Abstract
Background
The majority of sudden cardiac death (SCD) in patients with heart failure occurs in those with mild‐moderate left ventricular (LV) systolic dysfunction (LVEF 36–50%) who under current guidelines are ineligible for primary prevention implantable cardiac defibrillator (ICD) therapy. Recent data suggest that cardiac magnetic resonance (CMR) evidence of replacement fibrosis forms a substrate for malignant arrhythmia and therefore potentially identifies a subgroup at increased risk of SCD. Our hypothesis is that among patients with mild‐moderate LV systolic dysfunction, a CMR‐guided management strategy for ICD insertion based on the presence of scar or fibrosis is superior to a current strategy of standard care.
Methods/Design
CMR GUIDE is a prospective, multicenter randomized control trial enrolling patients with mild‐moderate LV systolic dysfunction and CMR evidence of fibrosis on optimal heart failure therapy. Participants will be randomized to receive either a primary prevention ICD or an implantable loop recorder (ILR). The primary endpoint is the time to SCD or hemodynamically significant ventricular arrhythmia (VF or VT) during an average 4‐year follow‐up. Secondary endpoints include quality of life assessed by Minnesota Living with Heart Failure Questionnaire, heart failure related hospitalizations, and a cost‐utility analysis. Clinical trials.gov identifier NCT01918215.
Discussion
CMR GUIDE trial will add substantially to our understanding of the role of myocardial fibrosis and the risk of developing life‐threatening ventricular arrhythmias. If the superiority of a CMR‐guided approach over standard care is proven, it may change international clinical guidelines, with the potential to considerably increase survival in this growing patient population.
Keywords: cardiac magnetic resonance imaging, implantable cardiac defibrillator, left ventricular systolic dysfunction, sudden cardiac death, syncope
Abbreviations
- ACE
angiotensin converting enzyme
- ATP
anti‐tachycardia pacing
- BPM
beats per minute
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance image
- EDV
end‐diastolic volume
- EOS
end of study
- ESV
end‐systolic volume
- HF
heart failure
- HLA
horizontal long axis
- ICD
implantable cardiac defibrillator
- ILR
implantable loop recorder
- LGE
late gadolinium enhancement
- LVEF
left ventricular ejection fraction
- LVOT
left ventricular outflow tract
- MACE
major adverse cardiac event
- MM
myocardial mass
- MMP
Matrix metalloproteinases
- NICM
Nonischemic cardiomyopathy
- OMT
optimal medical therapy
- QALY
quality‐adjusted life years
- ROI
region of interest
- RWM
regional wall motion
- SCD
sudden cardiac death
- SPECT
single photon emission computed tomography
- SV
stroke volume
- SVT
supraventricular tachycardia
- TIMPs
tissue inhibitors of metalloproteinases
- VLA
vertical long axis
1. Introduction
Heart failure is a rapidly expanding clinical syndrome having prevalence between 1% and 2% in adults and increasing to over 10% in those aged above 70 years (Kong et al., 2011; Soliman et al., 2010). Coronary artery disease and primary diseases of the myocardium (cardiomyopathies) account for approximately two‐thirds of cases in the western world. A significant contributor to mortality in heart failure is ventricular tachyarrhythmia leading to sudden cardiac death (SCD) (Epstein et al., 2013; de Vreede‐Swagemakers et al., 1997; Weisfeldt & Becker, 2002). The safest and most effective therapy for ventricular tachycardia/ventricular fibrillation (VT/VF) is defibrillation (Weisfeldt & Becker, 2002) and in this regard, the advent of implantable cardiac defibrillator (ICD) technology has transformed clinical practice where device therapy has become standard care in several patient populations (Epstein et al., 2013).
The most recent American Heart Association/Heart Rhythm Society guidelines assign a class I recommendation for ICD implantation for primary prevention of SCD in patients with a left ventricular ejection fraction (LVEF) ≤35% on optimal medical therapy (OMT) (Epstein et al., 2013). The LVEF cutpoint of 35% for ICD implantation is based on the inclusion criteria of major randomized trials (Epstein et al., 2013) which have consistently demonstrated a one‐third reduction in all‐cause mortality with the exception of the Multicenter Automatic Defibrillator Implantation Trial (MADIT‐1) trial where a 54% risk reduction was found (Moss et al., 1996). However, recent studies highlight that current treatment guidelines substantially underestimate the number of patients who would benefit from ICD implantation (Dagres & Hindricks, 2013). The majority of SCD occurs in patients with mildly impaired or preserved LVEF (Makikallio et al., 2005; de Vreede‐Swagemakers et al., 1997), a trend reflected in both historic and contemporary data. The Oregon Sudden Unexplained Death Study, a prospectively enrolled community sudden cardiac death registry, examined 2093 cases of sudden death in the period 2003–12 from an intake population of 1 million (Narayanan et al., 2013). Of the 448 SCD cases with a pre‐event LVEF estimate, 308 (68%) patients had a LVEF >35% and would have been excluded from ICD therapy under current guidelines. Of these cases, 79.5% of deaths were excluded from ICD therapy by current guidelines and 55% were excluded from ICD therapy because of a LVEF between 36 and 50%.
Current risk stratification tools in patients with a LVEF above 35% who may benefit from primary prevention ICD therapy are therefore suboptimal. Cardiac magnetic resonance (CMR) imaging can identify myocardial scar and replacement fibrosis, which forms a critical substrate for ventricular arrhythmia, and represents a potentially transformative investigative technology enabling enhanced risk stratification in patients in this subgroup, a population currently excluded from ICD therapy under major society guidelines.
The primary objective of CMR GUIDE is to assess the effectiveness of ICD therapy in patients with mild to moderate left ventricular systolic dysfunction with myocardial fibrosis on CMR imaging in preventing SCD or hemodynamically significant ventricular arrhythmia. Furthermore, we will examine the impact of ICD therapy on all‐cause mortality, quality of life, heart failure functional class, and heart failure‐related hospitalization and to perform a cost‐utility analysis to estimate the incremental cost per quality‐adjusted life year (QALYs) of ICD therapy versus standard care. In a substudy of CMR GUIDE, a registry will be established to examine the incidence of SCD and hemodynamically significant ventricular arrhythmia in patients with mild to moderate left ventricular dysfunction without myocardial fibrosis on CMR.
