Abstract
Background
Recently, numerous models and techniques have been developed for analyzing and extracting features from the T wave which could be used as biomarkers for drug‐induced abnormalities. The majority of these techniques and algorithms use features that determine readily apparent characteristics of the T wave, such as duration, area, amplitude, and slopes.
Methods
In the present work the T wave was down‐sampled to a minimal rate, such that a good reconstruction was still possible. The entire T wave was then used as a feature vector to assess drug‐induced repolarization effects. The ability of the samples or combinations of samples obtained from the minimal T‐wave representation to correctly classify a group of subjects before and after receiving d,l‐sotalol 160 mg and 320 mg was evaluated using a linear discriminant analysis (LDA).
Results
The results showed that a combination of eight samples from the minimal T‐wave representation can be used to identify normal from abnormal repolarization significantly better compared to the heart rate‐corrected QT interval (QTc). It was further indicated that the interval from the peak of the T wave to the end of the T wave (Tpe) becomes relatively shorter after IK r inhibition by d,l‐sotalol and that the most pronounced repolarization changes were present in the ascending segment of the minimal T‐wave representation.
Conclusions
The minimal T‐wave representation can potentially be used as a new tool to identify normal from abnormal repolarization in drug safety studies.
Keywords: electrocardiogram, QT interval, repolarization, Tpeak‐Tend, T wave
1. Introduction
Features extracted from the T wave of the electrocardiogram (ECG) form the basis for the diagnoses of several heart diseases and for the identification of drug‐induced repolarization abnormalities. In particular, drug‐induced QT prolongation caused by drugs with IKr‐blocking potential has been in focus for more than a decade since an increased duration of cardiac repolarization has been associated with the life‐threatening ventricular tachyarrhythmia torsades de pointes (TdP) (Roden, 2004). The QT interval is inversely correlated with the heart rate and, therefore, a heart rate‐corrected QT interval (QTc) must be estimated. There is still a debate about the best correction method (Bazett, 1920; Fridericia, 1920; Malik et al., 2002; Nielsen et al., 2011). Although QT prolongation is a sensitive sign for IKr‐blockers, it does not have a high specificity, and it is not a specific marker for TdP either: not all drugs that prolong the QT interval to the same extent carry the same risk for TdP (Roden, 2004). Also, although the QTc interval has the advantage of being intuitively straightforward and a clinically relevant measure, manual assessment of the QT interval shows considerable intra‐ and interobserver variations (Al Khatib et al., 2003; Nielsen et al., 2011).
Important features may be extracted from the whole QT interval and be used to assess repolarization abnormalities in addition to QTc. The use of several measures of the T‐wave changes could also be more specific than QTc prolongation to identify those IKr‐blocking drugs that are potentially proarrhythmic. Various parameters have been suggested as alternatives to QT interval measurements. QT dispersion was not only a widely investigated ECG feature a decade ago but it also proved to be the least successful in predicting the risks of drug‐induced TdP (Shah, 2005), probably for good reasons (Kors & Van Herpen, 1998). T‐wave alternans has been reported with congenital long QT syndrome (Cruz Filho et al., 2000) and appears to be an important indicator in that it is commonly observed just prior to the episodes of TdP. As the trailing edge of the T wave appears to shift in parallel with a varying QT interval, a parameter, deltaT50, which measures the temporal variability in the steep part of the trailing edge of the T‐wave, instead of the end of the T wave, was developed to assess the beat‐to‐beat variability in cardiac repolarization time (Abrahamsson et al., 2011). There is also evidence suggesting that a prolonged Tpeak‐Tend interval (Tpe), thought to represent repolarization heterogeneity (Yan & Antzelevitch, 1998), could play a role in the identification of drug‐related risk of TdP (Couderc et al., 2009), although there are some controversies as to what the Tpe interval actually represents (Nielsen et al., 2010). The results of a T‐wave area‐based study in subjects receiving sotalol show that drug‐induced shape changes can occur across the entire repolarization interval (Couderc et al., 2010) suggesting that abnormal T‐wave morphology may play a role in differentiating between safe and unsafe drugs. Indeed, there is a growing body of research on the analysis of T‐wave morphology and drug‐induced repolarization abnormalities (Kanters et al., 2004; Struijk et al., 2006; Zabel et al., 2000). The linear combination of T waves–derived biomarkers, such as notches (Lupoglazoff et al., 2001), asymmetry (Merri et al., 1989; Struijk et al., 2006), and the appearance of flat T waves (Couderc et al., 2006; Graff et al., 2009) has been used as a sensitive and apparently more specific marker of repolarization abnormalities for a number of drugs from different classes (Graff et al., 2009, 2009, 2010; Shakibfar et al., 2012).
