Abstract
Background
The use of heart rate variability as a tool capable of discriminating individuals with diabetes mellitus is still little explored, as its use has been limited to comparing those with and without the disease. Thus, the purpose of this study was to verify the use of heart rate variability as a tool for diagnostic and prognostic evaluation in person with diabetes and to identify whether there are cutoff points generated from the use of this tool in these individuals.
Methods
A search was conducted in the electronic databases MEDLINE, Cochrane Library, Web of Science, EMBASE, and LILACS starting from the oldest records until January 2015, by means of descriptors related to the target condition, evaluated tool, and evaluation method. All the studies were evaluated for methodological quality using the QUADAS‐2 instrument.
Results
Eight studies were selected. In general, the studies showed that the heart rate variability is useful to discriminate cardiac autonomic neuropathy in person with diabetes, and the sample entropy, SD1/SD2 indices, SDANN, HF, and slope of TFC have better discriminatory power to detect autonomic dysfunction, with sensitivity and specificity values ranging from 72% to 100% and 71% to 97%, respectively.
Conclusion
Although there are methodological differences in indices used, in general, this tool demonstrated good sensitivity and specificity and can be used as an additional and/or complementary tool to the conventional autonomic tests, in order to obtain safer and more effective diagnostic, collaborating for better risk stratification conditions of these patients.
Keywords: diabetes mellitus, autonomic nervous system, sensitivity and specificity, heart rate, electrocardiography
Heart rate variability (HRV) is a simple and noninvasive tool that describes the oscillations in the intervals between consecutive heart beats (RR intervals), which are related to the influences of the autonomic nervous system (ANS) on the sinus node, allowing assessment of ANS1 behavior. ANS is altered in several pathological conditions, such as acute myocardial infarction,2 hypertension,3 coronary insufficiency,4 chronic obstructive pulmonary disease,5 and diabetes mellitus (DM).6
In individuals with DM, alterations in autonomic behavior can be related to the presence of autonomic neuropathy (altered behavior of the autonomic functions linked to DM).7, 8 These individuals can present cardiac autonomic neuropathy (CAN), which causes abnormalities in the control of heart rate and cardiovascular dynamics due to damage to the autonomic nerve fibers that innervate the heart and blood vessels.9 This condition can be assessed using various methods, including HRV.
In subjects with DM, linear analysis through HRV has indicated that there is an overall reduction in both the ANS components,6 or a reduction in the parasympathetic component with increased sympathetic,7 when compared with healthy individuals, indicating a loss of cardiac autonomic modulation in these individuals.10 In addition, studies assessing HRV through nonlinear methods demonstrate that there is a reduction in the magnitude and complexity of the control mechanisms of heart rate in subjects with DM.11, 12
In addition, overall HRV in different age groups has been shown to be reduced in type 1 DM compared with healthy subjects,6, 8, 13, 14 presenting autonomic impairment in the early stages and progressively worsening over the years.15 One possible reason for this is that constant high blood glucose values could influence the ANS.16
Despite some studies having demonstrated the effectiveness of HRV to assess and identify autonomic changes, its use in clinical practice is still incipient.13, 17, 18 Recently, HRV has been indicated as an important diagnostic and prognostic practical marker in different populations and situations19, 20 independent of other common cardiovascular risk factors, such as blood pressure and resting heart rate.
Côrrea et al.19 showed that the indices of total DFA, approximate entropy, and Lyapunov exponent obtained through nonlinear analysis of HRV were more sensitive and specific to differentiate cases of postoperative myocardial revascularization surgery that evolved with lung infection. Pivatelli et al.20 observed that the HF ms², RMSSD, NN50 indices, and the approximate entropy have better discriminatory power for the presence of significant coronary obstruction in patients with stable angina undergoing coronary angiography.
Heart rate variability is suggested as one of the autonomic tests used for clinical diagnosis, being a tool capable of evaluating the influence of changes in ANS produced by DM.21 The use of HRV offers assessment advantages as it is a simple, easy to use tool and does not require the effective cooperation of the patient.22 Conversely, conventional autonomic clinical tests, such as the Valsalva maneuver and heart rate response to postural change, require patient cooperation and often are not able to be executed due to the presence of comorbidities that are contraindications or influence the test result.23, 24
As reported in other studies, the use of sophisticated, easy to use instruments, such as HRV in comparison to conventional autonomic clinical trials, enables early diagnosis of autonomic dysfunction and cardiovascular involvement.19, 20 However, the use of HRV as a tool capable of discriminating individuals with DM is still little explored, as its use has been limited to comparing those with and without the disease.6, 7, 14
Information of this nature will contribute additional information to the theme, collaborating in better risk stratification conditions for these patients and awareness for elaboration of new treatment strategies. Moreover, by understanding the discriminatory power of this tool in DM, clinicians and researchers will be able to use it as an additional and/or complementary tool to the conventional autonomic tests proposed by Ewing,23 to achieve safer and more effective diagnosis. With regard to clinical practice, if it is a precise tool, it could contribute to preventive actions in the diagnosis of CAN, as this disease has been associated with higher mortality in patients.25
Thus, the objectives of this review were to seek studies that have used HRV as a tool for diagnostic and prognostic evaluation in patients with DM and to identify whether there are cutoff points generated from the use of this tool in these individuals.
Methods
Search Strategy
The studies were selected from research in the following databases: MEDLINE (via Ovid), Cochrane Central Register of Controlled Trials (via Ovid) EMBASE, LILACS (via Bireme), and Web of Science, starting from the oldest records until January 2015. For the search strategy, studies of accuracy were selected using the keywords in English (MeSH) related to the target condition (Diabetes Mellitus), evaluated tool (HRV), and evaluation method (Sensitivity and Specificity), as well as cross‐referencing performed with the Boolean operators “OR” and “AND” described in Table 1.