2. Methods/Design
2.1. Hypothesis
The principal hypothesis of CMR GUIDE is that in patients with mild to moderate left ventricular systolic dysfunction and CMR evidence of myocardial fibrosis, a strategy of ICD insertion will be superior to standard care. The secondary hypothesis is that CMR‐guided ICD insertion in this patient population will be cost effective compared with standard care.
2.2. Endpoints
2.2.1. Primary endpoint
The primary endpoint is a composite of:
Sudden cardiac death (SCD) or
Hemodynamically significant ventricular arrhythmia producing syncope (defined by a loss of consciousness) or associated with hypotension (systolic blood pressure < 90 mmHg)
2.2.2. Secondary endpoints
Sudden cardiac death
Hemodynamically significant ventricular arrhythmia
All‐cause mortality
New York Heart Association functional class
Quality of Life (Minnesota Living with Heart Failure Questionnaire)
Heart failure‐related hospitalizations
Cost (health economic evaluation)
2.3. Study conduct
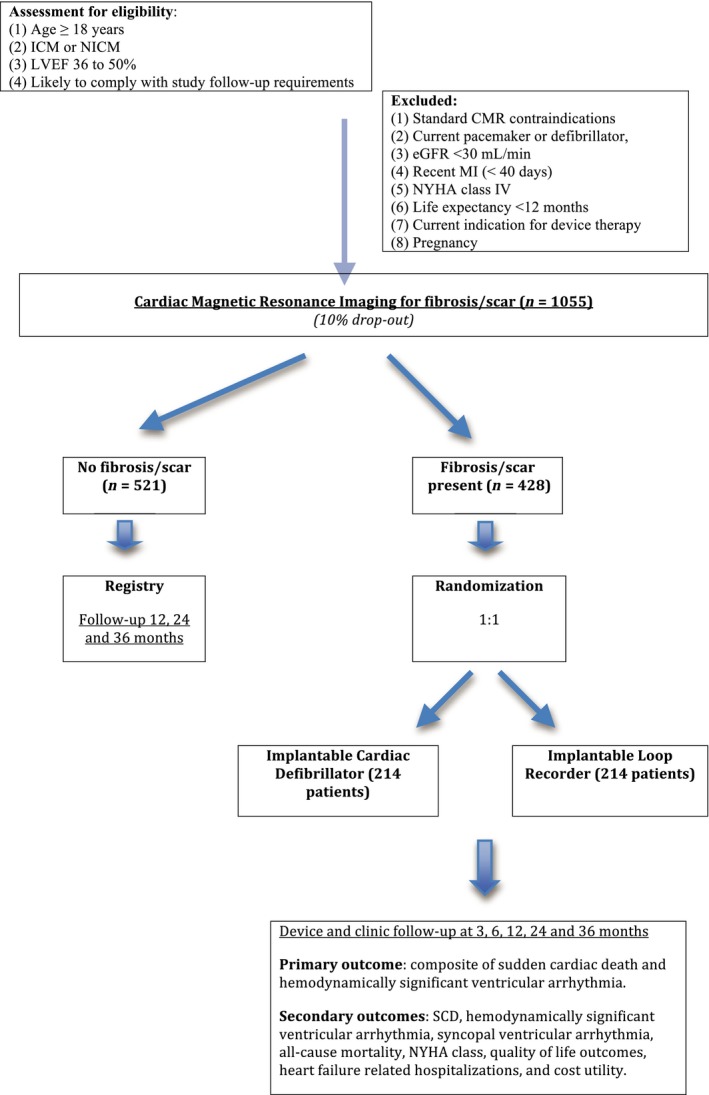
CMR GUIDE is an international, multicenter, combined registry and randomized trial to be conducted at 23 sites across Australia and Europe. All patients will provide written consent prior to enrollment in accordance with the principles of the declaration of Helsinki. The study protocol has been approved by the Southern Adelaide Ethics Committee (Australia) and registered on http://www.clinicaltrials.gov identifier NCT01918215. Sites with established heart failure and CMR imaging programs will be invited to participate in this trial. Each prospective site will have to submit 50 de‐identified case report forms to indicate that they have sufficient cases that meet study eligibility criteria. Only upon fulfilling this requirement will a site be deemed suitable to participate in this study. This approach will ensure recruitment targets are met in an efficient manner as only centers with sufficient CMR expertise and motivation (as demonstrated by the completed participant screening) will be involved in the trial. We will therefore know at a very early stage whether recruitment targets can be met, thus permitting timely change in the number of sites invited to participate. In addition, the identification of 50 potential participants per site even before trial commencement will minimize the risk of sites not recruiting.
Each participating center has approval by their respective Institutional Review Board or Ethics Committee. An independent academic executive committee led by J.S developed the protocol.
2.4. Patient population
Principle inclusion and exclusion criteria are shown in Table 1. CMR GUIDE will enroll male and female patients aged ≥18 years with a left ventricular ejection fraction between 36 and 50% due to coronary artery disease (CAD) or nonischemic cardiomyopathy (NICM). All patients will be on optimal medical therapy. Coronary artery disease is defined as evidence of one of three criteria: clinical history of prior myocardial infarction, significant (>70%) stenosis of a major epicardial coronary vessel by coronary angiography or prior revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Patients without clinical criteria for CAD with CMR findings of endocardial scar on CMR will not be enrolled. Nonischemic cardiomyopathy will be defined as Idiopathic, Familial, or Chronic Post Myocarditis based on the patients’ clinical and family history. The etiology of the systolic dysfunction (whether CAD or NICM) will be defined apriori on the basis of the patient's clinical diagnosis they have prior to entry into the trial. Patients are then recruited based on whether the CMR findings fulfill entry criteria for their preexisting diagnosis. Patients with infiltrative cardiomyopathy will be excluded due to the uncertain utility of ICDs in this population (Epstein et al., 2013). Participants must not have concomitant indications for a permanent pacemaker, biventricular pacemaker, or ICD therapy. Also excluded are patients who have had a myocardial infarction within 40 days or any coronary revascularization procedure (PCI or CABG) within 3 months of enrollment, as well as those with unstable angina or stable angina with Canadian Class 2 or above symptoms. In keeping with guidelines for ICD implantation in patients with LV ejection fraction ≤ 35%, patients will not be required to undergo functional testing for ischemia prior to recruitment for CMR GUIDE. Treating clinicians will be encouraged however to offer myocardial revascularization and appropriate medical therapy to patients, as indicated by international guidelines. Following myocardial revascularization patients can enroll in the trial but only if they fulfill the entry criteria after repeat assessment > 3 months after their revascularization procedure. Similarly, newly presenting NICM patients need to have at least 3 months of maximally tolerated medical therapy before being eligible for randomization.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
2.5. Study procedures and randomization
Table 2 illustrates the key phases of the trial. Participants will be recruited through heart failure clinics and echocardiography databases to undergo preliminary screening eligibility. Prior to enrollment, all participants are required to have had a LVEF measurement in the previous 6 months by any modality (echocardiography, left ventriculography, CMR, SPECT MPI). Consenting participants will then attend an initial clinic visit where a medical history (including their NYHA functional class), physical examination, ECG, and measurement of N‐terminal Pro‐BNP and Troponin T will be performed. A baseline Minnesota Living with Heart Failure questionnaire and EQ‐5D questionnaire to estimate quality‐adjusted life year (QALYs) will also be performed.