Although the morphology of the T wave can sometimes be of greater importance than the QT interval for the assessment of proarrhythmic risk, the T wave may contain more information than can be expressed by readily apparent characteristics such as duration, area, amplitude, and slopes. Some mathematical approaches such as principal component analysis (PCA) (Laguna et al., 1999) and Gaussian models (Clifford, 2006) have been applied to model the shape of the T wave. These methods can fit many waveforms quite well and reduce the dimension of the data. However, they cannot provide a framework for the physiological interpretation of the T‐wave morphology changes and they are not robust to small changes in T waves such as those caused by noise or inaccuracies in the determination of the end of the T wave.
In the present work we compared effects of the hERG blocker d,l‐sotalol on ST‐T subsegments with effects on the QT interval. D,l‐sotalol was selected because the drug prolongs QTc substantially [~30 ms for 160 mg and ~50 ms for 320 mg, (Graff et al., 2009; Sarapa et al., 2004)] and therefore would emphasize potential findings.
We reduced the dimension of the T wave by minimal sampling according to the sampling theorem (Nyquist, 1928; Shannon, 1949). Instead of using the typical 500 Hz sampling rate of many electrocardiographs we down‐sampled the T waves to a reduced number of samples so that a good reconstruction was still possible. Subsequently, we assessed which samples or combinations of samples were most relevant to describe sotalol‐induced changes of the T wave. This approach can add to our understanding of the relative changes of the repolarization subsegments of the QT interval, the J‐Tpeak and the Tpeak‐Tend intervals and their individual contributions to QT prolongation with pure hERG block. The work was inspired by recent detailed reports showing that the J‐Tpeak interval may be used in drug trials to discriminate between drugs that are selective hERG potassium channel blockers, and are associated with a high risk of TdP, and multichannel blockers with a low risk of TdP (Johannesen et al., 2014, 2014, 2016). Such findings suggest that assessment of the J‐Tpeak interval may add value in drug trials beyond only assessing the heart rate corrected QT interval.
2. Methods
2.1. Study design
Electrocardiogram data were obtained from 21 healthy subjects on baseline and after receiving 160 mg and 320 mg doses of the antiarrhythmic IKr‐blocking drug, d,l‐sotalol. Written informed consent was obtained from all subjects for the study protocol. The data and details of the protocol have been described by Sarapa et al. (Sarapa et al., 2004). We assessed the ability to distinguish between the repolarization segments at baseline and after 160 mg and 320 mg d,l‐sotalol based on the features obtained from a down‐sampled T wave. The results were compared with the commonly used measurement to assess repolarization changes, the Fridericia‐corrected QT interval (QTcF) (Fridericia, 1920).
2.2. Study population
All subjects were healthy males and between 18 and 45 years of age. Health status was confirmed by history, physical examination, normal blood pressure, and no use of concomitant medication. If the observed level of predose QTc prolongation was >410 ms or any QTc within 6 hr after dosing was >450 ms, subjects were withdrawn from the study. Detailed information can be found in Sarapa et al., (2004).
2.3. ECG acquisition
Electrocardiogram segments of 10‐s duration were obtained from 12‐lead digital Holter recordings (H12 Recorder, Mortara Instrument, Milwaukee, WI, USA). From each subject, 22.5‐hr recordings were obtained per day. We extracted 10‐s segments at study times corresponding to 0.5, 1, 1.5, 3, 3.5, 4, 16, and 16.5 hr after 160 and 320 mg d,l‐sotalol dosing on days 2 and 3 and at corresponding study times on the baseline day 1.
2.4. ECG preprocessing
Each ECG extracted from Holter was resampled from 180 Hz to 500 Hz, as previously described (Graff et al., 2009), to ensure compatibility with the software to be subsequently used. Median beats were then formed in the recorded leads using the MUSE/Interval Editor software (GE Healthcare, Milwaukee, WI, USA). Lead V5 was used to measure ECG parameters because, V5 as a lateral precordial lead, is likely to reliably reflect the electrical phenomena in the left ventricle during IKr‐blocker‐induced repolarization changes.
