Abstract
Background
Fragmented QRS reflects disturbances in the myocardium predisposing the heart to ventricular tachyarrhythmias. Recent studies suggest that fragmented QRS (fQRS) is associated with worse major arrhythmic events in hypertrophic cardiomyopathy (HCM). However, a systematic review and meta‐analysis of the literature has not been done. We assessed the association between fQRS and major arrhythmic events in hypertrophic cardiomyopathy by a systematic review of the literature and a meta‐analysis.
Methods
We comprehensively searched the databases of MEDLINE and EMBASE from inception to May 2017. Included studies were published prospective or retrospective cohort studies that compared major arrhythmic events (sustained ventricular tachycardia, sudden cardiac arrest, or sudden cardiac death) in HCM with fQRS versus non‐fQRS. Data from each study were combined using the random‐effects, generic inverse variance method of DerSimonian and Laird to calculate risk ratios and 95% confidence intervals.
Results
Five studies from January 2013 to May 2017 were included in this meta‐analysis involving 673 subjects with HCM (205 fQRS and 468 non‐fQRS). Fragmented QRS was associated with major arrhythmic events (pooled risk ratio = 7.29, 95% confidence interval: 4.00–13.29, p < .01, I 2 = 0%).
Conclusion
Baseline fQRS increased major arrhythmic events up to sevenfold. Our study suggests that fQRS could be an important tool for risk assessment in patients with HCM.
Keywords: fragmented QRS, hypertrophic cardiomyopathy, major arrhythmic events
1. INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a common inherited disease of the heart muscle. The prevalence of the disease in the general population is approximately 1 in 500 (Maron et al., 2014). Some patients may remain asymptomatic throughout life but others may experience heart failure symptoms or sudden cardiac arrest. Symptomatic HCM is a now much more manageable condition with improved options for SCA prevention (e.g., subcutaneous implantable defibrillator) and invasive treatments of outflow tract obstruction (Maron et al., 2014). Nonetheless, identification of patients at high risk of SCA or sudden cardiac death remains challenging.
The twelve‐lead ECG is one of the most common, useful and cost‐effective investigations performed in clinical settings. Fragmented QRS (fQRS) is a marker of myocardial scar and identifies high‐risk patients in various cardiac conditions, including acute coronary syndrome, cardiac sarcoidosis, Brugada syndrome, acquired long QT syndrome, or idiopathic dilated cardiomyopathy (Das, Khan, Jacob, Kumar, & Mahenthiran, 2006; Sha et al., 2011; Take & Morita, 2012). Recent studies suggest that fQRS is associated with increased major arrhythmic events in patients with HCM (Debonnaire et al., 2015; Femenia et al., 2013; Kang et al., 2014; Nomura et al., 2015; Ozyilmaz et al., 2017). However, no systematic review or meta‐analysis of literature has been done to address the association in this group of patients. Therefore, we performed a systematicmeta‐analysis to establish the association between fQRS and major arrhythmic events in HCM.
2. METHOD
2.1. Search strategy
Two investigators (CK and PC) independently searched for published studies indexed in MEDLINE and EMBASE databases from inception to January 2017 using a search strategy that included the terms “fragmented QRS”, “QRS fragmentation” and “hypertrophic cardiomyopathy” (described in Data S1). Only English language publications were included. A manual search for additional pertinent studies and review articles using references from retrieved articles was also completed.
2.2. Inclusion criteria
The eligibility criteria included the following:
Cohort studies (prospective or retrospective) reporting incidence of major arrhythmic events (MAE) including sustained ventricular tachycardia, sudden cardiac arrest, or sudden cardiac death) in HCM patients with and without fQRS.
Relative risk, hazard ratio, odds ratio, incidence ratio, or standardized incidence ratio with 95% confidence intervals or sufficient raw data for these calculations had to be provided.
HCM participants without fQRS were used as controls.
Study eligibility was independently determined by two investigators (TR and NK) and differences were resolved by mutual consensus. Newcastle–Ottawa quality assessment scale was used to evaluate each study in three domains: recruitment and selection of the participants, similarity and comparability between the groups, and ascertainment of the outcome of interest among cohort studies (Stang, 2010).