Table 2.
Trial time line
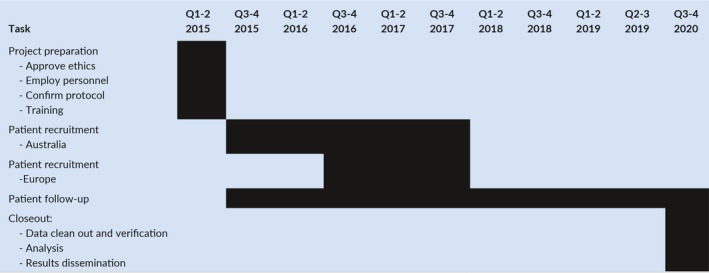
CMR screening will firstly confirm that the patient meets the inclusion criteria of a LVEF between 36 and 50% for enrollment in the trial. Secondly, it will identify the presence and extent of myocardial fibrosis with late gadolinium enhancement (LGE), which is defined for ICM as two or more transmural (>75%) myocardial segments and for NICM as evidence of any myocardial fibrosis. Patients with NICM whose CMR shows evidence of any scar or fibrosis will be randomized to the ICD implant group or the implantable loop recorder (ILR) group. Patients with ICM, need to have two or more myocardial segments (using 17 segment AHA model) of transmural (>75%) LGE to be eligible for randomization. Patients with isolated RV insertion point fibrosis will not be considered to have myocardial fibrosis as the prognostic importance in this setting is less certain. Patients who have already undergone a CMR for clinical reasons within the previous 2 months who fulfill the above criteria will be eligible for randomization into the trial without repeat screening CMR. CMR analysis will be performed at the CMR Core Lab, South Australian Health and Medical Research Institute (SAHMRI), Adelaide, Australia.
2.6. Study arm with myocardial fibrosis
Patients with CMR evidence of myocardial scar or fibrosis as previously defined, will be allocated to the device arm of CMR GUIDE and will be randomized via a centralized computer allocation (1:1) to receive either a BIOTRONIK single chamber implantable cardiac defibrillator (ICD) or a BIOTRONIK implantable loop recorder (ILR) (Figure 1). A maximum of 2 months will be allowed from the time of CMR screening to randomization. Programming parameters will be standardized at the time of implant and represent a combination of both the high rate and delayed therapy arms of the MADIT clinical trial (Moss et al., 2012) (Table 3). The monitor zone in patients with ICDs will allow rhythm correlation in those with syncope, allowing comparison with the ILR group. Anti‐tachycardia pacing will include two active tachycardia therapy zones and a further inactive monitor zone, which will be determined by tachycardia cycle length. All ICDs will have backup bradycardia pacing set at 40 bpm.
Figure 1.
Patient flow
Table 3.
Device therapy programming parameters
| ICD group |
|
A single chamber BIOTRONIK ICD will be implanted with the following parameters: |
|
|
|
|
| ILR group |
|
A BIOTRONIK implantable loop recorder will have the following parameters programmed |
|
|
2.6.1. Follow‐up
Patients in the device arm (ICD or ILR) will be followed as per standard post implantation practice at 3, 6, 12, 24, 36 months and depending on time of enrollment at 48 and 60 months. An end of study (EOS) visit will occur for patients who are >39 months following enrollment. At follow‐up visits, the Minnesota Living with Heart Failure questionnaire and EQ‐5D questionnaire to estimate quality‐adjusted life year (QALYs) will be performed. Also, an assessment of unexpected adverse events and study endpoints will be performed. Patients in the ILR implant group will have their device downloaded annually or following an event. At the conclusion of the trial, participants with an ICD inserted will have the option of keeping the device active or having it inactivated. All participants with an ILR inserted will have it removed at conclusion of the trial.
2.7. Study arm without myocardial fibrosis
Participants without CMR evidence of scar or fibrosis (according to the definitions above) will be enrolled into the observational registry.
2.7.1. Follow‐up
Collection and review of study endpoints for participants in the registry group will occur at 12, 24, and 36 months.
2.8. Study outcomes
The primary outcome will be incidence of sudden cardiac death, or first hemodynamically significant ventricular arrhythmia (VT/VF) during an average 4‐year follow‐up period (Table 4). Sudden cardiac death is a standard endpoint used in many ICD trials. We have defined sudden cardiac death (SCD) as an unexpected death due to cardiac causes occurring in a short time (generally within 1 hr of symptom onset) in a person with known or unknown cardiac disease. We have defined hemodynamically significant ventricular arrhythmia as ventricular arrhythmia associated with syncope (defined as loss of consciousness) or documented hypotension (systolic BP < 90 mmHg) during the dysrhythmia.
Table 4.
Primary and secondary endpoints
| Primary endpoints |
|
| Secondary endpoints |
|
The secondary outcomes consist of sudden cardiac death, hemodynamically significant VT, all‐cause mortality, quality of life assessed by Minnesota Living with Heart Failure Questionnaire, heart failure symptoms, heart failure‐related hospitalizations, and a cost‐utility analysis.
