Abstract
Background
Although the harmful effect of tobacco exposure on cardiovascular disease (CVD) and its risk factors are well established, the constituents of cigarette‐smoke and the pathophysiological mechanism involved are unknown. Recently, deep terminal negativity of P wave in V1 (DTNPV1) has emerged as a marker of left atrial abnormality that predicts atrial fibrillation, stroke, and death due to all‐cause or CVD. Therefore, we examined the association between serum cotinine levels with abnormal DTNPV1 using the Third National Health and Nutrition Examination Survey.
Methods
This analysis included 4,507 participants (mean age 58 ± 13 years, 53% women, 49% non‐Hispanic white) of NHANES III, without history of CVD or major electrocardiographic abnormalities and not on heart rate modifying medications. Multivariable logistic regression analysis was used to examine the association between serum cotinine and abnormal DTNPV1—defined from automatically processed electrocardiograms as values of the amplitude of the terminal negative phase of P wave in lead V1 exceeding 100 μV.
Results
Abnormal DTNPV1 was detected in 2.3% (n = 105) of the participants. In a model adjusted for demographics and CVD risk factors, each 10 ng/ml serum cotinine was associated with 2% increased odds of abnormal DTNPV1 (odds ratio 1.02, 95% confidence interval 1.01–1.03, p‐value < 0.001). This association was consistent in subgroups stratified by age, sex, race, smoking status, hypertension, diabetes, dyslipidemia, and chronic obstructive pulmonary disease.
Conclusion
Elevated serum cotinine levels are associated with an abnormal DTNPV1. This suggests that nicotine exposure can lead to left atrial abnormalities, a possible mechanism for increased risk of CVD.
Keywords: cotinine, left atrial abnormality, P‐wave deep terminal negativity V1, smoking
1. INTRODUCTION
Electrocardiographic (ECG) markers of left atrial abnormalities represent atrial remodeling. These markers have proved to be useful in improving prediction of cardiovascular disease (CVD), and in enhancing our understanding of the pathophysiological mechanisms linking atrial disease to CVD [1–4]. Deep terminal negativity of P wave in V1 (DTNPV1), an electrocardiographic marker of left atrial abnormality, is easily computed on the routine ECG and defined as the depth of the downward deflection (terminal portion) of the P wave in lead V1 (Hancock et al., 2009). DTNPV1 has been associated with left atrial fibrosis, dilation, and elevating filling pressure (Kasser & Kennedy, 1969; Tiffany Win et al., 2015). It also has been predictive of increased risk for development of incident ischemic stroke (Kohsaka et al., 2005; Soliman, Prineas, Case, Zhang, & Goff, 2009) and atrial fibrillation (A‐Fib) (Kamel et al., 2014). Several studies suggest that DTNPV1 signals left atrial pathophysiological processes that form a substrate for thromboembolism via pathways other than the dysrhythmia that characterizes A‐Fib (Kamel et al., 2014; Kamel, Bartz, et al., 2015; Kamel, Okin, Longstreth, Elkind, & Soliman, 2015).
Although cigarette smoking has been suggested to promote the occurrence of atrial arrhythmias, studies have not examined the influence of nicotine exposure on left atrial electrophysiology. Understanding the mechanisms by which tobacco exposure impacts left atrial abnormalities could enhance our understanding of the relationship between smoking and atrial cardiac arrhythmias and conduction defects. This may help explaining the inconsistent reports on the association between smoking and A‐Fib (Chamberlain et al., 2011; Heeringa, Kors, Hofman, van Rooij, & Witteman, 2008; Knuiman et al., 2014; Krahn, Manfreda, Tate, Mathewson, & Cuddy, 1995; Psaty et al., 1997; Suzuki et al., 2015).