The onset of the QRS complex was located manually as the point in the P‐R segment where the first wave of the QRS complex just begins to deviate, abruptly or gradually, from the isoelectric line, the latter being at 0 mV in the median beat.
The end of the T wave (Tend) was located semiautomatically using custom software as the point where the isoelectric line intersects a tangential line drawn at the maximal downslope of the positive T wave. All locations of T‐end were visually verified and modified if the tangential line was not the best fit to the descending limb of the T wave (which happened occasionally because of noise or irregularity in the trailing edge of the T wave).
The QT interval was measured from the onset of the QRS complex to the end of the T wave. The QT intervals were corrected for heart rate with Fridericia's formula: QTcF = QT/RR1/3.
The T‐wave interval was measured from the J‐point to Tend, where the J‐point was determined as the point after the QRS complex where the variance among the 12 leads was minimal.
2.5. ECG down‐sampling and scaling
The number of samples required to describe a T wave was minimized according to the sampling theorem which states that a signal can be perfectly reconstructed if the sampling frequency is at least twice the highest frequency in the signal.
Figure 1 shows the average spectral analysis of all T waves in the dataset. It is clear that there is very little signal above 20 Hz. A sampling frequency of 40 Hz is therefore sufficient to reliably represent the T‐wave shape.
Figure 1.
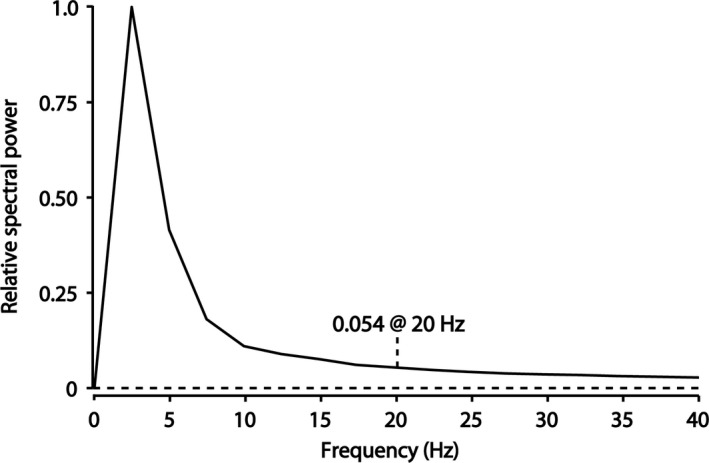
The average amplitude spectral density for all T waves in the dataset. There is little frequency content above 20 Hz so a sampling frequency of 40 Hz is sufficient to reliably represent the T wave. T waves were down‐sampled to 20 discrete sample values and since the interval between those 20 points was always <500 ms, this number of samples corresponded to a sample frequency of more than 40 Hz
Using the information about T‐wave frequency content, all T waves in the dataset were down‐sampled to a fixed number of 20 samples from the J‐point to Tend rather than sampling at a fixed rate, Fig. 2. Since the interval between those points was always <500 ms, this number of samples corresponded to a sampling frequency of more than 40 Hz. The amplitudes of the samples were normalized to the peak value of the T wave. Since all T waves were positive monophasic, the amplitudes thus scaled between 0 and 1.
Figure 2.
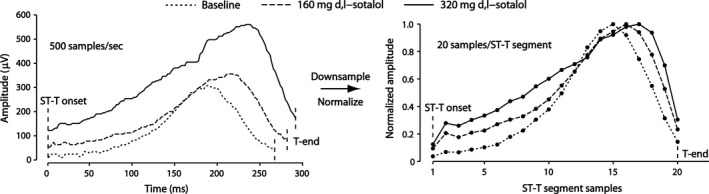
Examples of T waves of a subject in the study (left) and the corresponding down‐sampled and normalized T waves (right): The interval between J‐point and Tend was down‐sampled to 20 samples and normalized by its peak amplitude, thus yielding 20 features of each T wave
2.6. Statistical analyses
All statistical analyses were performed with Matlab version R2012b (Mathworks Inc., Natick, MA, USA). A linear discriminant analysis (LDA) was used at each instance to select those features from the minimal T‐wave representation that most clearly separated the T waves at baseline from the T waves after dosing of 160 and 320 mg d,l‐sotalol. Using the time of occurrence of the peak QTcF effect in this study which corresponds with the time of peak QTcF effect previously reported (Graff et al., 2009; Sarapa et al., 2004), a McNemar test of marginal frequencies was used to compare the number of ECGs which were correctly classified by QTcF and the minimal T‐wave representation as taken before (baseline) or after drug administration (160 and 320 mg d,l‐sotalol). A p‐value <.05 was regarded as significant.