The cost‐utility analysis will estimate the cost per quality‐adjusted life year (QALY) of the intervention over standard care. Costs will be assessed in terms of procedural cost, hospital admission, and readmission cost and ambulatory care cost within the context of an Australian Refined Diagnostic Group (AR‐DRG) weight. Nonhospital medical‐based services and drugs over the average 4‐year follow‐up will be estimated from Medicare data and the Pharmaceutical Benefits Schedule.
QALYs will be estimated from patient level measures of utility derived from the EQ‐5D instrument at each time point and integrated with survival curves to estimate quality‐adjusted life years in each trial arm. Based on the differences in costs and QALYs between the two arms, the incremental cost per QALY gained will be estimated.
2.9. Investigations
2.9.1. CMR scanning
There are two routes in which patients will have a CMR. The majority of patients will be enrolled on clinical ejection fraction measurements by echocardiography, MUGA, SPECT etc. and then have CMR as part of the study. These patients will have their study CMR reimbursed by the trial budget and will not have the scan paid for by hospital/health insurance. However, patients who have a clinical CMR which fulfills criteria can be enrolled via a clinical route and CMR not repeated (provided the clinical CMR is in last 2 months prior to enrollment). This is expected to be a minority of patients.
All scans will be performed on 1.5 or 3T scanners from a variety of vendors (Phillips, Siemens, and General Electric). Images will be acquired using phase array surface coils, ECG gating, and expiratory breath‐hold imaging. Cine images will be performed initially in long axis views including horizontal long axis (HLA), vertical long axis (VLA), left ventricular outflow tract (LVOT), and contiguous short axis slices that encompass the entire LV from base to apex. Tissue characterization of the LV for T1 mapping and late gadolinium enhancement (LGE) will be performed in matching short axis slices. T1 mapping will only be performed at selected sites using Siemens (1.5 T scanner), utilizing the Shortened Modified Look‐Locker Inversion recovery (ShMOLLI) sequence before administration of contrast. The acquisition parameters have been previously published (Piechnik et al., 2010). In brief, ShMOLLI T1‐maps are based on 5–7 images with specific TI~100–5000 ms, collected using SSFP readouts in a single breath‐hold, typically: TR/TE~201.32/1.07 ms, flip angle = 35°, matrix = 192 × 144, 107 phase encoding steps, interpolated voxel size = 0.9 × 0.9 × 8 mm, cardiac delay time TD = 500 ms; 206 ms acquisition time for single image. LGE images will then be carried out 6 min after injection of 0.10 mmol/kg of a 1 molar gadolinium‐based contrast agent using a T1‐weighted inversion gradient echo sequence. Images are to be acquired sequentially in the short axis, followed by long axis views. If any hyperenhancement is observed, all short‐axis slices are to be phase swapped. A single mid‐ventricular short axis slice, in the same position as the mid ventricle native T1 slice, will be acquired for post contrast T1 maps at 15 min after the administration of contrast as has been previously published (White et al., 2013).
2.9.2. CMR image analysis
CMR analysis will be performed at the CMR Core Lab South Australian Health and Medical Research Institute (SAHMRI), Adelaide, Australia. Images would be transferred to the core lab in digital form by being uploaded using the Filezilla Client system https://filezilla-project.org/. This will be accompanied by an Electronic Image Acquisition form. For sites that have firewall restrictions imposed by their hospitals, data will be accepted on CD‐ROMs that are express posted to the Core Lab. Sites will be notified of potential qualification by the core lab within 72 hr. Randomization will not be performed by the Core Lab.
Analysis will be performed with dedicated computer software (CMR42, Release 5.1, Circle Cardiovascular Imaging, Calgary, AB, Canada). Functional data will be assessed using the cine images, based on previously published criteria (Alfakih et al., 2004). In brief, endocardium and epicardium are contoured in end‐diastolic and end‐systolic phase to ascertain end‐diastolic (EDV) and end‐systolic volumes (ESV) and subsequently LV stroke volume (SV), ejection fraction (EF), and myocardial mass (MM) are calculated. All parameters will be indexed to body surface area.
For ischemic LV dysfunction; the LV cavity is divided into 17 segments according to a standardized model (Cerqueira et al., 2002). Regional wall motion (RWM) will be analyzed by a separate, experienced observer blinded to the LGE finding in accordance with previously published criteria (Selvanayagam et al., 2004).
Short axis images from native T1 maps will be manually contoured using third party software, specifically designed for analysis of ShMOLLI‐based T1 maps; software MC‐ROI (programmed by S.K.P. in Interactive Data Language, version 6.1, Exelis Visual Information Solutions, Boulder, CO, USA) (Ferreira et al., 2013). Specifically, the outline of the endocardium and epicardium will be obtained, and then divided into six segments per slice using the anterior right ventricular–left ventricular insertion point as reference and for comparing segments among sequences. Only regions of myocardium with a contiguous area of ≥40 mm2 above the specified thresholds will be considered relevant to minimize effects of noise as positive findings. All slices will be matched for cine images, precontrast T1 maps, and LGE images.
In evaluation of infarct size, using LGE images, a region of interest (ROI) will be placed within the hyperintense myocardium and used to define the maximum signal for full width half maximum (FWHM) threshold. The size of the ROI is dictated by the boundaries of the area to avoid any endocardium and epicardium surfaces. Furthermore, a manual correction will be made to exclude any hyperintense pixels that have been incorporated as part of blood pool, pericardial partial voluming, or artifact. Infarct size is to be quantified in each short axis and summed to provide total infarct size. Furthermore, areas of LGE will be graded in transmural extent (0, no LGE; 1, 1–25% LGE; 2, 26–50% LGE; 3, 51–75% LGE; and 4, ≥76% LGE) and quantified by use of computer‐assisted planimetry on each of the short axis images.
For evaluation of nonischemic pattern of hyperenhancement (midwall or subepicardial), two independent observers will evaluate each short axis slice as has been previously published (Gulati et al., 2013). Presence of scar should be visualized in at least two orthogonal planes by two experienced CMR readers. A third blinded observer will adjudicate cases of disagreement. Quantification of hyperenhancement will be performed utilizing the FWHM method as was described by Gulati et al., (2013).
