Abstract
Background
Noninvasive fetal electrocardiography (fECG), obtained positioning electrodes on the maternal abdomen, is important in safeguarding the life and the health of the unborn child. This study aims to provide a review of the state of the art of fECG, and includes a description of the parameters useful for fetus clinical evaluation; of the fECG recording procedures; and of the techniques to extract the fECG signal from the abdominal recordings.
Methods
The fetus clinical status is inferred by analyzing growth parameters, supraventricular arrhythmias, ST‐segment variability, and fetal‐movement parameters from the fECG signal. This can be extracted from an abdominal recording obtained using one of the following two electrode‐types configurations: pure‐abdominal and mixed. Differently from the former, the latter also provides pure maternal ECG tracings. From a mathematical point of view, the abdominal recording is a summation of three signal components: the fECG signal (i.e., the signal of interest to be extracted), the abdominal maternal ECG (amECG), and the noise. Automatic extraction of fECG includes noise removal by abdominal signal prefiltration (0.5–45 Hz bandpass filter) and amECG cancellation.
Conclusions
Differences among methods rely on different techniques used to extract fECG. If pure abdominal electrode configurations are used, fECG is extracted directly from the abdominal recording using independent component analysis or template subtraction. Eventually, if mixed electrode configurations are used, the fECG can be extracted using the adaptive filtering fed with the maternal ECG recorded by the electrodes located in the woman thorax or shoulder.
Keywords: noninvasive fetal ECG, abdominal ECG, fetal monitoring
The assessment of procedures for accurately monitoring the fetal cardiac activity has occupied a prominent role in the biomedical research for centuries because of its importance in safeguarding the life and the health of the unborn child. Indeed, congenital fetal heart defects are among the most common birth defects and the leading cause of birth deaths.1 Moreover, several pathologies and complications, even not directly linked to the fetus heart, such as fetal hypoxia (a deprivation of an adequate supply oxygen that, if prolonged, can lead to irreversible neurological diseases2), show abnormalities in the cardiac activity as side effect. Premature diagnosis of fetal cardiac defects and abnormalities during pregnancy may allow the treatment of the pathologies in the early stages of the fetus development and may prevent a permanent disease or, in the worst cases, a fatal outcome.3
Cardiotocography (also known as electronic fetal monitoring) consists of two simultaneous recordings performed by two separate transducers, an ultrasonic sensor that continuously emits ultrasound and detects motion of the fetal heart by the characteristic of the reflected sound, and a pressure transducer that provides information on the maternal uterine contractions. Although the transducers can be either external or internal, cardiotocography is usually performed in a noninvasive fashion, with the two sensors strapped to the mother abdominal wall. For what concerns fetal cardiac monitoring, cardiotocography4 has a high sensitivity for the detection of the early signs of fetal hypoxia. When introduced, this practice (which became almost universal for hospital births) was expected to reduce the incidence of fetal demise in labor and make for a reduction in cerebral palsy. Still, in recent years there has been some controversy as to the utility of the cardiotocography in low‐risk pregnancies, and the related belief that overreliance on the test has led to increased misdiagnoses of fetal distress and increased (and possibly unnecessary) cesarean deliveries.5
In the presence of risk factors in labor, when the chorioamniotic membranes is ruptured and there is a sufficient dilatation of the uterine cervix to allow the insertion of an electrode on the fetal scalp, cardiotocography has sometimes been combined with direct fetal electrocardiography (fECG)6 (Fig. 1, upper panel) to improve fetal hypoxia identification. However, its invasivity‐related limitations and the need of having a technique usable also during pregnancy have led to the introduction of noninvasive or indirect fECG,7 which consists in the recording of the electrical activity of the fetal heart by means of electrodes positioned on the maternal abdomen (Fig. 1, lower panel). The feature of noninvasiveness makes indirect fECG, a potentially promising method in the field of prenatal diagnostics. The signal‐to‐noise ratio (SNR) of these recordings is quite low so that their processing and interpretation are very challenging tasks. Consequently, despite the noninvasive fECG has been studied for over 40 years,8 such technique has not reached sufficient reliability to be used in daily clinical practice and is still a current research topic among physicians and biomedical engineers.
Figure 1.

Simultaneous direct (scalp) fECG recording (upper panel) and abdominal recording (lower panel), showing both maternal and fetal ECG components.