3. Results
The 20 down‐sampled and normalized T‐wave values and their mean values at study times 30 min after dose (0.5 hr) and peak QTcF effect (3.5 hr) are demonstrated in Fig. 3 for all subjects.
Figure 3.
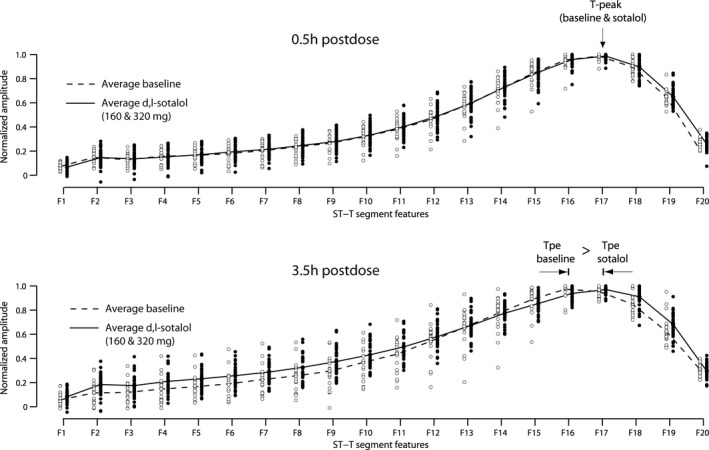
The average of all down‐sampled T waves (dotted line: average of 21 T waves at baseline, solid line: average of 42 T waves after sotalol). At the time of peak QTcF effect (3.5 hr postdose), most of the prolongation is caused by prolongation of the J‐Tpeak interval, whereas the Tpeak to Tend interval becomes relatively shorter
The T‐wave morphology at time 3.5 hr shows that most of the prolongation occurs from the J‐point to Tpeak (a shift of Tpeak toward the right), whereas Tpeak to Tend becomes relatively shorter after d,l‐sotalol. Also, the most important samples (F1–F13) are in the ascending segment of the T wave, where they have increased amplitude compared to baseline.
Table 1 shows the misclassification error obtained by linear discriminant analysis (baseline day vs both sotalol days combined) for QTcF and different subsets of T‐wave samples. The most effective samples are again from the ascending part of the J‐Tpeak interval. Linear combinations of samples from the ascending T‐wave segment have a lower number of misclassifications compared to QTcF.
Table 1.
Misclassification error for linear discriminant analysis (wrongfully classifying a baseline ECG as an ECG after d,l‐sotalol and vice versa) of QTcF and different subset features at different times after d,l‐sotalol administration
| Features | Times postdose (hr) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 1.5 | 3 | 3.5 | 4 | 16 | 16.5 | |
| Missclassification (%) | ||||||||
| QTcF | 30 | 34 | 32 | 29 | 24 | 24 | 31 | 34 |
| DS [6] | 32 | 34 | 32 | 32 | 30 | 21 | 31 | 33 |
| DS [7 17] | 32 | 34 | 33 | 18 | 22 | 29 | 31 | 34 |
| DS [7 9 13] | 34 | 31 | 32 | 16 | 20 | 23 | 31 | 33 |
| DS [7 9 13 17] | 30 | 29 | 32 | 19 | 16 | 38 | 33 | 34 |
| DS [3 7 9 12 17] | 29 | 31 | 33 | 19 | 16 | 23 | 33 | 33 |
| DS [1 2 3 10 12 13] | 30 | 34 | 30 | 18 | 14 | 25 | 25 | 27 |
| DS [1 2 3 11 12 13 16] | 27 | 34 | 26 | 16 | 12 | 24 | 30 | 34 |
| DS [1 2 3 8 9 10 12 13] | 31 | 29 | 30 | 13 | 11 | 23 | 25 | 30 |
DS = Discrete sample number of a T wave with 20 samples between the J‐point and the end of the T wave.