2.10. Cardiac biomarkers
After enrollment, patients in both the device arm and the observational registry will have baseline N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) and high sensitivity Troponin T (hsTnT) values measured. In addition, serum markers of fibrosis (MMP and TIMPs) will be measured. This will allow external validity of our patient population, and as risk predictors of SCD (Hussein et al., 2013; Scott et al., 2009) will help to ensure successful randomisation of patients with myocardial fibrosis to device therapy.
2.11. Data collection and monitoring
A centralized data management center will be based at the George Institute, Sydney Australia. An independent clinical events committee (CEC) will review and adjudicate all primary endpoint events and selected secondary endpoints in a blinded fashion based on study definitions. The primary objective of the CEC will be to manage the process of coordinating independent review and adjudication of suspected endpoints. Primary and specified secondary endpoint events that are adjudicated will require sites to complete specific event case report forms (CRFs) and provide source documents. An additional independent committee, the Device Data Committee (DDC), will adjudicate all device downloads (ILR and ICD). Data on sustained and nonsustained atrial and ventricular arrhythmia and appropriate and inappropriate ICD therapies will be collected in a prospective manner.
2.12. Study organization
Responsibility for the study will reside with the study Steering Committee, comprised of the chief investigators. The study will comply with the International Conference on Harmonization and Good Clinical Practice standards, and will be reported in accordance with the 2010 CONSORT guidelines. The study steering committee will be the main policy and decision‐making committee for the study and will meet by teleconference on a quarterly basis. The clinical trial coordination and the Data Management Center will be located at SAHMRI and the chief investigator will coordinate activities across all the participating sites.
The authors are solely responsible for the design and conduct of the study, all analysis, the drafting and editing of this article, and its final contents. Funding of the study will be provided by the South Australian Health and Medical Research Institute (SAHMRI) and all devices (ICD and ILR) will be provided by BIOTRONIK.
2.13. Statistical considerations and data analysis
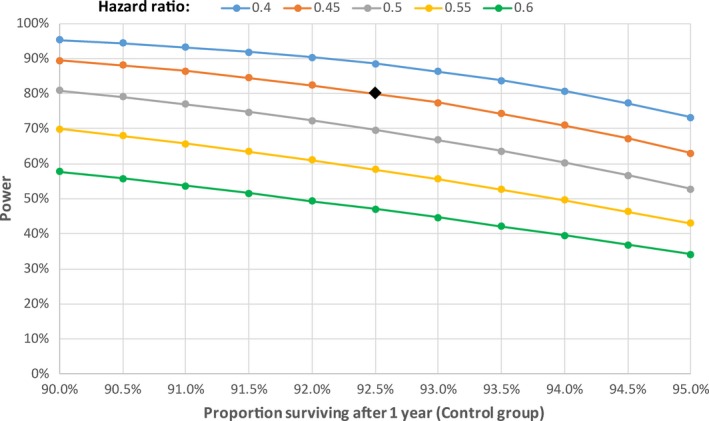
In total, 1055 patients (assuming a 10% dropout) will be the recruitment target consisting of 428 patients in the device‐group and 521 patients in the registry group. These sample size calculations are based on achieving 80% power. See Figure 1 for an illustration of participants flow through the trial. We estimate 7.5% annual rate of the primary endpoint (Gulati et al., 2013) and have made a conservative estimate of hemodynamically significant ventricular arrhythmia of 3.5%, based on observational and interventional ICD trials (Abello et al., 2006; Wilkoff et al., 2008). We have conservatively estimated a rate of SCD/aborted SCD at 4% per year. The power calculations for different hazard ratios and event rates is shown in Figure 2.
Figure 2.
Power calculations for primary endpoint based on different hazard ratios
In the Gulati et al. study, (2013) the rate of SCD or aborted SCD in patients with NICM and myocardial fibrosis was 5.2% per year. Syncope is common in the heart failure population, with an incidence of up to 6.8% per year, and is associated with a poor outcome (Bansch et al., 1998; Middlekauff et al., 1993). Although the etiology can be multifactorial (Cerqueira et al., 2002), it is often related to ventricular arrhythmia (Middlekauff et al., 1993; Pires et al., 2000).
We predict a hazard ratio of the primary endpoint of 0.45 in the ICD group compared with the ILR group. Previous ICD trials such as MADIT II have shown a hazard ratio reduction of similar or higher degree for SCD (HR 0.33 for SCD in MADIT II) (Greenberg et al., 2004). It seems reasonable to assume that although the incidence of SCD and hemodynamically significant ventricular arrhythmia may be lower in our patient population, the effect size will be similar. In total, this gives an estimated raw sample size of 246. CMR GUIDE is an endpoint‐driven trial and patients in the ILR group who have aborted SCD or who have hemodynamically significant ventricular arrhythmia (SBP <90 or syncope) will have met the primary endpoint and will receive an ICD as per standard practice. The presence of NSVT is not an indication for ICD and therefore we will encourage investigators not to crossover to ICD therapy. However, we recognize that in some patients with VT who do not have hemodynamic compromise may be offered an ICD. As such we have assumed a drop‐in rate of 20%, producing an adjusted sample size of 384. Assuming a further loss‐to‐follow‐up rate of 10%, the final adjusted sample size is 428. To achieve appropriate power for the primary endpoint of sudden cardiac death or syncope to ventricular arrhythmia, we plan recruitment over 2 years with an average follow‐up of 4 years.
Statistical analysis will be performed on an intention‐to‐treat basis. The main analysis of the time to SCD or ventricular arrhythmia leading to syncope will be performed using the log‐rank test. Also, competing risk approaches will also be used to account for deaths from other causes and the following baseline covariates will be used for prespecified adjustment of the treatment effect: age quartile, gender, heart failure etiology, and baseline LVEF.
3. Discussion
CMR GUIDE will add substantially to our understanding of the role of myocardial fibrosis in patients with mild to moderate LV dysfunction and the risk of developing life threatening ventricular arrhythmias. This subgroup represents the majority of patients who succumb to SCD in the community, and are currently excluded from primary preventative ICD therapy. The presence of myocardial late gadolinium enhancement (LGE) on CMR is used to identify highly specific patterns of fibrosis and scarring in many of the heart failure states (Leong et al., 2012; McCrohon et al., 2003; Selvanayagam et al., 2004, 2005). Potentially, the most significant application of the CMR in patients with cardiomyopathy is its emerging role in determining prognosis. Recent studies suggesting the presence of LGE in NICM patients might be associated with adverse clinical outcomes above traditional risk factors (Assomull et al., 2006; Wu et al., 2008). While the study by Wu et al., (2008) examined only patients with a LVEF <35%, a study by Assomull et al., (2006) suggested that CMR LGE may have prognostic implications for NICM patients with a LVEF 36–50%.