2.3. Data extraction
A standardized data collection form was used to obtain the following information from each study: title of study, name of first author, year of study, year of publication, country of origin, number of participants, demographic data of participants, method used to identify cases and controls, method used to diagnose the outcomes of interest (fQRS and major arrhythmic events), and average duration of follow‐up. Confounders were also assessed and adjusted effect estimates with 95% confidence interval 95% confidence intervals and covariates were included in the multivariable analysis.
To ensure accuracy, all investigators independently performed this data extraction process. Any data discrepancy was resolved by referring back to the original articles.
2.4. Statistical analysis
We performed a meta‐analysis of the included cohort studies using a random‐effects model. The extracted studies were excluded from the analysis if they did not present an outcome in each intervention group or did not have enough information required for continuous data comparison. We pooled the point estimates from each study using the generic inverse‐variance method of DerSimonian and Laird (1986). The heterogeneity of effect size estimates across these studies was quantified using the I 2 statistic and Q statistic. For the Q statistic, substantial heterogeneity was defined as p < .10. The I 2 statistic ranges in value from 0% to 100% (I 2 < 25%, low heterogeneity; I 2 = 25%–50%, moderate heterogeneity; and I 2 > 50%, substantial heterogeneity; Higgins, Thompson, Deeks, & Altman, 2003). A sensitivity analysis was performed to assess the influence of the individual studies on the overall results by omitting one study at a time. Publication bias was assessed using funnel plot and Egger's regression test (Sterne & Egger, 2001; p < .05 was considered significant). All data analyses were performed using the Stata SE 14.1 software from StataCorp LP.
3. RESULT
3.1. Description of included studies
Our search strategy yielded 15 potentially relevant articles (eight articles from EMBASE and seven articles from MEDLINE). After exclusion of three duplicate articles, 12 articles underwent title and abstract review. One article was excluded because it was an abstract presentation. Six articles were excluded at this stage since they were not cohort studies, did not report the outcome of interest (MAE) or were not conducted in patients with HCM, leaving five articles for full‐length article review. Therefore, three retrospective and two prospective cohort studies of HCM patients (205 fQRS and 468 non‐fQRS controls) were included in this meta‐analysis. Figure 1 outlines the search and literature review process. The clinical characteristics and summary of included studies are described in Table 1.
Figure 1.
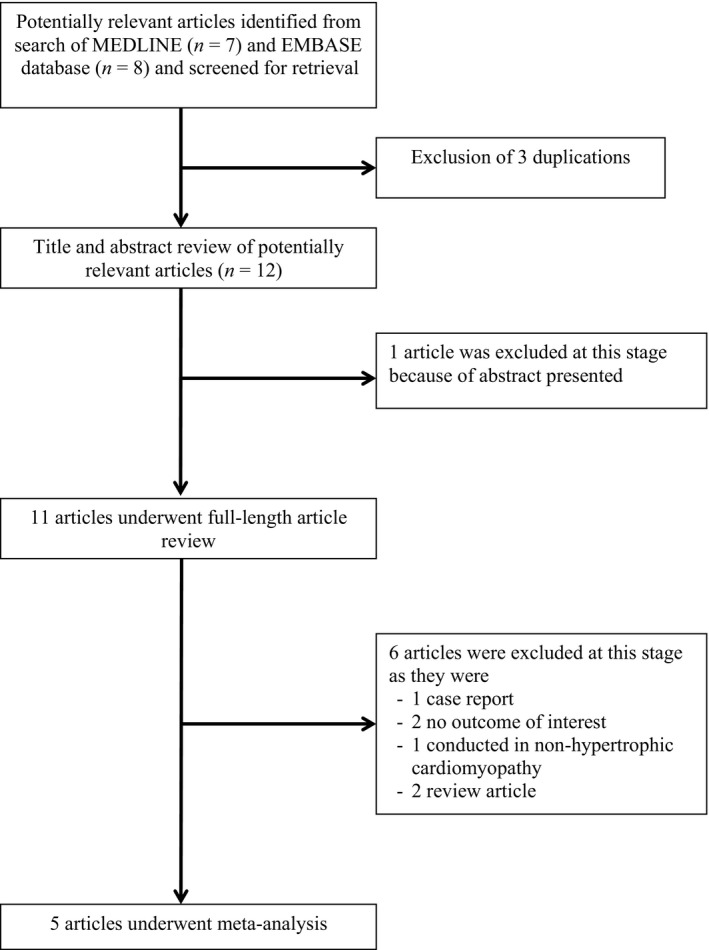
Search methodology and selection process
Table 1.