Table 2 compares the discriminating ability of the LDA with eight samples included [F1 F2 F3 F8 F9 F10 F12 F13] to QTcF at time 3.5 hr postdose using the McNemar test. An LDA with eight samples included is significantly better than QTcF (p = .04) at discriminating between the 21 baseline ECGs and the 42 ECGs recorded after d,l‐sotalol.
Table 2.
Classifier accuracy at peak QTcF effect 3.5 hr postdose (baseline vs 160 and 320 mg d,l‐sotalol)
| DS [1 2 3 8 9 10 12 13] and QTcF (correctly classified) | QTcF | Total | ||
|---|---|---|---|---|
| Yes | No | |||
| DS | Yes | 44 | 12 | 56 |
| No | 4 | 3 | 7 | |
| Total | 48 | 15 | 63 | |
| McNemar (binomial distribution): p = .04a | ||||
p‐value for the null hypothesis—correctly classified drug effects: DS = QTcF.
4. Discussion
In this study, the sampling theorem was used to reduce the number of samples required to accurately describe the T wave. Individual samples were then used to describe and classify drug‐induced changes of the T wave. This approach was motivated by the fact that currently a single parameter (corrected QT interval) is used for the evaluation of pro‐arrhythmic risk of drugs, whereas it is clear from many publications that the whole T wave may change following administration of a wide variety of drugs.
The QTc interval as a cardiac safety indicator ignores this information in the shape of the T wave, relying instead upon only one point of this complex curve.
Attempting to use measurements reflecting T‐wave morphology changes, a Morphology Combination Score (MCS) has been applied successfully for the identification of drug‐induced disturbed repolarization by linear combination of just a few fundamental features (asymmetry, flatness and the occurrence of notches) (Graff et al., 2009, 2009, 2010; Nielsen et al., 2009; Shakibfar et al., 2012). These features are relatively independent of heart rate (Andersen et al., 2008). The major difference between the method proposed in the current work and other ECG‐based repolarization measurements is that the method described in this work divides the whole J‐Tend interval into a fixed number of samples, making each sample independent of QT duration and thus much less dependent on heart rate. This implicit scaling of the time axis makes the individual samples more useful for the description of relative changes in the repolarization process. And in contrast to all other methods of analysis of T‐wave morphology changes, the method presented in this work takes into account the whole T wave and does not focus on predefined features. This property of the proposed method may be particularly useful when the ST‐T segment is noisy, has a biphasic T‐wave configuration, is low amplitude or has notched T waves where it can otherwise be difficult to delineate fiducial points for interval measurements and quantification of T‐wave morphology.
The most important samples turned out to be those of the ST segment and the up‐slope of the T wave, which is probably caused by the fact that the prolonged T wave is rescaled such that samples with higher values (during the up‐slope) appear earlier in the resampled signal.
Interestingly, it turned out that the peak of the T wave shifted to the right and that Tpe, thus relatively shortened due to the IKr‐blocker effects. This finding is in contrast to the common belief that the Tpe interval would be prolonged due to the effect of IKr inhibition.
The down‐sampling approach for T‐wave morphology analysis may be a promising new tool to assess drug‐induced abnormalities, and the reference data provided here could be a catalyst for further investigations of repolarization abnormalities.
The technique presented in this work might not necessarily be suitable for ECG signals under all conditions, in particular, drugs with much more subtle effects on the QT interval. Further work can be done to extend the current work to nonlinear parametric models that can better capture the nonlinear and nonstationary nature of the ECG.
5. Conclusions
The minimal T‐wave representation can potentially be used as a new tool to identify normal from abnormal repolarization in drug safety studies.
Conflict of Interest
Regarding this work the authors do not report any conflict of interest.
Authors’ Contributions
SSh carried out the data analysis and drafted the manuscript. HS and CG participated in the design of the study and supervised the statistical analysis and helped to draft the manuscript. JK carried out the ECG data requirement and advised on medical and clinical perspective of the study. SS and JN participated in its design and commented to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This study was partially supported by the Region Nordjyllands Sundhedsvidenskaelige Forskningsfond (Health Research Fund of Central Denmark Region). The authors thank Pfizer Inc. for providing the data for the sotalol study.