Serum cotinine is a more accurate highly sensitive and specific biomarker for tobacco exposure than self‐reported smoking status (Caraballo, Giovino, Pechacek, & Mowery, 2001). The availability serum cotinine levels in the Third National Health and Nutrition Examination (NHANES‐III) Survey as well as digital ECG data provides a unique opportunity to examine the association between nicotine exposure as measured by serum cotinine levels with left atrial abnormalities as measured by DTNPV1, in a large racially diverse human population.
2. METHODS
The NHANES, a periodic survey of a representative sample of the civilian un‐institutionalized United States population, aims to provide estimates of disease prevalence and the overall health status of the population. All participants gave a written informed consent at the time of the survey. Baseline data were collected during an in‐home interview and a subsequent visit to a mobile examination center in 1988–1994. Data collected during the in‐home interview included demographic and medication information. Blood samples were obtained at mobile centers, and basic laboratory values were recorded for each participant, including serum cotinine, total cholesterol, high‐density lipoprotein cholesterol, and plasma glucose. Diabetes was defined as a fasting plasma glucose level of ≥126 mg/dl, glycosylated hemoglobin A1C values ≥6.5, or a history of glucose‐lowering medications. Hypertension was defined as systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or use of blood pressure‐lowering medications. Body mass index was computed as the weight in kilogram divided by the square of the height in meter, and obesity was defined as a body mass index of >30 kg/m2. Age, gender, race/ethnicity, and smoking status were self‐reported. Chronic Obstructive Pulmonary Disease (COPD) was defined as patients with a combination of asthma and emphysema.
The ascertainment of electrocardiographic DTNPV1 in NHANES III has been described before (Tereshchenko, Shah, Li, & Soliman, 2014). Briefly, standard 12‐lead ECGs were recorded using a Marquette MAC 12 system (Marquette Medical Systems, Milwaukee, Wisconsin) by trained technicians during a visit to a mobile examination center. Computerized automated analysis of the electrocardiographic data was performed with visual inspection of outlier values by a trained technician in a central ECG core laboratory (EPICARE Center at the Wake Forest School of Medicine, Winston Salem, North Carolina). Amplitude of the terminal negative phase of P wave in lead V1 was measured automatically. We defined the presence of abnormal DTNPV1 if the amplitude of the terminal negative phase of P wave in lead V1 exceeded 100 μV. For the reference, 100 μV = 0.1 mV = 1 mm (one small box).
For the purpose of this analysis, we only included NHANES III participants who underwent ECG recording. We excluded participants with a history of CVD (coronary heart disease, heart failure, or stroke), major ECG abnormalities including electrocardiographic evidence of myocardial infarction or ischemia as defined by Minnesota Electrocardiogram Classification, those on anti‐arrhythmic drugs including beta blockers, calcium channel blockers, or those with missing cotinine level data.
2.1. Statistical analysis
Categorical variables were reported as frequency and percentage, whereas continuous variables were recorded as mean ± standard deviation. Statistical significance for continuous variables was tested using t test, whereas chi‐square was used for categorical variables. Logistic regression was used to calculate the odds ratios and 95% confidence intervals for the association between serum cotinine levels and DTNPV1. Serum cotinine levels were included in the models as a continuous variable (i.e., per 10 ng/ml increase). Multivariable adjusted model was constructed to adjust for age, gender, race/ethnicity, heart rate, obesity, diabetes, hypertension, dyslipidemia, and COPD. As there were important significant differences in the baseline characteristics between the normal and abnormal DTNPV1 group, we also performed propensity score matching analysis to compare with a more precise control group. We conducted subgroup analyses stratified by age (cutoff point by median—56 years), gender, race/ethnicity (whites and non‐white), smoking status (never, current, and former), hypertension, diabetes, dyslipidemia, and COPD. Moreover, we used receiver operating characteristic (ROC) curve to find the optimal cutoff point of predicted abnormal DTNPV1 by maximizing the Youden Index. We tested for interactions between our main effect variable and the subgroups using models adjusted for variables similar to those included in model 2. A two‐sided p value of <0.05 was considered significant for main effects and for interactions. Data were analyzed using Statistical Package for Social Sciences (SPSS) software (version 24, SPSS, Inc, Chicago, Illinois).