The clinical characteristics and summary of included studies
| Study | Debonnaire et al. (2015) | Femenia et al. (2013) | Kang et al. (2014) | Nomura et al. (2015) | Ozyilmaz et al. (2017) |
|---|---|---|---|---|---|
| Country | The Netherlands | Argentina, Spain, Belgium, Turkey, Venezuela, and Canada | South Korea | Japan | Turkey |
| Study design | Prospective cohort | Retrospective cohort | Retrospective cohort | Retrospective cohort | Prospective cohort |
| Year of publication | 2015 | 2013 | 2014 | 2014 | 2017 |
| Study subjects | HCM patient at Leiden University Medical Centre, The Netherlands | HCM patient with ICD implanted for primary or secondary prevention | HCM patient diagnosed from echocardiography between Feb 2001 and Apr 2007 | HCM patient followed at the Kanazawa University Hospital and its affiliated hospitals from Sep 2008 to Mar 2010 | HCM patients aged more than 17 who presented to the Mehmet Akif Ersoy Thoracic and Cardiovascular surgery Center, Training and Research Hospital and Bezmialem Vakif University School of Medicine between Dec 2012 and Mar 2016 |
| Exclusion criteria | HCM patients with ventricular pacing or bundle branch block at baseline ECG | N/A | Reduced LV function (LVEF <50%), QRS ≤120 ms, left or right bundle branch block, previous ICD placement (n and age <18 years | Unable to obtain appropriate ECG data at registration, clinical data missing, diagnosed with cardiac sarcoidosis after registration | Patients with previous history of aborted SCD or those who had previously undergone ICD implantation |
| Patients with history of septal ablation or myomectomy | |||||
| Patients with hypertension, renal failure, history of MI or aoritic valve stenosis | |||||
| Number of subjects (% male, mean age) | 195 patients (61% male, mean age 52 ± 13 years) | 102 patients (52% male, mean age 41.16 ± 18.25 years) | 273 patients (57% male, mean age 55 years) | 94 patients (60% male, mean age 58 ± 17 years) | 115 patients (58% male, mean age 46.5 ± 15.3 years) |
| Number of fQRS subjects | 145 | 54 | 67 | 31 | 65 |
| Number of non‐fQRS subjects | 50 | 48 | 100 | 63 | 50 |
| Median LV wall thickness (mm) | 21 | 24.79 ± 7.65 | 21 ± 4 | 17 ± 5 | N/A |
| Mean QTc duration (msec) | 427 ± 28 | 430.38 ± 22.98 | 438 ± 29 | 436 ± 36 | N/A |
| LA size (mm) | N/A | 42.72 ± 9.66 | N/A | N/A | 41.9 ± 4.3 |
| Hx of Non‐sustained VT | 52 | 39 | N/A | N/A | N/A |
| Unexplained syncope | 17 | 60 | 15 | N/A | 13 |
| Family Hx SCD | 91 | 33 | 33 | 11 | 48 |
| Prior personal history of SVT/VF/SCD | 13 | 43 | N/A | 7 | N/A |
| Abnormal BP during exercise | N/A | 13 | N/A | N/A | N/A |
| ICD implantation at baseline | 58 | 102 | N/A | 7 | 11 |
| fQRS definition criteria | Presence of various RSR′ patterns, notching in the R or S wave or presence of >1 additional R in ≥2 beats of a non‐aVR lead. | Presence of various RSR′ patterns, which included an additional R′ or notching of the R‐wave, notching of the down‐ or upstroke of the S‐wave, or the presence of >1R′ in two contiguous leads. | Presence of an additional R′, notching in the nadir of the R or S wave, or the presence of >1 R′ in 2 contiguous leads that corresponded to a single myocardial territory. | QRS duration <120 ms | Presence of R′ with or without a Q wave on 12‐lead ECG, the presence of notching on an R wave, the presence of notching on an S wave, or the presence of more than one R′ wave in two adjacent derivations corresponding to the feeding area of one of the major coronary arteries |