Table 1.
Presentation of the Terms Used and Cross‐Referencing Strategy Performed
|
When inserting the terms registered in MeSH into the Ovid platform, we obtained other corresponding terms as results (electrocardiography, ambulatory and tool use behavior), which were inserted into the search. This increase was carried out in the databases MEDLINE and Cochrane; all details of the strategy can be seen in Appendices 1 and 2, respectively. For the search realized in EMBASE and Web of Science, the cross‐referencing strategies contained in Appendices 3 and 4 were used, and for searching the LILACS database, the cross referencing of the three groups was performed, always encompassing the target condition versus evaluated tool versus evaluation method (Appendix 5).
All references were imported into a database (EndNote X7) to identify duplicate titles and to select potentially eligible articles to compose the review.
Inclusion Criteria
For the development of the research, the following inclusion criteria were stipulated: (1) be an accuracy study, determining the power of prognosis and diagnosis of HRV associated or not with another method through the analysis of sensitivity and specificity; (2) applied only in individuals with diabetes mellitus without restriction as to age, sex, or type of diabetes; (3) included independent of language and year of publication.
Exclusion Criteria
The following constituted exclusion criteria: (1) editorials, letters to the editor, case reports, review studies, and conference proceedings and (2) those which did not use HRV as a tool for analysis of the presence of diabetes and/or cardiac autonomic neuropathy.
Study Selection and Assessment of Methodological Quality
Titles that addressed: HRV, the prognostic power of this tool, sensitivity and specificity of the indices of HRV or methods associated with it, diagnostic accuracy, and risk predictor, as the main idea, were selected for this review. At the end of the search, repeated titles were excluded, as the search was realized in various databases, by two independent reviewers.
Next, detailed reading of the article abstracts was performed to select those that exclusively addressed the prognostic and diagnostic power of HRV, as well as the cutoff points obtained. Excluding the abstracts which did not deal with the topic, the full texts were evaluated and those which were not eliminated due to the exclusion criteria were included as the final result of the search. In addition, all references from the selected studies were reviewed to supplement the search. All search steps were performed by two independent evaluators, and in the event of disagreement, a third reviewer was invited to perform the evaluations, which was necessary in only one case.
The selected studies were also evaluated for their methodological quality, through a rating scale used in studies of diagnostic accuracy, called the Quality Assessment of Diagnostic Accuracy Studies (QUADAS)26 (Appendix 6) and adopted by the Cochrane Library.
Data Extraction
From the results, information regarding the type of diabetes of the volunteers, presence of cardiac autonomic neuropathy, sample size, tool used to evaluate the autonomic modulation, indices evaluated by the tool, and sensitivity and specificity was extracted.
Analysis of Results
Data were described quantitatively and qualitatively and tabulated according to the authors and year of study, description of the population (sample size, type of diabetes, and age), studied variables (methods used to obtain the series of RR intervals and evaluated indices), and results obtained (cutoff points and value of sensitivity and specificity). In addition, the methodological quality of the studies was also evaluated.
Results
The electronic search resulted in 4297 references, from which 41 duplicate titles were excluded followed by 4239 references as they were not related to the theme proposed by the review. After this step, 17 full texts were selected as fitting the preestablished inclusion criteria. Of these abstracts, eight articles were selected to compose the final selection of this review. The selected articles were in English, either made available in full or through direct contact with the author. The search and selection process is described in Figure 1.
Figure 1.
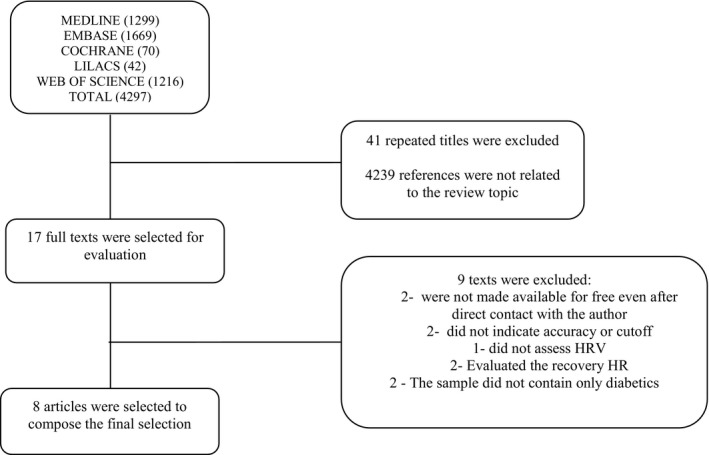
Flow diagram of the study inclusion process.
Table 2 shows the main results and conclusions of the studies included in this update.
Table 2.