The power calculations for CMR GUIDE are based on a longitudinal study of 472 patients with nonischemic cardiomyopathy referred for CMR imaging between November 2000 and December 2008. The study found that both the presence and extent of fibrosis were independently associated with SCD or aborted SCD (Gulati et al., 2013) with a median ejection fraction of 37.2% and a median follow‐up of 5.3 years. Among the 142 patients with midwall fibrosis, there were 38 deaths (26.8%) versus 35 deaths (10.6%) among the 330 patients without fibrosis (hazard ratio [HR], 2.96 [95% CI, 1.87–4.69]; absolute risk difference, 16.2% [95% CI, 8.2–24.2%]; p < .001). The arrhythmic composite of SCD or aborted SCD was reached by 42 patients with fibrosis (29.6%) and 23 patients without fibrosis (7.0%) (HR, 5.24 [95% CI, 3.15–8.72]; absolute risk difference, 22.6% [95% CI, 14.6–30.6%]; p < .001).
Among patients with a LVEF of 36–50%, LGE was present in 48 (22%) of the patients and during follow‐up 24 (11%) of the cohort achieved the endpoint of SCD/aborted SCD. There were 15 (31.3%) events within the LGE‐positive group as compared to 9 (5.3%) in the LGE‐negative group. (HR, 6.38; 95% CI 2.79 to 14.59 p < .0001. (S. Prasad, unpublished data). After adjustment for LVEF and other conventional prognostic factors, both the presence and extent of fibrosis was independently associated with SCD or aborted SCD (by fibrosis presence: HR, 4.61 [95% CI, 2.75–7.74], p.001; and by fibrosis extent HR, 1.10 [95% CI, 1.05–1.16], p < .001).
It is likely that the rates of SCD or aborted SCD in patients with CAD‐related heart failure and LGE would be even higher than that seen in NICM (Kwong et al., 2006). A recent meta‐analysis and review of the literature of this area by Zemrak et al. indicates that the presence of LGE on CMR is a strong predictor of mortality and major adverse events (major adverse cardiac events (MACE), sudden death, nonfatal MI, and new or worsening HF) in patients with coronary artery disease (Zemrak & Petersen, 2011). Meta‐analysis of available prospective studies showed that the presence of LGE on CMR increases both the hazards of death and MACE by almost four times in patients with coronary artery disease (Zemrak & Petersen, 2011). With increasing size of LGE (per gram or percent), the hazard ratio for death and MACE increased by 4% and 5%, respectively (18, 37, 39). In addition, the spatial extent of LGE is associated with increased hazard for mortality and MACE (Wu et al., 2008).
One study, “Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation” (DETERMINE), has attempted to prospectively randomize patients with mild to moderate heart failure to ICD versus standard therapy. (Kadish et al., 2009) This trial however failed to meet its recruitment target and was subsequently discontinued. CMR GUIDE differs from DETERMINE in that it will include patients with nonischemic cardiomyopathy (NICM), which will broaden the eligibility for enrollment and also improve the generalizability of our findings. Furthermore, we will be using different CMR criteria for identification of myocardial fibrosis in patients with CAD. DETERMINE recruited patients with LGE >10% LV mass which we believe has no evidential basis and the absolute LGE quantification in grams is more technically challenging and would likely preclude widespread implementation of the study findings into clinical practice.
There is wide variability in the literature in assessing and reporting the degree of LGE in patients with coronary artery disease. As reviewed by Zemrak & Petersen, (2011), prior studies have reported on infarct mass (Klem et al., 2012), relative infarct mass (% of total LV mass), number of segments with LGE, infarct transmurality, and infarct heterogeneity. In our study, we will recruit patients with CAD who have transmural (>75% of wall thickness) LGE in two or more myocardial segments. This is based on two recent studies (Desjardins et al., 2009; Scott et al., 2013) demonstrating that in patients with ischemic LV dysfunction scheduled for primary prevention ICD therapy, an increasing number of myocardial segments with >75% transmural LGE is associated with increased risk for life‐threatening arrhythmias and cardiac death. This was especially so in patients with two or more transmural segments. A recent study by Desjardins et al., (2009) fused electroanatomic mapping results with CMR images in postinfarct patients with recurrent ventricular arrhythmias and showed that electric instability was particularly increased in scar regions with high transmural involvement and large infarct cores. Hence, although both infarct size and transmurality are important in defining risk, infarct transmurality is an easier parameter to measure clinically and is therefore likely to be more robust. Hence, we will use infarct transmurality as the primary criteria for inclusion into the trial for CAD patients, but will nevertheless quantify infarct size in our secondary analysis. The latter analysis may identify patients at greater or lesser risk, and be hypotheses generating for future trials.
4. Summary
CMR GUIDE is a large prospective multicenter randomized trial of CMR‐guided primary prevention ICD insertion versus standard therapy in patients with mild to moderate left ventricular systolic dysfunction on optimal medical therapy. Our findings have the potential to significantly transform clinical practice with identification of a subgroup of patients at high risk for SCD, currently ineligible for defibrillator therapy under current society guidelines.
4.1. Trial status
Recruitment commenced in July 2015.
Conflict of Interest
None.
Funding
Devices in CMR GUIDE (ICD and ILR) are funded by BIOTRONIK SE & Co. KG (Woermannkehre 1, 12359 Berlin, Germany). The study design, collection, management, analysis, and interpretation of data, writing of the report, and decision to submit for publication will be made independently of BIOTRONIK. Ultimate authority of these activities resides with the chief investigator of J.S.
Authors’ Contribution
JS conceived the study and is the principle‐coordinating researcher. All authors have made substantial contribution to the design and analysis of this work and have approved the final manuscript.
Acknowledgment
Dr James Gunton ‐ Flinders Medical Centre, S.A, Australia 5042.