| R′, notching in nadir of the S wave, notching of R wave, or >1 R′ in 2 contiguous leads | |||||
| In patients with right or left bundle branch block (QRS duration ≥120 ms) | |||||
| RsR′ pattern with or without a Q wave, >2 notches in the R wave, >2 notches in the downstroke or upstroke of the S wave, in two contiguous leads | |||||
| Patients with mechanical pacing (QRS duration ≥120 ms) | |||||
| >2 R′ or >2 notches in the S waves in two contiguous leads | |||||
| Endpoints | Occurrence of malignant sustained VT, VF, or SCD | Appropriate ICD therapies (sustained VT or VF) | Major arrhythmic events (sustained VT and SCD) | Major arrhythmic event (sudden cardiac death, sustained VT and VF) | Sudden cardiac death |
| Mean follow‐up | Median 5.7 years (IQR 2.7–9.1) | 47.9 ± 39.3 months | 6.3 years | 4 years | 5 years |
| Conclusion | Extensive fQRS is associated with sustained VT/VF and or SCD in HCM patients. | fQRS is associated with a significant increase in arrhythmic events in HCM patients with ICD implant. | The presence of an fQRS, in particular in the inferior leads, wassignificantly associated with a higher risk of fetal ventricular arrhythmia events in HCM patients | fQRS is significantly associated with heart failure with hospitalization and lower heart failure‐free survival in HCM patient | fQRS significantly increase risk of ventricular arrhythmias and SCD in HCM patients. fQRS is an independent high‐risk indicator of SCD in HCM |
Bold terms indicate subgroup definition. ECG, electrocardiogram; fQRS, fragmented QRS; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; IQR, Interquartile range; N/A, not applicable; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
3.2. Quality assessment of included studies
Newcastle–Ottawa scales of the included studies are described in the Table S1. The Newcastle–Ottawa scale uses a star system (0–9) to evaluate included studies on three domains: selection, comparability, and outcomes. Higher scores represent higher study quality. Intra‐study risks of bias of included studies are also described in Table S2.
3.3. Meta‐analysis results
Five studies from January 2013 to May 2017 were included in this meta‐analysis. All of five studies did reveal an increased MAE among HCM patients with fQRS with four of the five studies achieving statistical significance. The pooled analysis demonstrated a statistically significant increased risk of MAE in HCM patients with fQRS compared to non‐fQRS HCM patients with the pooled risk ratio of 7.29 (95% confidence interval: 4.00–13.29, p < .001, I 2 = 0%). There was no statistical heterogeneity observed. Forest plot of our meta‐analysis is shown in Figure 2.
Figure 2.
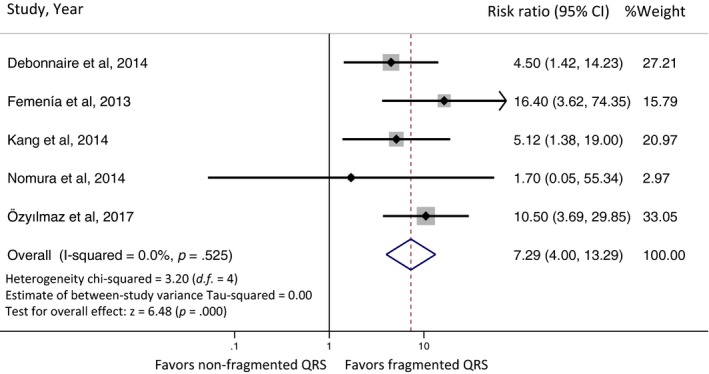
Forest plot of the included studies assessing the association between fragmented QRS and major arrhythmic events
3.4. Sensitivity analysis
To assess the stability of the results of the meta‐analysis, we conducted a sensitivity analysis by excluding one study at a time. The results were not significantly altered indicating that our findings were robust.