Aim of this study was to provide a review of noninvasive fECG, which includes information about genesis and characterization of the fECG signal from a physiologic point of view; technical aspects of the noninvasive fECG recording procedures, including skin‐electrode types and configurations proposed for maximizing the SNR; and signal processing algorithms specifically designed for noninvasive fECG extraction from the maternal abdominal recordings. The future of the noninvasive fECG will also be discussed. The PRISMA Flow Diagram (http://www.prisma-statement.org/2.1.4-PRISMAFlow2009Diagram.pdf) indicating the process that had let to the selection of the publications used in this study is displayed in Figure 2.
Figure 2.

PRISMA flow diagram indicating the process that had let to publications selection.
THE FETAL ECG SIGNAL
The Fetal ECG Morphology
The fetal heart is among the first organs that develop in the fetus and, after 7 weeks of gestation, is characterized by an anatomic conformation similar to that of an adult heart (four cameras, two atria and two ventricles).8 Consequently, from a morphological point of view, fetuses and adults have rather similar ECG signals containing the same basic waves: the P wave, associated to atrial depolarization; the QRS complex, associated to ventricular depolarization; and the T wave, associated to ventricular repolarization. Still, the mechanical function of the fetal heart differs from that of the adult heart because of some structural differences required by a different blood circulation in the prenatal period. It is well known that, after birth, the left ventricle pumps blood into the body for delivering oxygen whereas the right ventricle pumps blood into the lungs for acquiring oxygen. In the fetus, the oxygen is supplied by the placenta9 and therefore blood is not pumped into the lungs for this purpose. Both ventricles pump blood throughout the body (including the lungs). Particularly, the left ventricle supplies blood to the heart itself and to brain, whereas the right one to all the inferior parts of the body. The cardiac output of the right ventricle is greater than that of the left ventricle and this yields an abundance of cardiac muscle in the right part of the fetal heart. Thus, in the fetus the cardiac electrical axis points toward the right ventricle, whereas in the adult it points toward the left ventricle (being the ventricle with the largest mass). Consequently, the fetal vectocardiogram (VCG; i.e., the vector that indicates the magnitude and direction of the electrical forces generated by the heart during one complete cycle9) is oriented differently from the adult VCG, and each fetal ECG representation—being the projection of the fetal VCG onto the appropriate lead vector—differs from the corresponding adult ECG representation.9
Clinical Information from Fetal ECG
Several clinical evaluations, not necessarily directly related to the fetal heart, can be derived from the analysis of the fECG signal. Below the most common ones are reported.
Fetal Growth Parameters
Intrauterine growth restriction (IUGR) refers to a poor baby growth while in the mother's womb during pregnancy, and represents a condition of risk for hypoxia. Because fECG provides information about fetal growth rate and oxygenation, it can also be used for IUGR assessment.10 In the fECG signal, the P‐wave duration and the QRS‐complex duration indicate the time needed for atrial and ventricular depolarization, respectively. Such intervals are determined by both the size of the cardiac muscle and the conduction speed of the action potentials. Hypothesizing the latter to be constant, each wave has a duration that depends on the dimension of the related cardiac rooms. As the heart grows proportionally to the fetus, both the P‐wave duration and the QRS‐complex duration can be used to estimate the size of the fetal heart9 and, consequently, to assess the presence of IUGR.
Supraventricular Arrhythmias
In the fECG signal, the occurrence of supraventricular extrasystoles (SVES), which manifest as widened QRS complexes of opposite sign with absent P waves, are usually sporadic and innocent.9 However, in the cases in which the SVES are due to congenital heart diseases (like supraventricular tachycardia, bradycardia, or premature atrial contractions11, 12), the fECG is of vital importance since permits timely detection of the congenital fetal heart disease and its treatment during pregnancy or immediately after birth.