Studies Using HRV as a Tool for Diagnosis in Diabetics
| Author and Year | Sample | Analysis | Parameters and Methods | Sensibility (%) | Specificity (%) | Conclusion |
|---|---|---|---|---|---|---|
| Takasae et al. (1992)27 | Diabetes: N = 25; DMT2; with CAN (13) 58 ± 11 years; without CAN (12) 49 ± 16 years |
ECG 24 hours |
Mean of SD of iRR (SDANN) | 72 | 92 | SDANN < 30 ms produced a better sensitivity and specificity for diabetics with CAN |
| Ziegler et al. (2001)28 |
Diabetes: N = 108; DMT1 (89); DMT2 (19), CAN (3 stages); 45.3 ± 1.4 years Control: N = 37, 41.1 ± 2.0 years |
Finapress 10 minutes |
LF, HF | – | – | HF is more sensitive to detect early autonomic dysfunction to a cutoff value of 0.892 |
| Balcioglu et al. (2007)29 | Diabetes: N = 90; DMT2; with CAN (35) 56 ± 9 years; CAN borderline (55) 56 ± 9 years |
ECG 24 hours |
SDNN, SDANN, RMSSD, triangular index, TFC of start and TFC tilt | 97 | 71 | All indices were reduced in individuals with CAN. TFC of tilt is better sensitivity and specificity for detection of CAN at a cutoff value of 3.32. |
| Khandoker et al. (2009)30 | Diabetes: N = 17; DMT2; with CAN (9) 52 ± 12 years; without CAN (8) 56 ± 14 years |
ECG 20 minutes |
SDNN, RMSSD, LFun, HFun, LF/HF, SD1, SD2, SD1/SD2 and SampEn | 100 | 75 | SampEn and SD1/SD2 produced better sensitivity and specificity to distinguish between diabetics with and without CAN |
| Acharya et al. (2011)31 |
Diabetes: N = 15; 58.5 ± 6.42 years, CAN unknown Control: N = 15; 50 ± 8.8 years |
ECG 60 minutes |
Correlation dimension, Poincaré geometry, Recurrence plot/AdaBoost and SVM | 87.5 | 84.6 | The AdaBoost classifier obtained better accuracy to diagnose DM. The index DII was proposed based on the results to discriminate diabetic neuropathy. |
| Seyd et al. (2012)32 |
Diabetes: N = 70; DMT2; NAC unknown Control: N = 65 Age varied from 40 to 72 years for both groups |
ECG 60 minutes |
RMSSD, NN50, TINN, triangular index, SDNN, PNN50, LF, HF, VLF/artificial neural network | 89.23 | 96.92 | Training of artificial neural network using as base indices of HRV has good sensitivity and specificity to distinguish DM and healthy individuals |
| Acharya et al. (2013)33 |
Diabetes: N = 15; 58.5 ± 6.42 years, CAN unknown Control: N = 15; 50 ± 8.8 years |
ECG 60 minutes |
Recurrence plot, ApEn, DFA, Lyapunov exponet/AdaBoost, DT, FSC, k‐NN, PNN and SVM |
92.52 | 88.73 | The AdaBoost classifier obtained better performance than other classifiers to diagnose DM |
| Swapna et al. (2013)34 |
Diabetes: N = 15; 58.5 ± 6.42 years, CAN unknown Control: N = 15; 50 ± 8.8 years |
ECG 60 minutes |
Higher order spectra features/GMM, SVM, NBC, k‐NN, Fuzzy classifier, DT | 85.7 | 95.2 | The GMM classifier showed better accuracy than other classifiers to diagnose DM |
N = sample; DMT2 = type 2 diabetes mellitus; DMT1 = type 1 diabetes mellitus; CAN = cardiac autonomic neuropathy; ECG = electrocardiogram; SDANN = standard deviation of mean of normal RR intervals every 5 minutes for a period of time, expressed in ms; LF = low‐frequency component; HF = high‐frequency component; SDNN = standard deviation of all normal RR intervals recorded at an interval of time expressed in ms; RMSSD = square root of the mean of the square of differences between adjacent normal RR intervals, at an interval of time expressed in ms; TFC = heart rate turbulence; SD1 = standard deviation of the instantaneous variability beat to beat; SD2 = standard deviation of the long‐term variability; SampEn = Sample Entropy; NN50 = adjacent RR intervals with difference of duration of 50 ms; TINN = triangular interpolation of RR intervals; PNN50 = percentage of adjacent RR intervals with difference of duration greater than 50 ms; VLF = very low‐frequency components; SVM = support vector machine; ApEn = approximate entropy; DFA = detrended fluctuation analysis; DT = decision tree; FSC = fuzzy Sugeno classifier; k‐NN = k‐nearest neighbor algorithm; PNN = probabilistic neural network; GMM = Gaussian Mixture Model; NBC = NaïveBayes classifier; DII = diabetes integrated index.
The results obtained from the analysis of the methodological quality of the studies in this review are presented in Table 3.
Table 3.
Evaluation of the Methodological Quality of the Studies Collected in this Review Using the Criteria Established by QUADAS‐2 Tool
| Risk of Bias | Concern as to the Applicability | ||||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Takasae et al. (1992)27 | High | High | High | Uncertain | Low | Low | Uncertain |
| Ziegler et al. (2001)28 | High | High | Low | Uncertain | Low | Low | Low |
| Balcioglu et al. (2007)29 | High | High | Low | Low | Low | Low | Low |
| Khandoker et al. (2009)30 | High | Low | Low | Low | Low | Low | Low |
| Acharya 201131 | High | Uncertain | Uncertain | Uncertain | Low | Low | Uncertain |
| Seyd et al. (2012)32 | High | Low | High | Uncertain | Low | Low | Uncertain |
| Acharya et al. (2013)33 | High | Uncertain | Uncertain | Uncertain | Low | Low | Uncertain |
| Swapna et al. (2013)34 | High | Uncertain | Uncertain | Uncertain | Low | Low | Uncertain |
Discussion
In general, the studies presented in this review27, 28, 29, 30, 31, 32, 33, 34 indicate that HRV presents a good tool to discriminate CAN in person with diabetes and that certain indices (Entropy Sample, SD1/SD2, SDANN, HF, Recurrence Plot, Approximate Entropy, DFA, Lyapunov exponent, correlation dimension, and TFC of tilt) have better discriminatory power to detect autonomic dysfunction, indicating that this method can be used as a diagnostic marker and practical prognosis tool in this population. Furthermore, the use of HRV indices as a gateway to automated classifiers can discriminate person with diabetes from those without diabetes.31, 32, 33, 34
Of the eight studies included in this review, four27, 28, 29, 30 evaluated patients with CAN using HRV and observed a reduction in the autonomic behavior in person with diabetes with CAN, which corroborates other studies.6, 11, 35
The presence of CAN in the studies of this review was determined through prior autonomic tests28, 29, 30 and application of questionnaires relating to signs and symptoms.27 The prior assessment of the presence of CAN enabled evaluation of individuals who already had autonomic dysfunction associated with DM, which was confirmed using HRV, showing that this tool is effective and can be used in clinical practice to detect CAN. Seyd et al.32 did not report whether individuals were evaluated for the presence of CAN in their study.