Selvanayagam JB, Hartshorne T, Billot L, et al. Cardiovascular magnetic resonance GUIDEd management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): Study protocol for a randomized controlled trial. Ann Noninvasive Electrocardiol. 2017;22:e12420 10.1111/anec.12420
Funding information
Devices in CMR GUIDE (ICD and ILR) are funded by BIOTRONIK SE & Co. KG (Woermannkehre 1, 12359 Berlin, Germany).
References
- Abello, M. , Merino, J. L. , Peinado, R. , Gnoatto, M. , Arias, M. A. , Gonzalez‐Vasserot, M. , & Sobrino, J. A. (2006). Syncope following cardioverter defibrillator implantation in patients with spontaneous syncopal monomorphic ventricular tachycardia. European Heart Journal, 27, 89–95. [DOI] [PubMed] [Google Scholar]
- Alfakih, K. , & Sivananthan, M . (2004). Assessment of ventricular function and mass by cardiac magnetic resonance imaging. European Radiology, 14(10), 1813–1822. [DOI] [PubMed] [Google Scholar]
- Assomull, R. G. , Prasad, S. K. , Lyne, J. , Smith, G. , Burman, E. D. , Khan, M. , … Pennell, D. J. (2006). Cardiovascular magnetic reonance, fibrosis, and prognosis in dilated cardiomyopathy. Journal of the American College of Cardiology, 48(10), 1977–1985. [DOI] [PubMed] [Google Scholar]
- Bansch, D. , Brunn, J. , Castrucci, M. , Weber, M. , Gietzen, F. , Borggrefe, M. , … Block, M. (1998). Syncope in patients with an implantable cardioverter‐defibrillator: Incidence, prediction and implications for driving restrictions. Journal of the American College of Cardiology, 31(3), 608–615. [DOI] [PubMed] [Google Scholar]
- Cerqueira, M. D. , Weissman, N. J. , Dilsizian, V. , Jacobs, A. K. , Kaul, S. , Laskey, W. K. , … Registration for Cardiac, I . (2002). Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation, 105(4), 539–542. [DOI] [PubMed] [Google Scholar]
- Dagres, N. , & Hindricks, G. (2013). Risk stratification after myocardial infarction: Is left ventricular ejection fraction enough to prevent sudden cardiac death? European Heart Journal, 34(26), 1964–1971. [DOI] [PubMed] [Google Scholar]
- Desjardins, B. , Crawford, T. , Good, E. , Oral, H. , Chugh, A. , Pelosi, F. , … Bogun, F. (2009). Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm, 6(5), 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, A. E. , DiMarco, J. P. , Ellenbogen, K. A. , Estes, N. A., 3rd , Freedman, R. A. , Gettes, L. S. , … Heart Rhythm, S. (2013). 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation, 27(3), 283–352. [DOI] [PubMed] [Google Scholar]
- Ferreira, V. M. , Piechnik, S. K. , Dall'Armellina, E. , Karamitsos, T. D. , Francis, J. M. , Ntusi, N. ,… Neubauer, S. (2013). T(1) mapping for the diagnosis of acute myocarditis using CMR: Comparison to T2‐weighted and late gadolinium enhanced imaging. JACC: Cardiovascular Imaging, 6(10), 1048–1058. [DOI] [PubMed] [Google Scholar]
- Greenberg, H. , Case, R. B. , Moss, A. J. , Brown, M. W. , Carroll, E. R. , Andrews, M. L. , & Investigators, M.‐I. (2004). Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT‐II). Journal of the American College of Cardiology, 43(8), 1459–1465. [DOI] [PubMed] [Google Scholar]
- Gulati, A. , Jabbour, A. , Ismail, T. F. , Guha, K. , Khwaja, J. , Raza, S. , … Prasad, S. K. (2013). Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA, 309(9), 896–908. [DOI] [PubMed] [Google Scholar]
- Hussein, A. A. , Gottdiener, Nartz. T. M. , Bartz, T. M. , Sotoodehnia, N. , deFilippi, C. , Dickfeld, T. ,… Prasad, S. K. (2013). Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: The Cardiovascular Health Study. Journal of the American College of Cardiology, 62, 2112–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish, A. H. , Bello, D. , Finn, J. P. , Bonow, R. O. , Schaechter, A. , & Subacius, H. , … Goldberger, J. J. (2009). Rationale and design for the Defibrillators to reduce risk by Magnetic Resonance Imaging Evaluation (DETERMINE) trial. Journal of Cardiovascular Electrophysiology, 20(9), 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem, I. , Weinsaft, J. W. , Bahnson, T. D. , Hegland, D. , Kim, H. W. , Hayes, B. , … Kim, R. J. (2012). Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. Journal of the American College of Cardiology, 60(5), 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, M. H. , Fonarow, G. C. , Peterson, E. D. , Curtis, A. B. , Hernandez, A. F. , Sanders, G. D. , … Al‐Khatib, S. M. (2011). Systematic review of the incidence of sudden cardiac death in the United States. Journal of the American College of Cardiology, 57(7), 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, R. Y. , Chan, A. K. , Brown, K. A. , Chan, C. W. , Reynolds, H. G. , Tsang, S. , & Davis, R. B. (2006). Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event‐free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation, 113(23), 2733–2743. [DOI] [PubMed] [Google Scholar]
- Leong, D. P. , Chakrabarty, A. , Shipp, N. , Molaee, P. , Madsen, P. L. , Joerg, L. , … Selvanayagam, J. B. (2012). Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new‐presentation idiopathic dilated cardiomyopathy: Insights from cardiovascular magnetic resonance and echocardiography. European Heart Journal, 33(5), 640–648. [DOI] [PubMed] [Google Scholar]
- Makikallio, T. H. , Barthel, P. , Schneider, R. , Bauer, A. , Tapanainen, J. M. , Tulppo, M. P. , … Huikuri, H. V. (2005). Prediction of sudden cardiac death after acute myocardial infarction: Role of Holter monitoring in the modern treatment era. European Heart Journal, 26(8), 762–769. [DOI] [PubMed] [Google Scholar]
