Abstract
Background
Recently, it has become increasingly recognized that pulmonary hypertension (PH) is a particularly threatening result of left‐sided heart disease. However, there have been few investigations of the impact of cardiopulmonary exercise testing (CPX) variables on PH in dilated cardiomyopathy (DCM). We evaluated the usefulness of crucial CPX variables for detecting elevated pulmonary arterial pressure (PAP) in patients with DCM.
Methods
Ninety subjects with DCM underwent cardiac catheterization and CPX at our hospital. Receiver operator characteristic (ROC) analysis was performed to assess the ability of CPX variables to distinguish between the presence and absence of PH.
Results
Overall mean values were: mean PAP (mPAP), 18.0 ± 9.6 mmHg; plasma brain natriuretic peptide, 233 ± 295 pg/mL; and left ventricular ejection fraction, 30.2 ± 11.0%. Patients were allocated to one of two groups on the basis of mean PAP, namely DCM without PH [mean PAP (mPAP) <25 mmHg; n = 75] and DCM with PH (mPAP ≥25 mmHg; n = 15). A cutoff achieved percentage of predicted peak VO2 (%PPeak VO2) of 52.5% was the best predictor of an mPAP ≥25 mmHg in the ROC analysis (area under curve: 0.911). In the multivariate analysis, %PPeak VO2 was the only significant independent predictor of PH (Wald 6.52, odds ratio 0.892, 95% CI 0.818–0.974; P = 0.011).
Conclusions
%PPeak VO2 was strongly associated with the presence of PH in patients with DCM. Taken together, these findings indicate that CPX variables could be important for diagnosing PH in patients with DCM.
Keywords: cardiopulmonary exercise testing, dilated cardiomyopathy, peak VO2, pulmonary hypertension
Pulmonary hypertension (PH) occurs commonly in patients with left heart disease and is associated with increased morbidity and mortality.1, 2 PH associated with left heart disease is classified in group 2 of the Nice 2013 classification.2 and is believed to be the most common form of PH,3 with both passive and active components.1, 4 In patients with ischemic or nonischemic dilated cardiomyopathy (DCM), the presence of PH is also a predictor of morbidity or mortality.5 We previously reported that the presence of PH provides valuable prognostic information in ambulatory patients with DCM.6
Cardiopulmonary exercise testing (CPX) is an established assessment tool in heart failure (HF) populations.7 Most often, CPX is used to assess prognosis in HF patients being considered for heart transplantation.8 and to provide an additional evaluation of disease severity.7 Given the emerging importance of detecting PH in HF patients and the already established role of CPX in this patient population, examining the ability of this exertional assessment to unmask elevated pulmonary arterial pressure (PAP) is an important research endeavor. CPX has already been proved useful for diagnosing secondary PH in other patients, such as those with hypertrophic cardiomyopathy.9 or HF.10, 11 However, to our knowledge, there have been no investigations of the impact of CPX variables on PH in DCM. Here, we therefore aimed to evaluate the usefulness of crucial CPX variables for detecting elevated PAP in patients with DCM.
METHODS
Study Population
A total of 90 consecutive ambulatory patients with DCM (62 men (69%); mean age ± SD, 52 ± 13 years) were enrolled retrospectively in the study at Nagoya University Hospital, Japan. All patients were on optimal pharmacological therapy according to current guidelines for the treatment of HF.12 Individuals who had suffered an episode of acute HF within the previous month, who had renal dysfunction [estimated glomerular filtration rate (eGFR) <30 mL min–1 1.73 m–2], or who had received an implanted cardiac resynchronization therapy device or an implantable cardioverter defibrillator before cardiac catheterization were excluded from the study. Echocardiographic findings as a screening tool were left ventricular (LV) dilatation and systolic dysfunction, as defined by depressed LV ejection fraction. DCM was defined by the presence of both an LV ejection fraction of <50% (as revealed by contrast left ventriculography) and dilation of the LV cavity in the absence of coronary artery stenosis of >50% (as determined by coronary angiography), valvular heart disease, arterial hypertension, and secondary cardiac muscle disease attributable to any known systemic condition.13 No patients had histories of acute viral myocarditis or familial DCM, or evidence of immune triggers. The study protocol complied with the Declaration of Helsinki, and written informed consent was obtained from each study patient. The study protocol was approved by the Ethics Review Board of Nagoya University School of Medicine (approval no. 359).
Study Protocol
Physical examination, laboratory measurements, CPX, and biventricular catheterization were performed within 3 days of study enrollment. All patients were in a stable condition at the time of testing.
Cardiac Catheterization
All patients initially underwent diagnostic right and left heart catheterization. Patients were in a stable condition at the time of catheterization. For hemodynamic assessment, a 6F Swan‐Ganz catheter (Goodman Biosensors, Tokyo, Japan) was inserted by using a jugular approach. Coronary angiography and left ventriculography via the right radial artery were also performed. A 6F fluid‐filled pigtail catheter with a high‐fidelity micromanometer (CA‐61000‐PLB Pressure‐tip Catheter; CD Leycom, Zoetermeer, The Netherlands) was positioned in the left ventricle to measure LV pressure. Endomyocardial biopsy was performed in all patients to exclude myocarditis and specific heart muscle disease. Biopsy specimens were obtained from the septal wall of the right ventricle with a 6F bioptome.