Diagnostic accuracy tests in relation to the use of HRV, associated or not with other methods, were performed in all studies in this review with the objective of evaluating whether it is a sensitive tool to help in the discrimination of individuals with CAN from those without CAN27, 28, 29, 30 and whether, from the HRV indices, it would be possible to make an automatic diagnosis of diabetes using linear indices as a basis.31, 32, 33, 34
Of the eight studies analyzed, five29, 31, 32, 33, 34 used other assessment methods associated with HRV measures and pointed out that the use of classifiers such as the artificial neural network,32 AdaBoost,31, 33 Gaussian Mixture Model,34 and Diabetes integrated index31 are sensitive to diagnose DM, and the use of heart rate turbulence parameters can be used with good diagnostic and prognostic value for detecting CAN in individuals with DM.29
Takasae et al.27 showed that the values of SDANN lower than 30 ms generated better sensitivity (72%) and specificity (92%) than values lower than 20 ms (31% sensitivity and 100% specificity) for detecting autonomic dysfunction and cardiac events in person with diabetes with CAN, since the cutoff of 30 ms was used without a significant loss in sensitivity. The authors also indicated that SDANN values lower than 30 ms can also be used as a reference to assess risk of death in other pathological conditions such as ischemic heart disease and congestive heart failure.
Another study30 pointed out that the sample entropy and Poincaré plot (SD1/SD2) were able to better distinguish person with diabetes with CAN from those without CAN, with sensitivity of 100% and specificity of 75%. The authors emphasize that an initial assessment without the use of HRV indices pointed out that nine of the 17 patients with diabetes had clinical signs of CAN; however, when the individuals were evaluated using HRV, three more individuals were identified for the presence of CAN, suggesting that these individuals could have been in the early stages of CAN which conventional autonomic tests were not effective to detect. The authors emphasize that HRV can be safely used in clinical practice to detect CAN, even in individuals without clinical signs of neuropathy, having been shown to be a more robust method of analysis, as it was able to identify individuals with CAN without previous signs of this manifestation, enhancing the evaluation. Thus, the CAN could be treated as early as possible, increasing the chances of successful treatment.
According to Ziegler et al.,30 the HF index was more sensitive to detect early autonomic dysfunction in person with diabetes with CAN in a phase where autonomic function tests were not. This can be explained, at least in part, because the HF index reflects the behavior of the parasympathetic autonomic nervous system1 and this appears otherwise in DM.7, 36
Another tool found in the literature search to analyze autonomic behavior was the analysis of heart rate turbulence (TFC).29 This method indicates the heart's ability to react to changes in heart rate, as in the case of premature beats, reflecting good or poor adaptation of the ANS.37
Balcioglu et al.29 observed that both HRV indices and TFC are reduced in diabetic individuals with CAN compared with those without CAN. They also pointed out that the extent of TFC tilt, which indicates the speed at which the heart rate returns to normal (mediated by the parasympathetic ANS), was the parameter that most strongly correlated with DM duration and severity of the CAN, with good sensitivity (97%) and specificity (71%) for the detection of CAN to a cutoff value of 3.32 obtained by the ROC curve (receiver operating characteristic).
Seyd et al.32 showed that diabetes can be distinguished from healthy individuals, using a specific type of artificial neural network (constituted from computational systems creating graphics or algorithms that are able to predict the autonomic behavior of a person) based on linear indices of HRV with good diagnostic accuracy (89.23% sensitivity and 96.92% specificity).
Other studies have also used specific classifiers to assess the accuracy of an automated diagnostic of DM. Acharya et al.31 used a diabetes integrated index using nonlinear HRV indices for the diagnosis of diabetes and showed that this index was able to discriminate diabetic individuals from healthy, and also among the classifiers used, the AdaBoost presented better accuracy to diagnose DM with sensitivity of 87.5% and specificity of 84.6%, the same was pointed out in another study by Acharya et al.,33 wherein the AdaBoost classifier performed better than the others with a sensitivity of 92.52% and specificity of 88.73%. Swapna et al.34 pointed out that the Gaussian Mixture Model classifier showed better accuracy to diagnose DM than other classifiers used in their study.
The analysis of HRV was performed from linear methods, using indices in the time and frequency domains and geometric indices, except for Khandoker et al.29 and Acharya et al.31, 33 who used the sample entropy and the Poincaré plot, correlation dimension, recurrence plot, detrended fluctuation analysis, approximate entropy and Lyapunov exponent, considered nonlinear methods1, 4, 38, 39 for the analysis. The use of linear methods in the studies presented in this review could be related to ease of use and being the most commonly studied methods to date; however, when nonlinear methods are added to these analyzes, we have better discriminatory power, strengthening the sensitivity of these methods to detect autonomic dysfunction.9, 11, 39
Besides the variety of indices used, the studies made use of different methods and times to analyze the variation in RR intervals. Some studies used the electrocardiogram method (ECG), known as the gold standard for HR analysis, with duration of 20 minutes,29, 32 60 minutes,31, 33, 34 and 24 hours27, 28 of analysis, whereas only one28 used Finapress equipment, which records blood pressure continuously and through the software extracts RR interval series with a collection time of 10 minutes.28 Regarding the number of evaluated beats, only five28, 30, 31, 33, 34 of the eight included studies indicated the number of beats used for analysis (1024 iRR and 1000 iRR) in the methodology.