3. RESULTS
A total of 4,507 study participants (mean age 58 ± 13 years, 53% women, 49% non‐Hispanic white) were included in this analysis. A total of 105 (2.3%) participants had abnormal DTNPV1 at baseline. Study participants with abnormal (compared to normal) DTNPV1 were more likely to be older in age, current smokers, with higher prevalence of hypertension, higher resting heart rate, and higher mean cotinine levels. (Table 1).
Table 1.
Participants characteristics (Total N = 4,507)
| Characteristic | P‐wave deep terminal negativity V1 | ||
|---|---|---|---|
| Normal (n = 4,402) | Abnormal (n = 105) | p‐Value | |
| Age (years) | 57 ± 13 | 66 ± 11 | <0.001 |
| Women | 2,325 (52.8%) | 64 (61.0%) | 0.099 |
| Non‐Hispanic white | 2,167 (49.2%) | 52 (49.5%) | 0.952 |
| Smoking status | |||
| Never | 1962 (44.6%) | 37 (35.2%) | 0.06 |
| Current | 1,061 (24.1%) | 35 (33.3%) | |
| Past | 1,379 (31.3%) | 33 (31.4%) | |
| Diabetes mellitus | 406 (9.2%) | 10 (9.5%) | 0.916 |
| Hypertension | 1,097 (24.9%) | 46 (43.8%) | <0.001 |
| Systolic blood pressure (mm Hg) | 130 ± 18 | 138 ± 20 | <0.001 |
| Diastolic blood pressure (mm Hg) | 76 ± 10 | 77 ± 10 | 0.402 |
| Dyslipidemia | 955 (21.7%) | 18 (17.1%) | 0.263 |
| Obesity | 814 (18.5%) | 22 (21.0%) | 0.521 |
| Body mass index (kg/m2) | 27.4 ± 5.3 | 26.6 ± 5.7 | 0.174 |
| Chronic obstructive pulmonary disease | 116 (2.6%) | 5 (4.8%) | 0.183 |
| Heart rate (beats/minute) | 69 ± 11 | 72 ± 12 | 0.021 |
| Serum cotinine (ng/ml) | 70.5 ± 142.1 | 130.4 ± 222.7 | 0.005 |
Abnormal P‐wave deep terminal negativity V1 was defined as lesser than 100 μV.
Chronic Obstructive Pulmonary Disease is a combination of asthma and emphysema.
Obesity was defined as a body mass index of >30 kg/m2.
Participants with abnormal DTNPV1 had higher serum cotinine levels, p = 0.005 (Figure 1). In multivariable logistic regression model adjusted for demographics, each 10 ng/ml serum cotinine was associated with 2% increased odds of abnormal DTNPV1 (p‐value < 0.001). The propensity score adjusted OR was also similar; 1.02 (1.01, 1.03). This association was not attenuated after further adjustment for CVD risk factors (Table 2) and was consistent in subgroups stratified by age, sex, race, smoking status, hypertension, diabetes, dyslipidemia, and chronic obstructive pulmonary disease (Figure 2). Using a ROC curve analysis, a cut point for prediction of abnormal DTNPV1 was defined as a value ≥33 ng/ml.
Figure 1.