ST Segment Variability
The energy‐production and energy‐consumption balance controls the capability of the fetal heart to distribute blood to the body. Normally, the availability of oxygen exceeds its request, and the fetal heart utilizes aerobic (i.e., oxygen dependent) metabolism to generate energy. In this case, the energetic balance is positive and the fECG morphology is normal. On the contrary, if the available amount of oxygen decreases and the requested amount persists, the energy balance becomes negative and myocardial hypoxia emerges.13 In the fECG, the effect of myocardial hypoxia is commonly reflected in a morphological change of the ST segment, which becomes elevated or depressed (the Cochrane Library provides a support to interpret fetal ST waveform14). The fetus responds to the negative energy balance by suddenly increasing adrenalin to start glycogenolysis, a process in which stored glucose is utilized for generating energy. Changes in the ST segment will then indirectly reflect the fetal capacity of metabolic compensation.
Fetal Movements
Maternal perception of fetal movements is the oldest and most commonly used method to assess the well‐being of the fetus starting from the 20th week of gestation.15 At first, the fetal movements are weary and infrequent, but in the second half of pregnancy, they become stronger, more frequent, and increasingly linked to fetal heart‐rate patterns and fetal eye movements, and identify fetal behavioral states that are indicators of maturity and integrity of the fetal nervous system. Severe and sustained reductions of fetal movements indicate fetal distress, often preceding fetal death. Fetal movements only temporally influence the morphology of the fECG, whereas fetal distress, which associates to a sustained decrease of fetal movements, causes prolonged variations of the fECG, in particular of the ST segment. Abnormalities in the ST segment persisting for longer than 15 seconds have been associated to critical fetal states, whereas shorter episodes to the fetal movements.9
NONINVASIVE fECG RECORDING TECHNIQUES
Electrodes Features
The noninvasive fECG signal is obtained by applying standard ECG electrodes16, 17 on the pregnant woman abdomen. The skin area under the electrode must be slightly abraded to remove the most superficial stratum corneum, that is poorly conductive, and a transparent electrolyte gel containing Cl− is usually applied to maintain a good contact and reduce the impact of the skin on the impedance by making its dry outer layer ion conductive.18 In this way, the contact impedance between the sensor and the skin is minimized and the recorded signal quality is optimized.
Electrode Configurations
The morphology of the noninvasive abdominal fECG signal depends not only on electrodes placement, but also on fetus position,8 which is not always exactly predictable. Consequently, the definition of an optimal electrode configuration is rigorously not possible. Still, some different configurations have been proposed in the attempt to standardize the recording procedures. Some of them rely on the most likely fetus positions and consider a low number of leads (4–8) to optimize application simplicity. Others try to cover as much fetal positions as possible and consider a high number of leads (>8) to optimize feasibility of signal acquisition.
Globally, electrode configurations for noninvasive fECG recordings can be grouped into two classes: pure abdominal configurations and mixed configurations. Differently from the former, the latter also provide pure maternal ECG (mECG) tracings.
Pure Abdominal Electrode Configurations
Configuration with four electrodes
According to this configuration,19 one common electrode is located on the symphysis pubis whereas the other three are positioned to the left, above, and to the right of the navel (Fig. 2A).
Configuration with six electrodes
In this configuration, three electrodes are aligned on the navel (two to the right and one to the left), one is placed above the navel, a reference one on the pubic symphysis, and a common mode reference one, with active‐ground signal, on the left thigh20 (Fig. 2B) or on the back.21
Configuration with 10 electrodes
This configuration22 considers 10 electrodes, four vertically aligned at the center of the maternal abdomen (two above and two under the navel), two couples located on the right and on the left of the line identified by the previous four, the reference one located at the abdomen center near the navel, and the ground one located on the right thigh (Fig. 2C).
Configuration with 13 electrodes
The six‐pointed star electrode configuration23 (Fig. 2D) is obtained by placing 13 abdominal electrodes. The average of all recorded potentials represents the common reference.
Configuration with 32 electrodes
In this configuration,24 a set of 32 abdominal electrodes are placed on basis of anatomic landmarks (the navel, xiphoid process, pubic symphysis, axilla, and spine) in order to cover the maternal abdomen, sides and back (Fig. 2E).
Mixed Electrode Configurations
Mixed configuration with eight electrodes
This configuration25 considers eight electrodes, of which five are abdominal around the navel and three thoracic, under the left udder (Fig. 2F).
Mixed configuration with nine electrodes
This configuration26 includes nine electrodes, six abdominal located around the navel, and three thoracic vertically aligned in correspondence of the maternal heart (one above and two under the heart; Fig. 2G).