- McCrohon, J. A. , Moon, J. C. C. , Prasad, S. K. , McKenna, W. J. , Lorenz, C. H. , Coats, A. J. S. , & Pennell, D. J. (2003). Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium‐enhanced cardiovascular magnetic resonance. Circulation, 108, 54–59. [DOI] [PubMed] [Google Scholar]
- Middlekauff, H. R. , Stevenson, W. G. , Stevenson, L. W. , & Saxon, L. A. (1993). Syncope in advanced heart failure: High risk of sudden death regardless of origin of syncope. Journal of the American College of Cardiology, 21(1), 110–116. [DOI] [PubMed] [Google Scholar]
- Moss, A. J. , Hall, W. J. , Cannom, D. S. , Daubert, J. P. , Higgins, S. L. , Klein, H. , … Heo, M. (1996). Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. New England Journal of Medicine, 335(26), 1933–1940. [DOI] [PubMed] [Google Scholar]
- Moss, A. J. , Schuger, C. , Beck, C. A. , Brown, M. W. , Cannom, D. S. , Daubert, J. P. … Investigators, M.‐R. T. (2012). Reduction in inappropriate therapy and mortality through ICD programming. New England Journal of Medicine, 367(24), 2275–2283. [DOI] [PubMed] [Google Scholar]
- Narayanan, K. , Reinier, K. , Uy‐Evanado, A. , Teodorescu, C. , Chugh, H. , Marijon, E. , … Chugh, S. S. (2013). Frequency and determinants of implantable cardioverter defibrillator deployment among primary prevention candidates with subsequent sudden cardiac arrest in the community. Circulation, 128(16), 1733–1738. [DOI] [PubMed] [Google Scholar]
- Piechnik, S. K. , Ferreira, V. M. , Dall'Armellina, E. , Cochlin, L. E. , Greiser, A. , Neubauer, S. , & Robson, M. D. (2010). Shortened Modified Look‐Locker Inversion recovery (ShMOLLI) for clinical myocardial T1‐mapping at 1.5 and 3 T within a 9 heartbeat breathhold. Journal of Cardiovascular Magnetic Resonance, 12, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires, L. A. , May, L. M. , Ravi, S. , Parry, J. T. , Lal, V. R. , & Nino, C. L. (2000). Comparison of event rates and survival in patients with unexplained syncope without documented ventricular tachyarrhythmias versus patients with documented sustained ventricular tachyarrhythmias both treated with implantable cardioverter‐defibrillators. American Journal of Cardiology, 85(6), 725–728. [DOI] [PubMed] [Google Scholar]
- Scott, P. A. , Barry, J. , Roberts, P. R. , & Morgan, J. M. (2009). Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: A meta‐analysis. European Journal of Heart Failure, 11(1), 958–966. [DOI] [PubMed] [Google Scholar]
- Scott, P. A. , Rosengarten, J. A. , Murday, D. C. , Peebles, C. R. , Harden, S. P. , Curzen, N. P , & Morgan, J. M. (2013). Left ventricular scar burden specifies the potential for ventricular arrhythmogenesis: An LGE‐CMR study. Journal of Cardiovascular Electrophysiology, 24(4), 430–436. [DOI] [PubMed] [Google Scholar]
- Selvanayagam, J. B. , Kardos, A. , Francis, J. M. , Wiesmann, F. , Petersen, S. E. , Taggart, D. P. , & Neubauer, S. (2004). Value of delayed‐enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation, 110(12), 1535–1541. [DOI] [PubMed] [Google Scholar]
- Selvanayagam, J. B. , Porto, I. , Channon, K. , Petersen, S. E. , Francis, J. M. , Neubauer, S. , & Banning, A. P. (2005). Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: Insights from cardiovascular magnetic resonance maging. Circulation, 111(8), 1027–1032. [DOI] [PubMed] [Google Scholar]
- Soliman, O. I. , Theuns, D. A. , van Dalen, B. M. , Vletter, W. B. , Nemes, A. , Jordaens, L. … Geleijnse, M. L. (2010). Prediction of appropriate defibrillator therapy in heart failure patients treated with cardiac resynchronization therapy. American Journal of Cardiology, 105(1), 105–111. [DOI] [PubMed] [Google Scholar]
- de Vreede‐Swagemakers, J. J. , Gorgels, A. P. , Dubois‐Arbouw, W. I. , van Ree, J. W. , Daemen, M. J. , Houben, L. G. , & Wellens, H. J. (1997). Out‐of‐hospital cardiac arrest in the 1990's: A population‐based study in the Maastricht area on incidence, characteristics and survival. Journal of the American College of Cardiology, 30(6), 1500–1505. [DOI] [PubMed] [Google Scholar]
- Weisfeldt, M. L. , & Becker, L. B. (2002). Resuscitation after cardiac arrest: A 3‐phase time‐sensitive model. JAMA, 288(23), 3035–3038. [DOI] [PubMed] [Google Scholar]
- White, S. K. , Sado, D. M. , Fontana, M. , Banypersad, S. M. , Maestrini, V. , Flett, A. S. , … Moon, J. C. (2013). T1 mapping for myocardial extracellular volume measurement by CMR: Bolus only versus primed infusion technique. JACC: Cardiovascular Imaging, 6(9), 955–962. [DOI] [PubMed] [Google Scholar]
- Wilkoff, B. L. , Williamson, B. D. , Stern, R. S. , Moore, S. L. , Lu, F. , Lee, S. W. , … Investigators, P. S. (2008). Strategic programming of detection and therapy parameters in implantable cardioverter‐defibrillators reduces shocks in primary prevention patients: Results from the PREPARE (Primary Prevention Parameters Evaluation) study. Journal of the American College of Cardiology, 52(7), 541–550. [DOI] [PubMed] [Google Scholar]
- Wu, K. C. , Weiss, R. G. , Thiemann D. R., Kitagawa, K. , Schmidt, A. , Dalal, D. , … Lima, J. A. (2008). Late gadolinium enhancement by cardiovascular magnetic resonance heralds adverse prognosis in nonischemic cardiomyopathy. Journal of the American College of Cardiology, 54, 2414–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemrak, F. , & Petersen, S. E. (2011). Late gadolinium enhancement CMR predicts adverse cardiovascular outcomes and mortality in patients with coronary artery disease: Systematic review and meta‐analysis. Progress in Cardiovascular Diseases, 54(3), 215–229. [DOI] [PubMed] [Google Scholar]