Shakibfar S, Graff C, Kanters JK, Nielsen J, Schmidt S, Struijk JJ. Minimal T‐wave representation and its use in the assessment of drug arrhythmogenicity. Ann Noninvasive Electrocardiol. 2017;22:e12413. 10.1111/anec.12413
References
- Abrahamsson, C. , Dota, C. , Skallefell, B. , et al. (2011). DeltaT50 – a new method to assess temporal ventricular repolarization variability. Journal of Electrocardiology, 44, 477.e1‐477.e9 [DOI] [PubMed] [Google Scholar]
- Al Khatib, S. M. , LaPointe, N. M. A. , Kramer, J. M. , et al. (2003). What clinicians should know about the QT interval. JAMA, 289, 2120–2127. [DOI] [PubMed] [Google Scholar]
- Andersen, M. P. , Xue, J. Q. , Graff, C. , et al. (2008). New descriptors of T‐wave morphology are independent of heart rate. Journal of Electrocardiology, 41, 557–561. [DOI] [PubMed] [Google Scholar]
- Bazett, H. C. (1920). An analysis of time relations of electrocardiograms. Heart, 7, 353–370. [Google Scholar]
- Clifford, G. D. (2006). A novel framework for signal representation and source separation: Applications to filtering and segmentation of biosignals. Journal of Biological Systems, 14, 169–183. [Google Scholar]
- Couderc, J. P. , Kaab, S. , Hinterseer, M. , et al. (2009). Baseline values and sotalol‐induced changes of ventricular repolarization duration, heterogeneity, and instability in patients with a history of drug‐induced torsades de pointes. Journal of Clinical Pharmacology, 49, 6–16. [DOI] [PubMed] [Google Scholar]
- Couderc, J. P. , McNitt, S. , Xia, J. , et al. (2006). Repolarization morphology in adult LQT2 carriers with borderline prolonged QTc interval. Heart Rhythm, 3, 1460–1466. [DOI] [PubMed] [Google Scholar]
- Couderc, J. P. , Zareba, W. , Moss, A. J. , et al. (2010). Identification of sotalol‐induced changes in repolarization with T wave area‐based repolarization duration parameters. Journal of Electrocardiology, 36, 115–120. [DOI] [PubMed] [Google Scholar]
- Cruz Filho, F. E. , Maia, I. G. , Fagundes, M. L. , et al. (2000). Electrical behavior of T‐wave polarity alternans in patients with congenital long QT syndrome. Journal of the American College of Cardiology, 36, 167–173. [DOI] [PubMed] [Google Scholar]
- Fridericia, L. S. (1920). The duration of systole in the electrocardiogram of normal subjects and of patients with heart disease. Acta Medica Scandinavica, 53, 469–486. [Google Scholar]
- Graff, C. , Andersen, M. P. , Xue, J. Q. , et al. (2009). Identifying drug‐induced repolarization abnormalities from distinct ECG patterns in congenital LQT syndrome. Drug Safety, 32, 599–611. [DOI] [PubMed] [Google Scholar]
- Graff, C. , Matz, J. , Christensen, E. B. , et al. (2009). Quantitative analysis of T‐wave morphology increases confidence in drug‐induced cardiac repolarization abnormalities: Evidence from the investigational IKr inhibitor Lu 35‐138. Journal of Clinical Pharmacology, 49, 1331–1342. [DOI] [PubMed] [Google Scholar]
- Graff, C. , Struijk, J. J. , Matz, J. , et al. (2010). Covariate analysis of QTc and T‐wave morphology: New possibilities in the evaluation of drugs that affect cardiac repolarization. Clinical Pharmacology and Therapeutics, 88, 88–94. [DOI] [PubMed] [Google Scholar]
- Johannesen, L. , Vicente, J. , Gray, R. A. , et al. (2014). Improving the assessment of heart toxicity for all new drugs through translational regulatory science. Clinical Pharmacology and Therapeutics, 95, 501–508. [DOI] [PubMed] [Google Scholar]
- Johannesen, L. , Vicente, J. , Mason, J. W. , et al. (2014). Differentiating drug‐induced multichannel block on the electrocardiogram: Randomized study of dofetilide, quinidine, ranolazine, and verapamil. Clinical Pharmacology and Therapeutics, 96, 549–558. [DOI] [PubMed] [Google Scholar]