Mixed configuration with 14 electrodes
This configuration27 considers positioning 12 electrodes in two horizontal lines on the maternal abdomen, respectively, under and above the navel, and one electrode on each maternal shoulder (Fig. 2H).
AUTOMATIC FETAL ECG EXTRACTION: A UNIFIED FRAMEWORK
From a mathematical point of view, each lead of an abdominal recording (aLead) is a summation of three components: the fECG, the abdominal maternal ECG (amECG), and the noise (aNoise):
| (1) |
The fECG represents the signal of interest. It is characterized by a frequency band between 0.5 and 100 Hz, even though a bandwidth of 0.5–45 Hz results usually enough for most practical applications.7 Fetal QRS‐complex amplitude is strongly dependent on lead, gestational age, and fetus position. Typically, it does not overcome 60 μV. The amECG is the dominant physiological interference in the aLead. It is characterized by a frequency bandwidth similar to the one characterizing the fECG, but its amplitude can be up to 10 times higher.7 Typically, the amplitude of maternal QRS complexes reaches 100–150 μV. Eventually, aNoise is a mixture of interferences that can or cannot have a physiological origin. Physiological noise includes fetal and maternal electromyogram, electroencephalogram, and respiration. Nonphysiological noise includes instrumentation noise, poor shielding in the cables, and noise from the electrode/skin interface. In first approximation, aNoise can be further decomposed in low‐frequency noise (lfNoise), in‐band noise (ibNoise), and high‐frequency noise (hfNoise): the lfNoise, characterized by a frequency band between 0 and 0.5 Hz, typically includes baseline drifts and wandering due to respiration; instead, the hfNoise includes interferences that are characterized by frequency components wide above 40 Hz (till few hundreds of Hz) such as the line interference (50 or 60 Hz), maternal and fetal electroencephalogram and electromyogram (up to 200 Hz), and others.7 Eventually, ibNoise is characterized by all those noise frequency components that fall into the fECG bandwidth, and thus are the most difficult to be eliminated.
Automatic extraction of fECG from aLead usually includes two main steps: abdominal signal prefiltration and amECG cancellation (see Fig. 3), as described later.
Figure 3.
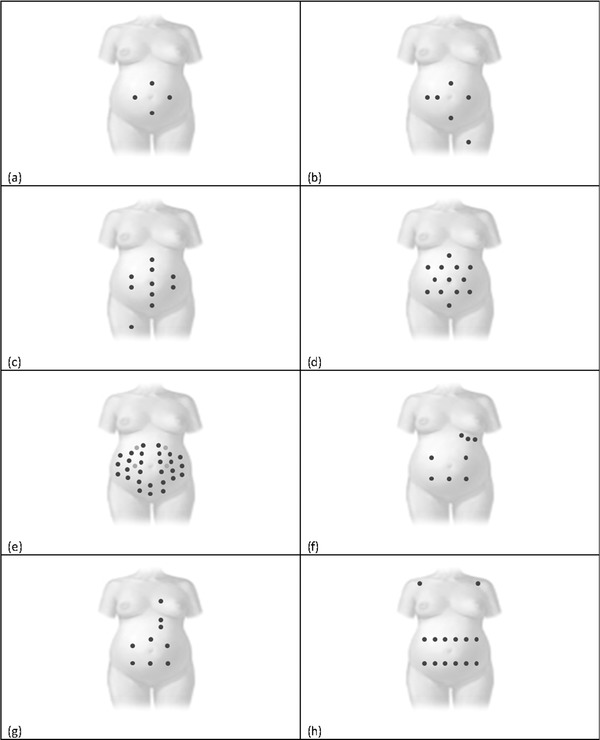
Electrode configurations for abdominal recordings. Pure abdominal configurations consider (A) four, (B) six, (C) ten, (D) thirteen, and (E) thirty two electrodes, respectively. Instead mixed configurations consider (F) eight, (G) nine, and (F) fourteen electrodes.
Abdominal Recording Prefiltration
Prefiltration of the aLead signal is usually linearly performed by applying a bandpass (0.5–45 Hz) filter in order to remove lfNoise and hfNoise, respectively. The resulting filtered signal (faLead; Fig. 4) is then composed by fECG, amECG, and ibNoise:
| (2) |
Figure 4.

Automatic fECG extraction from an abdominal recording (aLead) consisting in an initial prefiltration and subsequent maternal ECG (amECG) cancellation.