3.5. Publication bias
To investigate potential publication bias, we examined the contour‐enhanced funnel plot of the included studies in assessing change in log OR of death or composite outcome (Figure 3). The vertical axis represents study size (standard error) while the horizontal axis represents effect size (log odds ratio). From this plot, distribution of studies on both sides of the mean was symmetrical. The Egger's test was significant (p = .568) and confirmed no small study bias.
Figure 3.
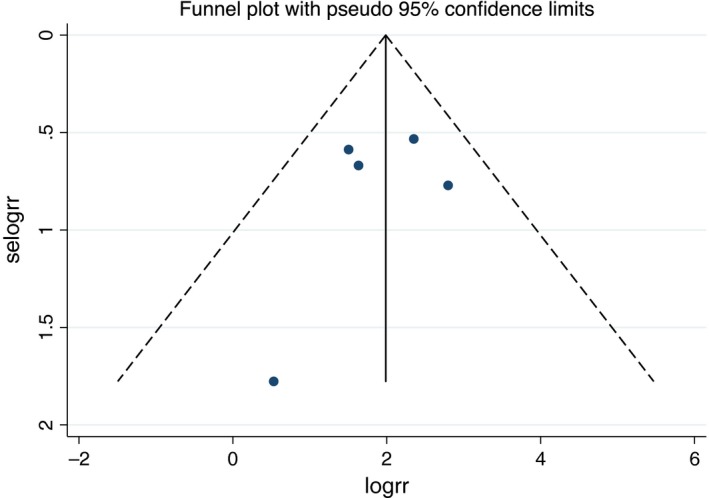
Funnel plot of fragmented QRS and major arrhythmic events. Circles represent observed published studies
4. DISCUSSION
Hypertrophic cardiomyopathy is a complex inherited heart disease and one of the most common causes of sudden cardiac death (SCD) in young adults. Multiple approaches to management of the patient should be taken into consideration. Prevention of SCD is a critical part of caring for the HCM patient. The overall risk of SCD in HCM patients is about 1% per year (Gersh et al., 2011). Recommended prevention strategies include exercise restriction, anti‐arrhythmic drugs, and ICD. Identifying those who would benefit from an ICD is challenging but an essential part of clinical decision‐making. Recommendations are based on cohort studies that identify relationships between clinical characteristics and prognostic outcomes.
Our meta‐analysis summarized all available evidence of MAE in HCM from five studies, a total of 779 patients. Fragmented QRS was present in 46% of the studied population. Our study revealed that HCM patients with fQRS have a statistically significant increased risk of MAE compared to those without fQRS (pooled risk ratio of 7.29, 95% CI 4.00–13.29, p < .001, I 2 = 0%). This result stresses the importance of integrating fQRS into risk stratification of HCM for SCD in clinical practice.
The importance of fQRS, which is suggestive of myocardial scarring and fibrosis, has been increasingly supported by recent studies of MAE in various cardiac diseases (Das et al., 2006; Konno et al., 2015; Lu et al., 2017; Sha et al., 2011; Take & Morita, 2012), including HCM (Debonnaire et al., 2015; Konno et al., 2015; Lu et al., 2017). Fibrosis or tissue heterogeneity increases susceptibility to reentry and arrhythmias. The presence of fQRS is more common in patients with high‐risk characteristics, such as family history of SCD, history of syncope, and HCM patients with a calculated HCM Risk‐SCD score >6% (Ozyilmaz et al., 2017). The strength of the association of fQRS with MAE may depend on observed territory of distribution (Femenia et al., 2013; Kang et al., 2014). A previous study of HCM patients found that the presence of fQRS in more than two territories has a positive predictive value of 33% and negative predictive value of 88% of MAE or sudden cardiac death (Debonnaire et al., 2015).