Diagnosis of PH
PH was defined hemodynamically as a mean PAP (mPAP) of ≥25 mmHg at rest, as assessed by right heart catheterization without inhalation of nitric oxide and supplemental oxygen. Mean PAP, mean right atrial pressure, mean pulmonary artery wedge pressure, and the respective oxygen saturations, along with those in the main pulmonary artery, were measured. Cardiac output was assessed by thermodilution and was expressed in liters per minute.
CPX Procedure
Each patient underwent CPX at a progressively increasing work rate to maximal tolerance on a cycle ergometer. The test protocol was in accordance with the recommendations of the American Thoracic Society and American College of Chest Physicians.14 The oxygen and carbon dioxide sensors were calibrated before each test by using gases with known oxygen, nitrogen, and carbon dioxide concentrations. The flow sensor was also calibrated before each test by using a 3‐L syringe. All patients started at 10 W for a 3‐min warm‐up, followed by a 10‐W/min ramp increment protocol up to the termination criteria. Test termination criteria consisted of patient request, volitional fatigue, ventricular tachycardia, ≥2 mm horizontal or downsloping ST‐segment depression, or a drop in systolic blood pressure (BP) of ≥20 mmHg during exercise. A qualified exercise physiologist conducted each test, with physician supervision. A 12‐lead electrocardiogram was monitored continuously, and BP was measured every minute during exercise and throughout the recovery period. Respiratory gas exchange variables, including VO2, VCO2, and minute ventilation (VE), were acquired continuously throughout the exercise testing by using an Oxycon Pro ergospirometer (Care Fusion; San Diego, CA, USA); gas‐exchange data were obtained breath‐by‐breath. Peak VO2 was expressed as the highest 30‐s average value obtained during the last stage of the exercise test, and the peak respiratory exchange ratio was the highest 30‐s average value during the last stage of the test. The VE/VCO2 slope was determined by using linear regression analysis of the VE and VCO2 obtained during exercise.15 Exercise oscillatory ventilation (EOV) was assessed by using the criteria previously reported by Leite et al.16 The achieved percentage of predicted peak VO2 (%PPeak VO2) was calculated as [obtained peak VO2 / age‐, gender‐, and weight‐adjusted predicted peak VO2 in mL min‐1 kg‐1] × 100.17, 18 The ratio of the increase in VO2 to the increase in work rate (WR) [ΔO2/ΔWR] was calculated by least‐squares linear regression from the data recorded between 30 s after the start of incremental exercise and 30 s before the end of exercise.
Statistical Analysis
Data are presented as means ± SD. Variables were compared between the DCM with PH and DCM without PH groups by using Student's t‐test for unpaired data. The chi‐square test was used to assess the significance of differences between dichotomous variables. The impact of mPAP on outcome was analyzed by using the presence or absence of PH as a categorical determinant of adverse events. Other baseline predictors of events were determined by performing univariate Cox proportional hazard regression analysis with age, gender, creatinine, plasma brain natriuretic peptide (BNP), heart rate, cardiac index, systolic BP, LV end‐diastolic pressure, pulmonary arterial wedge pressure, pulmonary vascular resistance, peak VO2, and VE/VCO2 slope as potential determinants. The hazard ratio and 95% confidence interval were defined. Receiver‐operator characteristic (ROC) curve analysis was used to assess the ability of CPX variables to identify subjects with an mPAP ≥25 mmHg. Binary logistic regression assessed the univariate and multivariate (with P < 0.1) ability of CPX variables to identify subjects with mPAP ≥25 mmHg. All analyses were performed with the SPSS 17.0 software package (SPSS, Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
RESULTS
Patient Characteristics
The mean age was 52 years, and 69% of subjects were male. At the time of cardiac catheterization, beta blockers were used by 87% of all patients, angiotensin‐converting‐enzyme inhibitors (ACE‐Is) or angiotensin II receptor blockers (ARBs) by 86%, diuretics by 69%, and spironolactone by 46%. The mean (25th, 75th percentile) plasma BNP level was 233 (55, 306) pg/mL, and the mean LV ejection fraction was 30.2 ± 11.0%.
Clinical characteristics and important hemodynamic parameters of all patients are shown in Table 1. Subjects were allocated to one of two groups on the basis of the absence (DCM without PH group, n = 75) or presence (DCM with PH group, n = 15) of PH. PH was present in 17% of patients with DCM, and the median (25th, 75th percentile) mPAP for all DCM patients was 17.9 (11.8, 20.0) mmHg. DCM patients with PH were significantly younger than those without PH. Diuretics, beta‐blockers, and spironolactone were used significantly more frequently in DCM with PH than in DCM without PH, but there were no differences between the 2 groups in the use of ACE‐Is or ARBs at the time of cardiac catheterization. Although plasma BNP levels were significantly higher in DCM with PH, eGFR, and serum hemoglobin levels did not differ between the two groups. On cardiac catheterization, the systolic, diastolic, and mean PAP, as well as the pulmonary vascular resistance and right arterial pressure, were significantly higher in DCM with PH than in DCM without PH, whereas the cardiac index and mixed venous oxygen saturation were significantly lower in the former group. LV end‐diastolic pressure, LV end‐diastolic volume index, and LV end‐systolic volume index were significantly higher, and LV ejection fraction was significantly lower, in DCM with PH.