Usually ibNoise cannot be neglected. Typically, at most one or very few leads of a multilead recording show an SNR sufficiently good (i.e., an ibNoise level sufficiently low) for a successful fECG extraction.
Fetal ECG Extraction
The frequency bands of fECG and amECG are strongly overlapped so that linear filtering cannot be applied for their separation.7 Rather, extraction procedures of fECG from faLead involve amECG cancellation, mathematically represented by a subtraction even though the process may or may not involve amECG estimation:
| (3) |
Many techniques have been proposed for fECG extraction. If pure abdominal electrode configurations are used, fECG extraction can occur directly from faLead by means of linear28, 29, 30, 31, 32, 33 or nonlinear34, 35 decomposition techniques, among which the independent component analysis (ICA)31 is the most commonly used, or by template subtraction.20, 36 Instead, if mixed electrode configuration are used, fECG can be extracted using adaptive filtering that, in addition to faLead, also uses the mECG recorded by the electrodes located in the woman thorax or shoulder.37, 38 A detailed description of each single technique reported in the literature is impossible to report here and out of the scope of this work. Consequently, only technical hints of the most commonly used technique will be provided.
Independent Component Analysis
The ICA is a blind source separation technique that can be used for fECG extraction under the hypothesis of statistical independence of fECG and amECG. The ICA can be applied in case of multi‐lead abdominal recording, and works under the assumption that the signals from different leads are linear combinations of the independent source signals generated by the maternal heart and fetal heart.31 To separate the various source signals, the so called separating matrix S is used:
| (4) |
where and are matrices containing fECG signals and faLead signals of all available leads. ICA finds the independent components by maximizing the statistical independence of the estimated components.31 The higher the number of available abdominal recordings, the better fetal ECG extraction is. However, recording a large number of channels makes the procedure difficult to apply and not comfortable to the pregnant woman. Consequently, the clinical use of such approach is quite limited.
Template Subtraction
The single‐lead amECG suppression by template subtraction, which can be applied to a single‐lead abdominal recording, exploits the repeatability of mECG beats to obtain the fECG.36 Each mECG beat is reconstructed using a common ECG beat waveform called template. The determination of the template occurs directly from the faLead, and involves various signal processing steps.20 First, the maternal R peaks (fiducial points) are identified, usually (but not necessarily) using the Pan‐Tompkins’ procedure39 combined with a threshold criteria finalized to distinguish maternal R peaks from fetal ones. Then, each maternal beat is segmented to have the corresponding PQRST complexes which are, in the most classic approaches, averaged to get the template.36 More rarely, the template is computed from all segmented beats using particular adaptive filters.40 Once the template has been constructed, all maternal beats are reconstructed and concatenated to estimate amECG, which is eventually subtracted from faLead.36
Adaptive Filtering for Fetal ECG Extraction
Adaptive filtering for fECG extraction is usually performed using the extended Kalman filter (EKF),37 an extension of standard Kalman filter41 to nonlinear systems. To make possible the fECG extraction from faLead with adaptive filtering, the filter needs two measurements: a primary input, represented by faLead that contains the signal of interest and the disturbing interference (amECG), and a secondary input, represented by mECG, highly correlated with amECG. The transformation of mECG into amECG can be determined minimizing the mean square error between the primary input faLead and mECG.37
Neural network is another technique for adaptively extracting fECG from faLead.38 The input signal is considered as mECG and the target signal is faLead. The suppression of amECG from faLead occurs by correlative detraction so that the output can be considered as only fECG.40
CLINICAL APPLICATIONS OF fECG EXTRACTION PROCEDURE
Most of the studies on the fECG extraction were performed in very few recordings (few units) and were finalized to propose and validate a new algorithm. Thus, they represent methodological rather than clinical studies and significant statistics on their performances on large (of at least few tens of units) dataset of clinical data are not available. As far as we know, the only exception to this observation is a study that used the ICA technique in 20 pregnant women23 at 38 weeks of gestational ages to labor finalized to fetal heart rate from the fECG. The found rate of success was 85%.