- Johannesen, L. , Vicente, J. , Mason, J. W. , et al. (2016). Late sodium current block for drug‐induced long QT syndrome: Results from a prospective clinical trial. Clinical Pharmacology and Therapeutics, 99, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanters, J. K. , Fanoe, S. , Larsen, L. A. , et al. (2004). T‐wave morphology analysis distinguishes between KvLQT1 and HERG mutations in long QT syndrome. Heart Rhythm, 1, 285–292. [DOI] [PubMed] [Google Scholar]
- Kors, J. A. , & Van Herpen, G. (1998). Measurement error as a source of QT dispersion: A computerised analysis. Heart, 80, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna, P. , Moody, G. B. , Garcia, J. , et al. (1999). Analysis of the ST‐T complex of the electrocardiogram using the karhunen‐loeve transform: Adaptive monitoring and alternans detection. Medical and Biological Engineering and Computing, 37, 175–189. [DOI] [PubMed] [Google Scholar]
- Lupoglazoff, J. M. , Denjoy, I. , Berthet, M. , et al. (2001). Notched T waves on Holter recordings enhance detection of patients with LQT2 (HERG) mutations. Circulation, 103, 1095–1101. [DOI] [PubMed] [Google Scholar]
- Malik, M. , Farbom, P. , Batchvarov, V. , et al. (2002). Relation between QT and RR intervals is highly individual among healthy subjects: Implications for heart rate correction of the QT interval. Heart, 87, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merri, M. , Benhorin, J. , Alberti, M. , et al. (1989). Electrocardiographic quantitation of ventricular repolarization. Circulation, 80, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Nielsen, J. , Andersen, M. P. , Graff, C. , et al. (2010). The effect of sertindole on QTD and TPTE. Acta Psychiatrica Scandinavica, 121, 385–388. [DOI] [PubMed] [Google Scholar]
- Nielsen, J. , Graff, C. , Hardahl, T. , et al. (2009). Sertindole causes distinct electrocardiographic T‐wave morphology changes. European Neuropsychopharmacology, 19, 702–707. [DOI] [PubMed] [Google Scholar]
- Nielsen, J. , Graff, C. , Kanters, J. K. , et al. (2011). Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs, 25, 473–490. [DOI] [PubMed] [Google Scholar]
- Nyquist, H. (1928). Certain topics in telegraph transmission theory. AIEE Transactions, 47, 617–644. [Google Scholar]
- Roden, D. M. (2004). Drug induced prolongation of the QT interval. New England Journal of Medicine, 350, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Sarapa, N. , Morganroth, J. , Couderc, J. P. , et al. (2004). Electrocardiographic identification of drug‐induced QT prolongation: Assessment by different recording methods. Annals of Noninvasive Electrocardiology, 9, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, R. R. (2005). Drug‐induced QT dispersion: Does it predict the risk of Torsade de pointes? Journal of Electrocardiology, 38, 10–18. [DOI] [PubMed] [Google Scholar]
- Shakibfar, S. , Graff, C. , Ehlers, L. H. , et al. (2012). Assessing common classification methods for the identification of abnormal repolarization using indicators of T‐wave morphology and QT interval. Computers in Biology and Medicine, 42, 485–491. [DOI] [PubMed] [Google Scholar]
- Shannon, C. E. (1949). Communication in the presence of noise. Proceedings of the IRE, 37, 10–21. [Google Scholar]
- Struijk, J. J. , Kanters, J. K. , Andersen, M. P. , et al. (2006). Classification of the long‐QT syndrome based on discriminant analysis of T‐wave morphology. Medical and Biological Engineering and Computing, 44, 543–549. [DOI] [PubMed] [Google Scholar]
- Yan, G. X. , & Antzelevitch, C. (1998). Cellular basis for the normal T wave and the electrocardiographic manifestations of the long‐QT Syndrome. Circulation, 98, 1928–1936. [DOI] [PubMed] [Google Scholar]
- Zabel, M. , Acar, B. , Klingenheben, T. , et al. (2000). Analysis of 12‐lead T‐wave morphology for risk stratification after myocardial infarction. Circulation, 102, 1252–1257. [DOI] [PubMed] [Google Scholar]