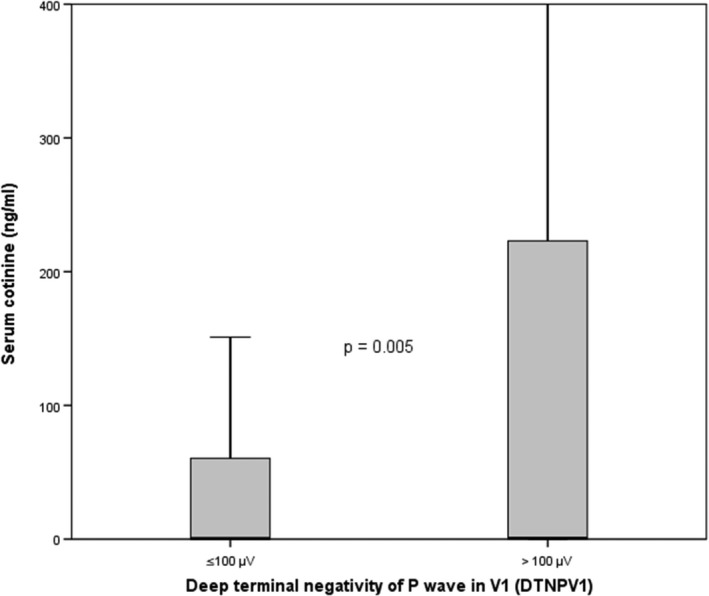
Box plot distribution of cotinine levels across normal and abnormal deep terminal negativity groups
Table 2.
Association between serum cotinine levels and abnormal P‐wave deep terminal negativity in V1
| Odds ratio (95% CI) | p‐Value | |
|---|---|---|
| Per 10 ng/ml cotininea | 1.02 (1.01, 1.03) | <0.001 |
Adjusted for age, sex, race, and heart rate, obesity, diabetes, hypertension dyslipidemia, and chronic obstructive pulmonary disease.
Figure 2.
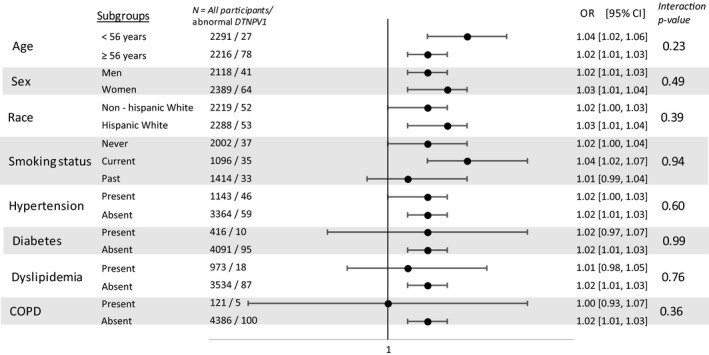
Association between by cotinine levels and abnormal deep terminal negativity in subgroups. COPD: Chronic obstructive pulmonary disease; DTNPV1: deep terminal negativity. Model adjusted for age, sex, race, heart rate, obesity, diabetes, hypertension, dyslipidemia, and chronic obstructive pulmonary disease
4. DISCUSSION
Abnormal DTNPV1 is an easy‐to‐measure electrocardiographic marker of left atrial abnormality (Alpert & Munuswamy, 1989; Kasser & Kennedy, 1969; Tiffany Win et al., 2015) that has been linked to increased risk of poor CVD outcomes (Kamel et al., 2014; Kohsaka et al., 2005; Soliman et al., 2009). DTNPV1 is considered as an intermediate outcome that falls between cardiovascular risk factors and serious cardiac events. Therefore, detection of abnormal DTNPV1 provides an opportunity to evaluate the subclinical left atrial remodeling from cigarette smoking and the possible mechanism for increased cardiac events. In this analysis from the NHANES III survey, we showed that higher levels of serum cotinine levels, a measure of tobacco exposure, were associated with abnormal DTNPV1 among subjects without CVD. There was no evidence of effect modification by demographics or risk factors including smoking status. These findings underscore the harmful effect of tobacco exposure on the left atrium and provide a possible mechanism by which smoking leads to increased risk of atrial‐related CVD such as AF and stroke.