DISCUSSION AND CONCLUSION
Availability of noninvasive fECG would allow provision of important information about the fetus health state. Still, practical clinical use of fECG monitoring is very limited because of some limitations affecting it. The first limitation has a physiological origin. The fetus is surrounded by different anatomical layers with different electrical conductivities, of which amniotic fluid and vernix caseosa are the ones with the highest and the lowest conductivity, respectively.8 Specifically, vernix caseosa is formed between the 28th and 32nd weeks of gestation, and it almost electrically shields the fetus making fECG testing difficult (only one study has reported a rate of success of 60%.23 Later on, during the 37th to 38th weeks of a normal (nonpremature) pregnancy, the vernix caseosa slowly dissolves and noninvasive fECG testing becomes easier.8
Despite fECG has been known for decades, the acquisition protocol has not been standardized yet (none of the above‐described electrode configurations can be considered as standard at the present time). Electrodes standardization in fECG testing is not as crucial as in thoracic ECG testing, which involve electrodes located in standard positions with respect to the heart. In the abdominal recordings, the electrodes are located on the mother's body surface, and the same electrode configuration can provide morphologically different fECG tracings due to different fetal positions. If it is true that some statistical considerations on the most likely fetus position at a certain gestation age may indicate an electrode configuration as preferable over the others, it is also true that it is impossible to force the fetus to assume that position and keep it still. Because of the relatively long time (minutes) needed to perform an abdominal recording, it is recommended to position the woman comfortably, either sitting upright or laterally; then ascertain the fetus position. Eventually, to help the electrodes placement, these can be grouped in a single strip so that it can be easily put on and off and the contact between the mother's skin and the surface of the electrodes can be ensured.
The choice of an electrode configuration over another is linked to the used technique for automatic fECG extraction. In this study, three major classes have been identified, namely the ICA,31 template subtraction,20, 36, 40 and adaptive filtering,37, 38 even though several others have been proposed in the literature.42, 43 The suitability of ICA technique for fECG extraction is not obvious considering that the purely mathematical basis of the method does not consider the several characteristics of the involved physiological signals.31 It has been reported44 that a good ICA performance requires: the fECG to be visible above the noise floor; and the number of channels to be larger than the number of desired independent signals due to the variable number of interference sources. The template subtraction technique36 is a single‐channel approach usually recursively applied to abdominal recordings with few channels. Eventually, adaptive filtering either requires a reference (thoracic) mECG channel or several linearly independent channels to morphologically reconstruct the maternal interference waveform.8
Despite the above‐described issues, good‐quality fECG tracings from any lead could still provide precious information on the fetus health, especially in view of delivery, that is a very critical moment for both fetus and mother. Good‐quality fECG tracings, however, are very difficult to obtain, and this limits its practical use more than anything else. In real applications, noise other than mECG components is only reduced by the prefiltering process (ibNoise in Eq. 2 is not negligible). Moreover, if using techniques that estimate amECG and extract fECG by subtraction processes, very little time shifts in the estimated amECG with respect to the real amECG can cause high‐amplitude artifact (especially in correspondence of the high‐frequency QRS complexes; Fig. 5), which may jeopardize fECG quality.
Figure 5.

Example of an artifact generated during the amECG cancellation (by subtraction) from faLead due to a nonperfect synchronization between estimated amECG and real amECG in correspondence of a high‐frequency QRS complex.
Given its potentialities on providing clinically useful information on the fetus health state and despite all its limitations, abdominal fECG monitoring is still of a great interest in the biomedical field, as demonstrates the Computing in Cardiology Physionet Challenge 2013 (http://physionet.org/challenge/2013), in which several preliminary ideas for improving fetal ECG extraction have been proposed and compared. In this context, this work provides an overview of the noninvasive fECG monitoring, useful to both get into the topic and better understand new technological developments.
Conflict of Interest Statement:
Angela Agostinelli, Marla Grillo, Alessandra Biagini, Corrado Giuliani, Luca Burattini, Francesco Di Nardo, Stefano R. Giannubilo, and Andrea Ciavattini have no financial and/or personal relationships with people or organizations that could inappropriately influence (bias) this work.
Laura Burattini and Sandro Fioretti declare their partnership to the academic spin‐off B.M.E.D. SRL (Bio‐Medical Engineering Development, Department of Information Engineering, Polytechnic University of Marche, Ancona, Italy, http://www.bmed-bioengineering.com ).