The methodological quality in relation to the risk of bias and concern as to the applicability of the study was also evaluated in this review using the QUADAS‐2 tool.26 Of the eight studies evaluated, none were rated low risk of bias for all domains and three27, 28, 29 presented high risk of bias for the “index test” domain and two for the “reference standard” domain.27, 32 In addition, uncertain risk determination was attributed to three studies for the domains “index test” and “reference standard” and for six studies for the “flow and timing” domain.27, 28, 31, 32, 33, 34 The concern about applicability was assessed as low in all domains, except for the reference standard domain for which five studies were classified as uncertain risk.27, 31, 32, 33, 34
The determination of high risk of bias for the “index test” in some studies27, 28, 29 occurred due to the evaluation of the diagnostic methods tested being carried out after the reference standard. The assignment of uncertain risk was given in three studies31, 33, 34 to this domain because the authors did not indicate the use of a reference standard to confirm the diagnosis. For the “reference standard” domain, one of the studies27 was regarded as high risk for having determined the CAN through the presence of at least one symptom of neuropathy; however, it is known that individuals with CAN can be asymptomatic,41 which could have produced errors in individuals that did not yet have such clinical manifestations. Another study32 was classified as high in this domain for using blood glucose levels as a previous criterion of diagnostic confirmation of the presence of diabetes, but without specifying how this test was realized and the period of fasting of the individuals. Uncertain risk was also pointed out in this domain in three studies since the authors31, 33, 34 did not suggest the use of a reference standard in their studies.
Finally, the “flow and timing” domain was considered with an uncertain risk of bias for six studies27, 28, 31, 32, 33, 34 by not specifying if there was an interval between the application of both tests, not using a reference test or not using the test in the entire sample, only in person with diabetes.
Regarding concerns about the methodological applicability, five studies27, 31, 32, 33, 34 were classified as uncertain (“reference standard”), since important information on the obtained result of the diabetes diagnosis method was absent, which prevented clarification of the correct classification of these individuals.
The HRV is an indirect measure of autonomic function because it reflects influences on the sinus node.1 In patient with diabetes, it has been used to recognize incipient CAN and to determine the severity of the disease.42 This decrease is the first sign of CAN and is suggested as one of the diagnostic tests in a statement by the American Diabetes Association,43 possibly contributing to the initial diagnosis and thus enabling preventive actions contributing to improvement in health levels, avoiding significant long‐term complications of DM,9 and minimizing negative effects on quality of life,44 cardiovascular events,45 and sudden death.25
The use of HRV to assess cardiac autonomic modulation is already well documented in the literature6, 8, 11, 13; however, few studies have evaluated the usefulness of this tool to discriminate pathological individuals that may have complications, as observed in this review.
This review presents some strong points that must be described. The first point is the search for studies that evaluated HRV as a discriminatory method in DM, which is a noninvasive method that enabled the knowledge that exists in the literature on this theme. The second point is related to methods of analysis used in the studies, because despite knowing that there are several HRV indices that reflect performance of the ANS, it was not possible to know before the review whether all the available indices could be useful for diagnosing DM or the presence of CAN with good accuracy in these individuals. Finally, we assessed the methodological quality of the studies, which demonstrated that some domains evaluated as high/uncertain risk of bias should be reviewed. It is suggested in future studies that the risk of bias of the studies analyzed and appointed by the QUADAS‐2 scale in this literature review be considered and possible gaps about this theme be better clarified in studies on this and other populations.
Although this review presents significant strengths, limitations also occurred. In the present review, studies using HRV, independent of DM type, were used; however, there was a predominance of studies with type 2 DM,27, 29, 30, 32 so we cannot affirm that the results presented in this review can be used as a reference for all types of DM. Furthermore, we cannot affirm that the indices identified as being more sensitive and specific can be used as a reference for all age groups, as the majority of the samples consisted of individuals over 40 years of age.
The present review enables clinicians and researchers to include HRV in their diagnostic methods as an alternative tool able to identify, with good sensitivity and specificity, the presence of autonomic alterations in individuals with DM, using it as either an additional criterion or complementary to conventional autonomic tests for diagnosis of DM. Based on the results, it is suggested that linear and nonlinear methods are used together since this seems to leverage the diagnostic power.
Conclusion
This review approaches studies using HRV as a tool for diagnostic evaluation in individuals with DM. The results demonstrated that individuals with DM had reduced HRV and loss of complexity and that nonlinear indices, such as Sample Entropy and SD1/SD2, extracted from the Poincaré plot and linear indices, such as SDANN, HF, and the TFC tilt, presented better discriminatory power to determine autonomic dysfunction than the other indices presented in this review and that the use of classifiers from HRV indices can contribute to the automated diagnosis of DM. In addition, although some studies showed high risk of bias in some domains, methodological differences and indices used, the use of this tool can be used effectively in the practical prognosis and diagnosis of individuals with DM.
Acknowledgments
The authors thank FAPESP (Support Foundation for Research of São Paulo State) for providing financial support for this study (case number: 2013/19055‐0).
Appendix 1. Medline (Ovid) search strategy
[Target condition]
-
1
Diabetes Mellitus, Type 1/ or diabetes.mp. or Diabetes Mellitus, Type 2/ or Diabetes Mellitus/
[Evaluated tool]
-
2
Autonomic nervous system.mp. or Autonomic Nervous System/
-
3
Sympathetic nervous system.mp. or Sympathetic Nervous System/
-
4
Parasympathetic nervous system.mp. or Parasympathetic Nervous System/
-
5
Heart Rate.mp. or Heart Rate/
-
6
Heart Rate Variability.mp.
