiernan, C. F. (2011). Towards a unifying hypothesis for the pathogenesis of peripartum cardiomyopathy. International Journal of Cardiology, 153(1), 1–3. 10.1016/j.ijcard.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Forster, O. , Hilfiker‐Kleiner, D. , Ansari, A. A. , Sundstrom, J. B. , Libhaber, E. , Tshani, W. , … Sliwa, K. (2008). Reversal of IFN‐gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. European Journal of Heart Failure, 10(9), 861–868. 10.1016/j.ejheart.2008.07.005 [DOI] [PubMed] [Google Scholar]
- George, A. , Arumugham, P. S. , & Figueredo, V. M. (2010). aVR ‐ The forgotten lead. Experimental and Clinical Cardiology, 15(2), e36–e44. [PMC free article] [PubMed] [Google Scholar]
- Goland, S. , McNamara, D. M. , Elkayam, U. , Alharethi, R. , Damp, J. , Hsich, E. , … IPAC Investigators (2015). Clinical outcomes for peripartum cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy‐Associated Cardiomyopathy). Journal of the American College of Cardiology, 66(8), 905–914. 10.1016/j.jacc.2015.06.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goland, S. , Modi, K. , Bitar, F. , Janmohamed, M. , Mirocha, J. M. , Czer, L. S. , … Elkayam, U. (2009). Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail, 15(8), 645–650. 10.1016/j.cardfail.2009.03.008 [DOI] [PubMed] [Google Scholar]
- Karaye, K. M. , & Henein, M. Y. (2013). Peripartum cardiomyopathy: A review article. International Journal of Cardiology, 164(1), 33–38. 10.1016/j.ijcard.2011.11.069 [DOI] [PubMed] [Google Scholar]
- Misumida, N. , Kobayashi, A. , Fox, J. T. , Hanon, S. , Schweitzer, P. , & Kanei, Y. (2016). Predictive value of ST‐segment elevation in lead aVR for left main and/or three‐vessel disease in non‐ST‐segment elevation myocardial infarction. Annals of Noninvasive Electrocardiology, 21(1), 91–97. 10.1111/anec.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto, O. , Matsuda, M. , Nakamoto, K. , Nishiyama, H. , Kuraoka, K. , Taniyama, K. , … Kawamoto, T. (2012). Peripartum cardiomyopathy presenting with syncope due to Torsades de pointes: A case of long QT syndrome with a novel KCNH2 mutation. Internal Medicine, 51(5), 461–464. 10.2169/internalmedicine.51.5943 [DOI] [PubMed] [Google Scholar]
- Okuda, K. , Watanabe, E. , Sano, K. , Arakawa, T. , Yamamoto, M. , Sobue, Y. , … Ozaki, Y. (2011). Prognostic significance of T‐wave amplitude in lead aVR in heart failure patients with narrow QRS complexes. Annals of Noninvasive Electrocardiology, 16(3), 250–257. 10.1111/j.1542-474X.2011.00439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautaharju, P. M. , Surawicz, B. , Gettes, L. S. , Bailey, J. J. , Childers, R. , Deal, B. J. , … Heart Rhythm Soceity (2009a). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology, 53(11), 982–991. 10.1016/j.jacc.2008.12.014 [DOI] [PubMed] [Google Scholar]
- Rautaharju, P. M. , Surawicz, B. , Gettes, L. S. , Bailey, J. J. , Childers, R. , Deal, B. J. , … Heart Rhythm Soceity (2009b). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation, 119(10), e241–250. 10.1161/CIRCULATIONAHA.108.191096 [DOI] [PubMed] [Google Scholar]
- Sato, Y. , Hayashi, T. , Joki, N. , & Fujimoto, S. (2017). Association of lead aVR T‐wave amplitude with cardiovascular events or mortality among prevalent dialysis patients. Therapeutic Apheresis and Dialysis, 21(3), 287–294. 10.1111/1744-9987.12512 [DOI] [PubMed] [Google Scholar]
- Separham, A. , Sohrabi, B. , Tajlil, A. , Pourafkari, L. , Sadeghi, R. , Ghaffari, S. , & Nader, N. D. (2018). Prognostic value of positive T wave in lead aVR in patients with non‐ST segment myocardial infarction. Annals of Noninvasive Electrocardiology, 23(5), e12554 10.1111/anec.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki, K. , Tamura, A. , & Kadota, J. (2011). Associations of positive T wave in lead aVR with hemodynamic, coronary, and left ventricular angiographic findings in anterior wall old myocardial infarction. Journal of Cardiology, 57(2), 160–164. 10.1016/j.jjcc.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Sliwa, K. , Hilfiker‐Kleiner, D. , Petrie, M. C. , Mebazaa, A. , Pieske, B. , Buchmann, E. , … Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy (2010). Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. European Journal of Heart Failure, 12(8), 767–778. 10.1093/eurjhf/hfq120 [DOI] [PubMed] [Google Scholar]
- Tan, S. Y. , Engel, G. , Myers, J. , Sandri, M. , & Froelicher, V. F. (2008). The prognostic value of T wave amplitude in lead aVR in males. Annals of Noninvasive Electrocardiology, 13(2), 113–119. 10.1111/j.1542-474X.2008.00210.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, Y. , Konno, T. , Tamura, Y. , Tsuda, T. , Furusho, H. , Takamura, M. , … Hayashi, K. (2017). Impact of T wave amplitude in lead aVR on predicting cardiac events in ischemic and nonischemic cardiomyopathy patients with an implantable cardioverter defibrillator. Annals of Noninvasive Electrocardiology, 22(6), 10.1111/anec.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibazarwa, K. , Lee, G. , Mayosi, B. , Carrington, M. , Stewart, S. , & Sliwa, K. (2012). The 12‐lead ECG in peripartum cardiomyopathy. Cardiovascular Journal of Africa, 23(6), 322–329. 10.5830/CVJA-2012-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe, K. , Tamura, A. , Kawano, Y. , Shinozaki, K. , Kotoku, M. , & Kadota, J. (2012). Upright T waves in lead aVR are associated with cardiac death or hospitalization for heart failure in patients with a prior myocardial infarction. Heart and Vessels, 27(6), 548–552. 10.1007/s00380-011-0193-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of the study are included in the article. Any additional data will be available on request.