Recently, HCM Risk‐SCD, a tool developed to estimate the 5‐year risk of SCD in HCM patients, was introduced to guide ICD placement for primary prevention of SCD (Authors/Task Force Members, 2014). ICDs may be considered in patients with HCM Risk‐SCD score of ≥4% to 6% 5‐year risk and should be considered if 5‐year risk ≥6%. Variables currently used in HCM Risk‐SCD are age, family history of sudden cardiac death, unexplained syncope, left ventricular outflow gradient, maximum LV wall thickness, LA diameter, nonsustained ventricular tachycardia (Authors/Task Force Members, 2014). fQRS could provide important additional risk assessment and in fact fQRS has been shown to increase the positive predictive value from 8% to 20% and specificity from 72% to 92% in patients with one risk factor (NEED REF). Specificity was increased from 88% to 94% in patients with at least two risk factors (Kang et al., 2014). Addition of fQRS to traditional markers significantly improved the specificity and positive predictive value of future MAEs in “low risk” patients as well (Kang et al., 2014).
4.1. Limitation
In our meta‐analysis, there were some limitations that could limit its generalizability. First, all of the studies included were cohorts which are observational in nature. Three out of five studies were retrospective. Secondly, our meta‐analysis was only focused on MAE. Given the published outcomes available for study, we did not take overall mortality or total cardiovascular mortality into account. However, the recent study from Lu et al. (2017) shows that fQRS is associated with increased all‐cause mortality, cardiovascular disease mortality, and heart failure related death. In addition, our data is not sufficient to determine the relationship between fQRS and other ECG parameters such as prolonged QTc or territory of fQRS to the outcome of interest. According to Debonnaire et al. (2015), prolonged QTc is an independent predictor of ventricular arrhythmias and sudden cardiac death. Finally, there was some heterogeneity amongst the studies. However, we used sensitivity analysis methods in the random‐effects model and found no difference between the imputed risk ratio and its 95% confidence interval.
5. CONCLUSION
In conclusion, we found that baseline fQRS significantly increased the risk of MAE in HCM patients. fQRS should be considered as an important factor when considering primary prevention ICD implantation in HCM patients. Further study is needed to establish the role of fQRS in risk stratification for SCD in patients with HCM.
CONFLICT OF INTEREST
None to declare.
AUTHOR'S CONTRIBUTION
Pattara Rattanawong: Conception design, data interpretation, statistic analysis, revise manuscript, final approval, corresponding; Tanawan Riangwiwat, Chanavuth Kanitsoraphan: Data acquisition, data interpretation, draft manuscript; Pakawat Chongsathidkiet, Napatt Kanjanahattakij: Data acquisition, data interpretation; Wasawat Vutthikraivit: Data interpretation, draft manuscript; Eugene H. Chung: Revise manuscript, critical reading.
Supporting information
ACKNOWLEDGMENT
None.
Rattanawong P, Riangwiwat T, Kanitsoraphan C, et al. Baseline fragmented QRS increases the risk of major arrhythmic events in hypertrophic cardiomyopathy: Systematic review and meta‐analysis. Ann Noninvasive Electrocardiol. 2018;23:e12533 10.1111/anec.12533
Data from this paper will be presented at the 2017 American Heart Association Scientific Sessions.