-
7
Cardiovascular system.mp. or Cardiovascular System/
-
8
Electrocardiography, Ambulatory/ or Electrocardiography/ or Electrocardiography.mp.
-
9
Instrument.mp.
-
10
Tool.mp. or “Tool Use Behavior”/
-
11
2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
[Analysis method]
-
12
(Sensitivity and Specificity).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
-
13
Limit of detection.mp. or “Limit of Detection”/
-
14
Roc curve.mp. or ROC Curve/
-
15
Predictive Value of Tests.mp. or “Predictive Value of Tests”/
-
16
Reproducibility of Results.mp. or “Reproducibility of Results”/
-
17
Diagnostic evaluation.mp.
-
18
12 or 13 or 14 or 15 or 16 or 17
[Combining results: Evaluated tool + Analysis method]
-
19
11 and 18
[Final combination]
-
20
1 and 19
Appendix 2. Cochrane Central Register of Controlled Trials (Ovid) search strategy
[Target condition]
-
1
Diabetes Mellitus, Type 1/ or diabetes.mp. or Diabetes Mellitus, Type 2/ or Diabetes Mellitus/
[Evaluated tool]
-
2
Autonomic nervous system.mp. or Autonomic Nervous System/
-
3
Sympathetic nervous system.mp. or Sympathetic Nervous System/
-
4
Parasympathetic Nervous System.mp. or Parasympathetic Nervous System/
-
5
Heart Rate.mp. or Heart Rate/
-
6
Heart Rate/ or Heart Rate Variability.mp.
-
7
Cardiovascular system.mp. or Cardiovascular System/
-
8
Electrocardiography, Ambulatory/ or Electrocardiography/ or Electrocardiography.mp.
-
9
Instrument.mp.
-
10
Tool.mp. or “Tool Use Behavior”/
-
11
2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
[Analysis method]
-
12
(Sensitivity and Specificity).mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
-
13
Limit of detection.mp. or “Limit of Detection”/
-
14
Roc curve.mp. or ROC Curve/
-
15
Predictive Value of Tests.mp. or “Predictive Value of Tests”/
-
16
Reproducibility of Results.mp. or “Reproducibility of Results”/
-
17
Diagnostic evaluation.mp.
-
18
12 or 13 or 14 or 15 or 16 or 17
[Combining results: Evaluated tool + Analysis method]
-
19
11 and 18
[Final combination]
-
20
1 and 19
Appendix 3. Embase search strategy
[Target condition]
-
1
“Diabetes mellitus”/exp OR “diabetes mellitus”
-
2
“Diabetes”/exp AND [humans]/lim AND [embase]/lim
-
3
#1 OR #2
[Evaluated tool]
-
4
“Electrocardiography”/exp OR “electrocardiography”
-
5
“Cardiovascular system”/exp OR “cardiovascular system”
-
6
“Heart rate variability”/exp OR “heart rate variability”
-
7
“Autonomic nervous system”/exp OR “autonomic nervous system”
-
8
“Heart rate”/exp OR “heart rate”
-
9
“Sympathetic nervous system”/exp OR “sympathetic nervous system”
-
10
“Parasympathetic nervous system”/exp OR “parasympathetic nervous system”
-
11
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
[Analysis method]
-
12
“Reproducibility of results”/exp OR “reproducibility of results”
-
13
“Limit of detection”/exp OR “limit of detection”
-
14
“Roc curve”/exp OR “roc curve”
-
15
“Sensitivity and specificity”/exp OR “sensitivity and specificity”
-
16
“Diagnostic evaluation”
-
17
“Predictive value of tests”/exp OR “predictive value of tests”
-
18
#12 OR #13 OR #14 OR #15 OR #16 OR #17
[Combining results: Evaluated tool + Analysis method]
-
19
#11 AND #18
[Final combination]
-
20
#3 AND #19
Appendix 4. Web of Science search strategy
[Target condition]
-
1
TS=(Diabetes Mellitus)
[Evaluated tool]
-
2
TS=(Electrocardiography)
-
3
TS=(Sympathetic Nervous System)
-
4
TS=(Parasympathetic Nervous System)
-
5
TS=(Autonomic Nervous System)
-
6
TS=(Heart Rate)
-
7
TS=(Cardiovascular System)
-
8
TS=(Heart Rate Variability)
-
9
TS=(Instrument)
-
10
TS=(Tool)
-
11
#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
[Analysis method]
-
12
TS=(Sensitivity and Specificity)
-
13
TS=(Limit of Detection)
-
14
TS=(Roc Curve)
-
15
TS=(Reproducibility of Results)
-
16
TS=(Diagnostic Self Evaluation)
-
17
TS=(Predictive Value of Tests)
-
18
#12 OR #13 OR #14 OR #15 OR #16 OR #17
[Combining results: Evaluated tool + Analysis method]
-
19
#18 AND #11
[Final combination]
-
20
#1 AND #19
Appendix 5. LILACS (Bireme) search strategy
[Target condition]
-
1
Diabetes Mellitus
[Evaluated tool]
-
2
Electrocardiography
-
3
Cardiovascular System
-
4
Heart Rate Variability
-
5
Autonomic Nervous System
-
6
Heart Rate
-
7
Sympathetic Nervous System
-
8
Parasympathetic Nervous System
[Analysis method]
-
9
Reproducibility of Results
-
10
Limit of Detection
-
11
Roc Curve
-
12
Sensitivity and Specificity
-
13
Diagnostic Evaluation
-
14
Predictive Value of Tests
[Combining results: Target condition + Evaluated tool + Analysis method]
-
15
1 AND 2 AND 9/‐14
-
16
1 AND 3 AND 9/‐14
-
17
1 AND 4 AND 9/‐14
-
18
1 AND 5 AND 9/‐14
-
19
1 AND 6 AND 9/‐14
-
20
1 AND 7 AND 9/‐14
-
21
1 AND 8 AND 9/‐14
Appendix 6. QUADAS‐2 – Quality Assessment of Diagnostic Accuracy Studies
Domain 1: Patient Selection
-
Risk of bias: Could the selection of patients have introduced bias?