The pathophysiological mechanism involved in the cigarette smoking‐induced cardiac arrhythmia is complicated, as the tobacco smoke consists of a mixture of more than 4,000 chemicals. Several studies have suggested that nicotine, carbon monoxoide, and hydrocarbons are the main components of tobacco smoke that exert the arrhythmogenic potential of smoking (D'Alessandro, Boeckelmann, Hammwhoner, & Goette, 2012). Nicotine, in particular, due to its sympathomimetic effect on the cardiac autonomic function and oxidative stress (Haass & Kubler, 1997), has been implicated to development of atrial fibrosis leading to arrhythmias (Goette, 2009; Jensen et al., 2012). In addition, nicotine is a potent inhibitor of cardiac potassium channels, which may contribute nicotine induced arrhythmias (Wang, Shi, et al., 2000; Wang, Yang, Zhang, Xu, & Wang, 2000). However to date, no previous large‐scale study investigated the effect of nicotine and cigarette smoking exposure on left atrium remodeling and a possible mechanism for cardiac arrhythmias. Our finding of the significant association between serum cotinine and abnormal DTNPV1 not only explains but also provides further supports the arrhythmogenic nature of tobacco exposure and its deleterious effect on the atrium.
Interestingly, we found no effect modification by smoking status, that is, the association between serum cotinine and abnormal DTNPV1 was not significantly different between smokers and non‐smokers This suggests that nicotine exposure from other sources, such as second‐ and third‐hand smoking or newer nicotine delivery devices (typically not considered as “smoking”) are also at risk for left atrial remodeling. This could provide possible pathophysiological link of second‐hand smoking and increased risk of A‐Fib (Dixit et al., 2016) and stroke (Malek, Cushman, Lackland, Howard, & McClure, 2015).
4.1. Limitations
Serial measurements of serum cotinine and ECG may better reflect tobacco exposure and left atrial abnormalities than a one‐time measurement. Although we have adjusted for several potential confounders, we recognize the possibility of residual confounding that is similar to other studies with a cross‐sectional design. The possible association of cotinine and long‐term outcomes, such as arrhythmias and stroke, or even atrial fibrotic markers needs to be tested in other database as NHANES does not collect that information in its follow‐up period. Our analyses were limited due to the small number of participants with abnormal DTNPV1 and also possibly lacked statistical power to detect differences between certain subgroups. Also, we relied on automated measurement of DTNPV1 that is not routinely reported by current ECG systems. However, it can easily be measured manually (Magnani et al., 2010; Soliman, Juma, & Nkosi, 2010). Despite these limitations, this is the first study examining the association between serum cotinine, an objective measure of tobacco exposure, and DTNPV1.
5. CONCLUSIONS
We found in this large racially diverse sample of the U.S. population, that elevated serum cotinine levels are associated with DTNPV1; an ECG marker of left atrial abnormality and a strong predictor of CVD such as AF, stroke, and death. This suggests chronic nicotine exposure leads to left atrial abnormality which serves possibly as a substrate for increased cardiovascular events. Further research is needed to understand the preventive and therapeutic implications of these findings.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
None.
Irfan A, Li Y, Bhatnagar A, Soliman EZ. Association between serum cotinine levels and electrocardiographic left atrial abnormality. Ann Noninvasive Electrocardiol. 2019;24:e12586 10.1111/anec.12586
REFERENCES
- Alpert, M. A. , & Munuswamy, K. (1989). Electrocardiographic diagnosis of left atrial enlargement. Archives of Internal Medicine, 149(5), 1161–1165. 10.1001/archinte.1989.00390050119024 [DOI] [PubMed] [Google Scholar]