REFERENCES
- 1. Velayo C, Sato N, Ito T, et al. Understanding congenital heart defects through abdominal fetal electrocardiography: Case reports and clinical. J Obstet Gynaecol 2011;37:428–435. [DOI] [PubMed] [Google Scholar]
- 2. Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: A review. Int J Pediatr 2010;2010:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Regitz‐Zagrosek V, Blomstrom‐Lundqvist C, Borghi C, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy. The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2001;32:3147–3197. [DOI] [PubMed] [Google Scholar]
- 4. Steer PJ. Fetal distress. Curr Obstetr Gynaecol 2002;12:15–21. [Google Scholar]
- 5. Goddard R. Electronic fetal monitoring. Brit Med J 2001;322:1436–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norén H, Amer‐Wåhlin I, Hagberg H, et al. Fetal electrocardiography in labor and neonatal outcome: Data from the Swedish randomized controlled trial on intrapartum fetal monitoring. Am J Obstet Gynecol 2003;188:183–192. [DOI] [PubMed] [Google Scholar]
- 7. Hasan M, Reaz M, Ibrahimy MI, et al. Detection and processing techniques of FECG signal for fetal monitoring. Biol Proced Online 2009;11:263–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sameni R, Clifford G. A review of fetal ECG signal processing; issues and promising directions. Electrophysiol Ther J 2010;3:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vullings R. Non‐invasive fetal electrocardiogram: Analysis and interpretation. PhD Thesis. Technische Universteit Eindhoven 2010.
- 10. Baschat AA. Integrated fetal testing in growth restriction: Combining multivessel Doppler and biophysical parameters. Ultrasound Obst Gyn 2003;21:1–8. [DOI] [PubMed] [Google Scholar]
- 11. Hornberger LK, Sahn DJ. Rhythm abnormalities of the fetus. Heart 2007;93:1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kleinman CS, Nehgme RA. Cardiac arrhythmias in the human fetus. Pediatr Cardiol 2004;25:234–251. [DOI] [PubMed] [Google Scholar]
- 13. Spilka J. Complex approach to fetal heart rate analysis: A hierarchical classification model. Doctoral thesis. Czech Technical University Prague 2013.
- 14. Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database Syst Rev 2013;5:1–29. [DOI] [PubMed] [Google Scholar]
- 15. Sadovsky E, Polishuk WZ. Fetal movements in utero nature, assessment, prognostic value, timing of delivery. Obstet Ginecol 1977;50:49–55. [PubMed] [Google Scholar]
- 16. Lee S, Kruse J. Biopotential Electrode Sensors in ECG/EEG/EMG Systems. Analog Devices 2008. Available at: http://www.analog.com/MedicalICs.
- 17. Neuman MR. Biopotential electrodes. Med Instrum 2008;5:189–240. [Google Scholar]
- 18. Gruetzmann A, Hansen S, Muller J. Novel dry electrodes for ECG monitoring. Physiol Meas 2007;8:1375–1390. [DOI] [PubMed] [Google Scholar]
- 19. Karvounis EC, Tsipouras MG, Fotiadis DI, et al. An automated methodology for fetal heart rate extraction from the abdominal electrocardiogram. IEEE Trans Inf Technol B 2007;11:628–638. [DOI] [PubMed] [Google Scholar]
- 20. Jezewski J, Matonia A, Kupka T, et al. Determination of fetal heart rate from abdominal signals: Evaluation of beat‐to‐beat accuracy in relation to the direct fetal electrocardiogram. Biomed Tech 2012;57:383–394. [DOI] [PubMed] [Google Scholar]
- 21. Bergveld P, Meijer WJH. A new technique for the suppression of the MECG. IEEE Trans Bio‐Med Eng 1981;28:348–354. [DOI] [PubMed] [Google Scholar]
- 22. Marossero D, Euliano T, Euliano N, J. Principe. Maternal‐fetal monitoring system. Patent No.: US 2005/0267377 A1.