REFERENCES
- Authors/Task Force Members , Elliott, P. M. , Anastasakis, A. , Borger, M. A. , Borggrefe, M. , Cecchi, F. , … Watkins, H. (2014). SC guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). European Heart Journal, 39, 2733–2779. [DOI] [PubMed] [Google Scholar]
- Das, M. K. , Khan, B. , Jacob, S. , Kumar, A. , & Mahenthiran, J. (2006). Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation, 113(21), 2495–2501. 10.1161/CIRCULATIONAHA.105.595892 [DOI] [PubMed] [Google Scholar]
- Debonnaire, P. , Katsanos, S. , Joyce, E. , Brink, O. V. , Atsma, D. E. , Schalij, M. J. , … Marsan, N. A. (2015). QRS fragmentation and QTc duration relate to malignant ventricular tachyarrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy. Journal of Cardiovascular Electrophysiology, 5, 547–555. 10.1111/jce.12629 [DOI] [PubMed] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Femenia, F. , Arce, M. , Van Grieken, J. , Trucco, E. , Mont, L. , Abello, M. , … Hopman, W. M. (2013). Fragmented QRS as a predictor of arrhythmic events in patients with hypertrophic obstructive cardiomyopathy. Journal of Interventional Cardiac Electrophysiology, 38(3), 159–165. 10.1007/s10840-013-9829-z [DOI] [PubMed] [Google Scholar]
- Gersh, B. J. , Maron, B. J. , Bonow, R. O. , Dearani, J. A. , Fifer, M. A. , Link, M. S. , … American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines (2011). 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology, 58(25), e212–e260. 10.1016/j.jacc.2011.06.011 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. British Medical Journal, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, K. W. , Janardhan, A. H. , Jung, K. T. , Lee, H. S. , Lee, M. H. , & Hwang, H. J. (2014). Fragmented QRS as a candidate marker for high‐risk assessment in hypertrophic cardiomyopathy. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 11(8), 1433–1440. 10.1016/j.hrthm.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Konno, T. , Hayashi, K. , Fujino, N. , Oka, R. , Nomura, A. , Nagata, Y. , … Yamagishi, M. (2015). Electrocardiographic QRS fragmentation as a marker for myocardial fibrosis in hypertrophic cardiomyopathy. Journal of Cardiovascular Electrophysiology, 26(10), 1081–1087. 10.1111/jce.12742 [DOI] [PubMed] [Google Scholar]
- Lu, X. , Wang, W. , Zhu, L. , Wang, Y. , Sun, K. , Zou, Y. , … Song, L. (2017). Prognostic significance of fragmented QRS in patients with hypertrophic cardiomyopathy. Cardiology, 138(1), 26–33. 10.1159/000471845 [DOI] [PubMed] [Google Scholar]
- Maron, B. J. , Ommen, S. R. , Semsarian, C. , Spirito, P. , Olivotto, I. , & Maron, M. S. (2014). Hypertrophic cardiomyopathy: Present and future, with translation into contemporary cardiovascular medicine. Journal of the American College of Cardiology, 64(1), 83–99. 10.1016/j.jacc.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Nomura, A. , Konno, T. , Fujita, T. , Tanaka, Y. , Nagata, Y. , Tsuda, T. , … Fujino, N. (2015). Fragmented QRS predicts heart failure progression in patients with hypertrophic cardiomyopathy. Circulation Journal, 79(1), 136–143. [DOI] [PubMed] [Google Scholar]
- Ozyilmaz, S. , Akgul, O. , Uyarel, H. , Pusuroglu, H. , Karayakali, M. , Gul, M. , … Bakır, İ. (2017). Assessment of the association between the presence of fragmented QRS and the predicted risk score of sudden cardiac death at 5 years in patients with hypertrophic cardiomyopathy. Anatolian Journal of Cardiology, 18, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha, J. , Zhang, S. , Tang, M. , Chen, K. , Zhao, X. , & Wang, F. (2011). Fragmented QRS is associated with all‐cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Annals of Noninvasive Electrocardiology, 16(3), 270–275. 10.1111/j.1542-474X.2011.00442.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang, A. (2010). Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. European Journal of Epidemiology, 25(9), 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- Sterne, J. A. , & Egger, M. (2001). Funnel plots for detecting bias in meta‐analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology, 54(10), 1046–1055. 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- Take, Y. , & Morita, H. (2012). Fragmented QRS: What is the meaning? Indian Pacing and Electrophysiology Journal, 12(5), 213–225. 10.1016/S0972-6292(16)30544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