- Caraballo, R. S. , Giovino, G. A. , Pechacek, T. F. , & Mowery, P. D. (2001). Factors associated with discrepancies between self‐reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. American Journal of Epidemiology, 153(8), 807–814. 10.1093/aje/153.8.807 [DOI] [PubMed] [Google Scholar]
- Chamberlain, A. M. , Agarwal, S. K. , Folsom, A. R. , Duval, S. , Soliman, E. Z. , Ambrose, M. , … Alonso, A. (2011). Smoking and incidence of atrial fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) study. Heart Rhythm: the Official Journal of the Heart Rhythm SocietyThe Official Journal of the Heart Rhythm Society, 8(8), 1160–1166. 10.1016/j.hrthm.2011.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro, A. , Boeckelmann, I. , Hammwhoner, M. , & Goette, A. (2012). Nicotine, cigarette smoking and cardiac arrhythmia: An overview. European Journal of Preventive Cardiology, 19(3), 297–305. 10.1177/1741826711411738 [DOI] [PubMed] [Google Scholar]
- Dixit, S. , Pletcher, M. J. , Vittinghoff, E. , Imburgia, K. , Maguire, C. , Whitman, I. R. , … Marcus, G. M. (2016). Secondhand smoke and atrial fibrillation: Data from the Health eHeart Study. Heart Rhythm: the Official Journal of the Heart Rhythm SocietyThe Official Journal of the Heart Rhythm Society, 13(1), 3–9. 10.1016/j.hrthm.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goette, A. (2009). Nicotine, atrial fibrosis, and atrial fibrillation: Do microRNAs help to clear the smoke? Cardiovascular Research, 83(3), 421–422. 10.1093/cvr/cvp188 [DOI] [PubMed] [Google Scholar]
- Haass, M. , & Kubler, W. (1997). Nicotine and sympathetic neurotransmission. Cardiovascular Drugs and Therapy, 10(6), 657–665. 10.1007/BF00053022 [DOI] [PubMed] [Google Scholar]
- Hancock, E. W. , Deal, B. J. , Mirvis, D. M. , Okin, P. , Kligfield, P. , Gettes, L. S. , … Heart Rhythm, S. (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology, 53(11), 992–1002. 10.1016/j.jacc.2008.12.015 [DOI] [PubMed] [Google Scholar]
- Heeringa, J. , Kors, J. A. , Hofman, A. , van Rooij, F. J. , & Witteman, J. C. (2008). Cigarette smoking and risk of atrial fibrillation: The Rotterdam Study. American Heart Journal, 156(6), 1163–1169. 10.1016/j.ahj.2008.08.003 [DOI] [PubMed] [Google Scholar]
- Jensen, K. , Nizamutdinov, D. , Guerrier, M. , Afroze, S. , Dostal, D. , & Glaser, S. (2012). General mechanisms of nicotine‐induced fibrogenesis. The FASEB Journal, 26(12), 4778–4787. 10.1096/fj.12-206458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel, H. , Bartz, T. M. , Longstreth, W. T. Jr , Okin, P. M. , Thacker, E. L. , Patton, K. K. , … Soliman, E. Z. (2015). Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke, 46(3), 711–716. 10.1161/STROKEAHA.114.007762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel, H. , Okin, P. M. , Longstreth, W. T. Jr , Elkind, M. S. , & Soliman, E. Z. (2015). Atrial cardiopathy: A broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiology, 11(3), 323–331. 10.2217/fca.15.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel, H. , Soliman, E. Z. , Heckbert, S. R. , Kronmal, R. A. , Longstreth, W. T. Jr , Nazarian, S. , & Okin, P. M. (2014). P‐wave morphology and the risk of incident ischemic stroke in the Multi‐Ethnic Study of Atherosclerosis. Stroke, 45(9), 2786–2788. 10.1161/STROKEAHA.114.006364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasser, I. , & Kennedy, J. W. (1969). The relationship of increased left atrial volume and pressure to abnormal P waves on the electrocardiogram. Circulation, 39(3), 339–343. 10.1161/01.CIR.39.3.339 [DOI] [PubMed] [Google Scholar]
- Knuiman, M. , Briffa, T. , Divitini, M. , Chew, D. , Eikelboom, J. , McQuillan, B. , & Hung, J. (2014). A cohort study examination of established and emerging risk factors for atrial fibrillation: The Busselton Health Study. European Journal of Epidemiology, 29(3), 181–190. 10.1007/s10654-013-9875-y [DOI] [PubMed] [Google Scholar]