- 23. Martens SMM, Rabotti C, Mischi M, et al. A robust fetal ECG detection method for abdominal recordings. Physiol Meas 2007;28:373–388. [DOI] [PubMed] [Google Scholar]
- 24. Clifford G, Sameni R, Ward J, et al. Clinically accurate fetal ECG parameters acquired from maternal abdominal sensors. Am J Obstet Ginecol 2011;47:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahmoud A, Zeng X. A novel technique for extraction foetal electrocardiogram using adaptive filtering and simple genetic algorithm. Am J Biostat 2010;2:75–81. [Google Scholar]
- 26. Sameni R, Clifford GD, Jutten C, et al. Multichannel and noise modeling: Application to maternal and fetal ECG signals. EURASIP J Adv Sig Pr 2007;2007:1–14. [Google Scholar]
- 27. Vullings R, Peters C, Mischi M, et al. Maternal ECG removal from non‐invasive fetal ECG recordings. Proc Ann Int Conf IEEE EMBS 2006;1:1394–1397. [DOI] [PubMed] [Google Scholar]
- 28. Mochimaru F, Fujimoto Y, Ishikawa Y. Detecting the fetal electrocardiogram by wavelet theory‐based methods. Prog Biomed Res 2002;7:185–193. [Google Scholar]
- 29. Hassanpour H, Parsaei A. Fetal ECG Extraction using Wavelet Transform. International Conference on Computational Intelligence for Modelling Control and Automation, and International Conference on Intelligent Agents, Web Technologies and Internet Commerce 2006:1–4. [Google Scholar]
- 30. Kanjilal PP, Palit S, Saha G. Fetal ECG extraction from single‐channel maternal ECG using singular value decomposition. IEEE Trans Biomed Eng 1997;44:51–59. [DOI] [PubMed] [Google Scholar]
- 31. Sameni R, Jutten C, Shamsollahi MB. What ICA provides for ECG processing: application to noninvasive fetal ECG extraction. IEEE ISSPIT 2006:656–661. [Google Scholar]
- 32. Azzerboni B, La Foresta F, Mammone N, et al. A new approach based on wavelet‐ICA algorithms for fetal electrocardiogram extraction. ESANN 2005:193–198. [Google Scholar]
- 33. Gao P, Chang Ee‐C, Wyse L. Blind separation of fetal ECG from single mixture using SVD and ICA. ICICS PMC 2003;3:1418–1422. [Google Scholar]
- 34. Schreiber T, Kaplan DT. Signal separation by nonlinear projections: The fetal electrocardiogram. APS 1996;53:4326–4329. [DOI] [PubMed] [Google Scholar]
- 35. Richter M, Schreiber T, Kaplan DT. Fetal ECG extraction with nonlinear state‐space projections. IEEE Trans Biomed Eng 1998;45:133–137. [DOI] [PubMed] [Google Scholar]
- 36. Matonia A, Jezewski J, Horoba K, et al. The maternal ECG suppression algorithm for efficient extraction of the fetal ECG from abdominal signal. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2006:3106–3109. [DOI] [PubMed] [Google Scholar]
- 37. Sameni R, Shamsollahi MB, Jutten C. Filtering electrocardiogram signals using the extended kalman filter. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2005:5639–5642. [DOI] [PubMed] [Google Scholar]
- 38. Hasan MA, Ibrahimy MI, Reaz MBI. Fetal ECG extraction from maternal abdominal ECG using neural network. J Softw Eng Appl 2009:2:330–334. [Google Scholar]
- 39. Pan J, Tompkins WJ. A real‐time QRS detection algorithm. IEEE Trans Bio‐Med Eng 1985;32:230–236. [DOI] [PubMed] [Google Scholar]
- 40. Ungureanu M, Bergmans JWM, Oei SG, et al. Fetal ECG extraction during labor using an adaptive maternal beat subtraction technique. Biomed Tech 2007;52:56–60. [DOI] [PubMed] [Google Scholar]
- 41. Welch G, Bishop G. An introduction to the Kalman filter. Siggraph 2001:1–48. [Google Scholar]
- 42. Guerrero‐Martínez JF, Martínez‐Sober M, Bataller‐Mompean M, et al. New algorithm for fetal QRS detection in surface abdominal records. CINC 2006;33:441–444. [Google Scholar]
- 43. Romero I, Geng D, Berset T. Adaptive filtering in ECG denoising: A comparative study. CINC 2012;39:45–48. [Google Scholar]
- 44. Ifeachor EC, Outram NJ, Henderson GT, et al. Nonlinear methods for biopattern analysis: role and challenges. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2004:5400–5406. [DOI] [PubMed] [Google Scholar]