Was a consecutive or random sample of patients enrolled?
Was a case–control design avoided?
Did the study avoid inappropriate exclusions?
Applicability: Are there concerns that the included patients and setting do not match the review question?
Domain 2: Index Test
-
Risk of bias: Could the conduct or interpretation of the index test have introduced bias?
Were the index test results interpreted without knowledge of the results of the reference standard?
If a threshold was used, was it prespecified?
Applicability: Are there concerns that the index test, its conduct, or its interpretation differ from the review question?
Domain 3: Reference Standard
-
Risk of bias: Could the reference standard, its conduct, or its interpretation have introduced bias?
Is the reference standard likely to correctly classify the target condition?
Were the reference standard results interpreted without knowledge of the results of the index test?
Applicability: Are there concerns that the target condition as defined by the reference standard does not match the question?
Domain 4: Flow and Timing
-
Risk of bias: Could the patient flow have introduced bias?
Was there an appropriate interval between the index test and reference standard?
Did all patients receive the same reference standard?
Were all patients included in the analysis?
Financial support: The study was funded by the Support Foundation for Research of São Paulo State (FAPESP).
Conflicts of interest: The authors declare that they have no conflicts of interest.
References
- 1. Vanderlei LCM, Pastre CM, Hoshi RA, et al. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Rev Bras Cir Cardiovasc 2009;24:205–217. [DOI] [PubMed] [Google Scholar]
- 2. Larosa C, Sgueglia GA, Sestito A, et al. Predictors of impaired heart rate variability and clinical outcome in patients with acute myocardial infarction treated by primary angioplasty. J Cardiovasc Med (Hagerstown) 2008;9:76–80. [DOI] [PubMed] [Google Scholar]
- 3. Pavithran P, Mithun R, Jomal M, et al. Heart rate variability in middle‐aged men with new‐onset hypertension. Ann Noninvasive Electrocardiol 2008;13:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Godoy M, Tanakamura I, Correa P. Relevância da análise do comportamento dinâmico nãolinear (Teoria do Caos) como elemento prognóstico de morbidade e mortalidade em pacientes submetidos à cirurgia de revascularização miocárdica. Arq Ciênc Saúde 2005;12:167–171. [Google Scholar]
- 5. Sin DD, Wong E, Mayers I, et al. Effects of nocturnal noninvasive mechanical ventilation on heart rate variability patients with advanced COPD. Chest 2007;131:156–163. [DOI] [PubMed] [Google Scholar]
- 6. Guzik P, Piskorski J, Contreras P, et al. Asymmetrical properties of heart rate variability in type 1 diabetes. Clin Auton Res 2010;20:255–257. [DOI] [PubMed] [Google Scholar]
- 7. Jaiswal M, Urbina EM, Wadwa RP, et al. Reduced heart rate variability among youth with type 1 diabetes: The Search CVD study. Diabetes Care 2013;36:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kardelen F, Akçurin G, Ertuğ H, et al. Heart rate variability and circadian variations in type 1 diabetes mellitus. Pediatr Diabetes 2006;7:45–50. [DOI] [PubMed] [Google Scholar]
- 9. Khatoon N, Kumar BS, Abdul M. Cardiovascular autonomic neuropathy in patients with diabetes mellitus. Int J Pharma Bio Sci 2010;1:1–7. [Google Scholar]
- 10. Pumprla J, Howorka K, Groves D, et al. Functional assessment of heart rate variability: Physiological basis and practical applications. Int J Cardiol 2002;84:1–14. [DOI] [PubMed] [Google Scholar]
- 11. Javorka M, Trunkvalterova Z, Tonhajzerova I, et al. Short‐term heart rate complexity is reduced in patients with type 1 diabetes mellitus. Clin Neurophysiol 2008;119:1071–1081. [DOI] [PubMed] [Google Scholar]
- 12. Javorka M, Trunkvalterova Z, Tonhajzerova I, et al. Recurrences in heart rate dynamics are changed in patients with diabetes mellitus. Clin Physiol Funct Imaging 2008;28:326–331. [DOI] [PubMed] [Google Scholar]
- 13. Javorka M, Javorková J, Tonhajzerová I, et al. Heart rate variability in young patients with diabetes mellitus and healthy subjects explored by Poincaré and sequence plots. Clin Physiol Funct Imaging 2005;25:119–127. [DOI] [PubMed] [Google Scholar]
- 14. Chessa M, Butera G, Lanza GA, et al. Role of heart rate variability in the early diagnosis of diabetic autonomic neuropathy in children. Herz 2002;27:785–790. [DOI] [PubMed] [Google Scholar]
- 15. Schroeder EB, Chambless LE, Liao D, et al. Diabetes, glucose, insulin, and heart rate variability: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2005;28:668–674. [DOI] [PubMed] [Google Scholar]
- 16. Yeap BB, Russo A, Fraser RJ, et al. Hyperglycemia affects cardiovascular autonomic nerve function in normal subjects. Diabetes Care 1996;19:880–882. [DOI] [PubMed] [Google Scholar]
- 17. Carvalho TD, Pastre CM, Rossi RC, et al. Índices geométricos de variabilidade da frequência cardíaca na doença pulmonar obstrutiva crônica. Rev Port Pneumol 2011;17:260–265. [DOI] [PubMed] [Google Scholar]