- Kohsaka, S. , Sciacca, R. R. , Sugioka, K. , Sacco, R. L. , Homma, S. , & Di Tullio, M. R. (2005). Electrocardiographic left atrial abnormalities and risk of ischemic stroke. Stroke, 36(11), 2481–2483. 10.1161/01.STR.0000185682.09981.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn, A. D. , Manfreda, J. , Tate, R. B. , Mathewson, F. A. , & Cuddy, T. E. (1995). The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. American Journal of Medicine, 98(5), 476–484. 10.1016/S0002-9343(99)80348-9 [DOI] [PubMed] [Google Scholar]
- Magnani, J. W. , Mazzini, M. J. , Sullivan, L. M. , Williamson, M. , Ellinor, P. T. , & Benjamin, E. J. (2010). P‐wave indices, distribution and quality control assessment (from the Framingham Heart Study). Annals of Noninvasive Electrocardiology, 15(1), 77–84. 10.1111/j.1542-474X.2009.00343.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek, A. M. , Cushman, M. , Lackland, D. T. , Howard, G. , & McClure, L. A. (2015). Secondhand smoke exposure and stroke: The reasons for geographic and racial differences in stroke (REGARDS) Study. American Journal of Preventive Medicine, 49(6), e89–e97. 10.1016/j.amepre.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaty, B. M. , Manolio, T. A. , Kuller, L. H. , Kronmal, R. A. , Cushman, M. , Fried, L. P. , … Rautaharju, P. M. (1997). Incidence of and risk factors for atrial fibrillation in older adults. Circulation, 96(7), 2455–2461. 10.1161/01.CIR.96.7.2455 [DOI] [PubMed] [Google Scholar]
- Soliman, E. Z. , Juma, H. , & Nkosi, N. (2010). A simple electrocardiogram marker for risk stratification of ischemic stroke in low‐resources settings. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 19(5), 388–392. 10.1016/j.jstrokecerebrovasdis.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Soliman, E. Z. , Prineas, R. J. , Case, L. D. , Zhang, Z. M. , & Goff, D. C. Jr (2009). Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke, 40(4), 1204–1211. 10.1161/STROKEAHA.108.534735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, S. , Otsuka, T. , Sagara, K. , Kano, H. , Matsuno, S. , Takai, H. , … Yamashita, T. (2015). Association between smoking habits and the first‐time appearance of atrial fibrillation in Japanese patients: Evidence from the Shinken Database. Journal of Cardiology, 66(1), 73–79. 10.1016/j.jjcc.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Tereshchenko, L. G. , Shah, A. J. , Li, Y. , & Soliman, E. Z. (2014). Electrocardiographic deep terminal negativity of the P wave in V1 and risk of mortality: The National Health and Nutrition Examination Survey III. Journal of Cardiovascular Electrophysiology, 25(11), 1242–1248. 10.1111/jce.12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany Win, T. , Ambale Venkatesh, B. , Volpe, G. J. , Mewton, N. , Rizzi, P. , Sharma, R. K. , … Tereshchenko, L. G. (2015). Associations of electrocardiographic P‐wave characteristics with left atrial function, and diffuse left ventricular fibrosis defined by cardiac magnetic resonance: The PRIMERI Study. Heart Rhythm: the Official Journal of the Heart Rhythm SocietyThe Official Journal of the Heart Rhythm Society, 12(1), 155–162. 10.1016/j.hrthm.2014.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Shi, H. , Zhang, L. , Pourrier, M. , Yang, B. , Nattel, S. , & Wang, Z. (2000). Nicotine is a potent blocker of the cardiac A‐type K(+) channels. Effects on cloned Kv4.3 channels and native transient outward current. Circulation, 102(10), 1165–1171. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Yang, B. , Zhang, L. , Xu, D. , & Wang, Z. (2000). Direct block of inward rectifier potassium channels by nicotine. Toxicology and Applied Pharmacology, 164(1), 97–101. 10.1006/taap.2000.8896 [DOI] [PubMed] [Google Scholar]