- 18. Vanderlei LCM, Pastre CM, Júnior IF, et al. Fractal correlation of heart rate variability in obese children. Auton Neurosci 2010;155:125–129. [DOI] [PubMed] [Google Scholar]
- 19. Corrêa PR, Catai AM, Takakura IT, et al. Variabilidade da Frequência Cardíaca e Infecções Pulmonares Pós Revascularização Miocárdica. Arq Bras Cardiol 2010;95:448–456. [DOI] [PubMed] [Google Scholar]
- 20. Pivatelli FC, Santos MA, Fernandes GB, et al. Sensitivity, specificity and predictive values of linear and nonlinear indices of heart rate variability in stable angina patients. Int Arch Med 2012;5:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spallone V, Ziegler D, Freeman R, et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 2011;27:639–653. [DOI] [PubMed] [Google Scholar]
- 22. Pagani M. Heart rate variability and autonomic diabetic neuropathy. Diabetes Nutr Metab 2000;13:341–346. [PubMed] [Google Scholar]
- 23. Ewing DJ, Martyn CN, Young RJ, et al. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985;8:491–498. [DOI] [PubMed] [Google Scholar]
- 24. Ewing DJ, Campbell IW, Murray A, et al. Immediate heart‐rate response to standing: Simple test for autonomic neuropathy in diabetes. Br Med J 1978;1:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–397. [DOI] [PubMed] [Google Scholar]
- 26. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 27. Takase B, Kurita A, Noritake M, et al. Heart rate variability in patients with diabetes mellitus, ischemic heart disease, and congestive heart failure. J Electrocardiol 1992;25:79–88. [DOI] [PubMed] [Google Scholar]
- 28. Ziegler D, Laude D, Akila F, et al. Time‐ and frequency‐domain estimation of early diabetic cardiovascular autonomic neuropathy. Clin Auton Res 2001;11:369–376. [DOI] [PubMed] [Google Scholar]
- 29. Balcioğlu S, Arslan U, Türkoğlu S, et al. Heart rate variability and heart rate turbulence in patients with type 2 diabetes mellitus with versus without cardiac autonomic neuropathy. Am J Cardiol 2007;100:890–893. [DOI] [PubMed] [Google Scholar]
- 30. Khandoker AH, Jelinek HF, Palaniswami M. Identifying diabetic patients with cardiac autonomic neuropathy by heart rate complexity analysis. Biomed Eng Online 2009;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Acharya UR, Faust O, Sree SV, et al. An integrated diabetic index using heart rate variability signal features for diagnosis of diabetes. Comput Methods Biomech Biomed Engin 2013;16:222–234. [DOI] [PubMed] [Google Scholar]
- 32. Pt AS, Joseph PK, Jacob J. Automated diagnosis of diabetes using heart rate variability signals. J Med Syst 2012;36:1935–1941. [DOI] [PubMed] [Google Scholar]
- 33. Acharya UR, Faust O, Adib Kadri N, et al. Automated identification of normal and diabetes heart rate signals using nonlinear measures. Comput Biol Med 2013;43:1523–1529. [DOI] [PubMed] [Google Scholar]
- 34. Swapna G, Acharya UR, VinithaSree S, et al. Automated detection of diabetes using higher order spectral features extracted from heart rate signals. Intell Data Anal 2013;17:309–326. [Google Scholar]
- 35. Schönauer M, Thomas A, Morbach S, et al. Cardiac autonomic diabetic neuropathy. Diab Vasc Dis Res 2008;5:336–344. [DOI] [PubMed] [Google Scholar]
- 36. Javorka M, Javorkova J, Tonhajzerova I, et al. Parasympathetic versus sympathetic control of the cardiovascular system in young patients with type 1 diabetes mellitus. Clin Physiol Funct Imaging 2005;25:270–274. [DOI] [PubMed] [Google Scholar]
- 37. Stein PK, Barzilay JI. Relationship of abnormal heart rate turbulence and elevated CRP to cardiac mortality in low, intermediate, and high‐risk older adults. J Cardiovasc Electrophysiol 2011;22:122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richman JS, Moorman JR. Physiological time‐series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 2000;278:H2039–H2049. [DOI] [PubMed] [Google Scholar]
- 39. Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng 2001;48:1342–1347. [DOI] [PubMed] [Google Scholar]
- 40. Voss A, Schroeder R, Truebner S, et al. Comparison of nonlinear methods symbolic dynamics, detrended fluctuation, and Poincaré plot analysis in risk stratification in patients with dilated cardiomyopathy. Chaos 2007;17:015120. [DOI] [PubMed] [Google Scholar]
- 41. AC Farmacêutica . Diretrizes da Sociedade Brasileira de Diabetes: 2013–2014. São Paulo, AC Farmacêutica, 2014. [Google Scholar]
- 42. Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: The enduring and the new. Curr Opin Cardiol 2004;19:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care 2005;28:956–962. [DOI] [PubMed] [Google Scholar]
- 44. Zochodne DW. Diabetes mellitus and the peripheral nervous system: Manifestations and mechanisms. Muscle Nerve 2007;36:144–166. [DOI] [PubMed] [Google Scholar]
- 45. Schmid H. Impacto Cardiovascular da Neuropatia Autonômica do Diabetes Mellitus. Arq Bras Endocrinol Metab 2007;51:232–243. [DOI] [PubMed] [Google Scholar]