Table 1.
Patient Characteristics
| DCM without PH | DCM with PH | ||
|---|---|---|---|
| (n = 75) | (n = 15) | P | |
| Age (years) | 54 ± 13 | 45 ± 14 | 0.038 |
| Male (n, %) | 53 (71) | 9 (60) | 0.459 |
| BMI (kg/m2) | 23.5 ± 4.2 | 21.6 ± 4.1 | 0.133 |
| NYHA | 1.57 ± 0.76 | 2.50 ± 1.23 | 0.131 |
| Medication | |||
| Diuretics (n, %) | 47 (63) | 15 (100) | <0.001 |
| ACE‐I/ARBs (n, %) | 63 (84) | 14 (93) | 0.753 |
| β‐Blockers (n, %) | 63 (84) | 15 (100) | <0.001 |
| Digitalis (n, %) | 9 (12) | 9 (17) | 0.710 |
| Statins (n, %) | 9 (12) | 1 (7) | 0.669 |
| Amiodarone (n, %) | 7 (9) | 5 (33) | 0.271 |
| Spironolactone (n, %) | 29 (39) | 12 (80) | 0.002 |
| Laboratory | |||
| BNP (pg/mL) | 175 (37–274) | 550 (178–840) | 0.013 |
| eGFR (mL/min/1.73 m2) | 72 ± 26 | 74 ± 15 | 0.707 |
| Hb (mg/dL) | 14.0 ± 1.6 | 14.0 ± 2.3 | 0.962 |
| T. Chol (mg/dL) | 194 ± 34 | 175 ± 46 | 0.152 |
| TG (mg/dL) | 156 (77–177) | 111 (68–152) | 0.046 |
| HbA1c (%) | 5.74 ± 1.15 | 5.63 ± 0.80 | 0.648 |
| Cardiac catheterization | |||
| PAWP (mmHg) | 10.0 ± 4.6 | 25.3 ± 6.9 | <0.001 |
| Systolic PAP (mmHg) | 24.2 ± 6.8 | 49.0 ± 15.4 | <0.001 |
| Diastolic PAP (mmHg) | 9.5 ± 4.0 | 28.8 ± 10.0 | <0.001 |
| Mean PAP (mmHg) | 14.4 ± 4.3 | 35.5 ± 9.6 | <0.001 |
| PVR (Wood units) | 0.96 ± 0.92 | 3.25 ± 2.64 | 0.005 |
| RAP (mmHg) | 5.0 ± 3.1 | 7.7 ± 2.6 | 0.018 |
| SvO2 (%) | 71.8 ± 5.8 | 61.8 ± 7.3 | 0.024 |
| TPG (mmHg) | 4.4 ± 4.0 | 10.3 ± 5.7 | 0.002 |
| DPG (mmHg) | –0.5 ± ‐0.6 | 3.5 ± 3.2 | <0.001 |
| Heart rate (bpm) | 76.5 ± 13.1 | 85.0 ± 23.9 | 0.303 |
| Cardiac output (L/min) | 4.73 ± 1.20 | 3.75 ± 1.46 | 0.025 |
| Cardiac index (L/min/m2) | 2.75 ± 0.59 | 2.27 ± 0.69 | 0.022 |
| Systolic BP (mmHg) | 122 ± 19 | 104 ± 30 | 0.051 |
| Diastolic BP (mmHg) | 74 ± 12 | 70 ± 14 | 0.287 |
| LVEDP (mmHg) | 14.4 ± 7.1 | 25.1 ± 8.8 | 0.001 |
| LVEDVI (ml/m2) | 129.5 ± 41.3 | 183.7 ± 65.4 | 0.004 |
| LVESVI (ml/m2) | 90.6 ± 37.8 | 150.1 ± 59.2 | 0.004 |
| LVEF (%) | 32.0 ± 10.1 | 22.7 ± 10.4 | 0.001 |
Data are presented as mean values ± SD and medians [interquartile range or n (%)].
ACE‐I = angiotensin‐converting enzyme inhibitor; ARB = angiotensin‐receptor blocker; BMI = body mass index; BNP = brain natriuretic peptide; BP = blood pressure; DCM = dilated cardiomyopathy; DPG = diastolic pulmonary vascular gradient; eGFR = estimated glomerular filtration rate; Hb = hemoglobin; LVEDP = LV end‐diastolic pressure; LVEDVI = LV end‐diastolic volume index; LVESVI = LV end‐systolic volume index; LVEF = LV ejection fraction; NYHA = New York Heart Association; PAP = pulmonary arterial pressure; PAWP = pulmonary artery wedge pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; RAP = right atrial pressure; SvO2 = mixed venous oxygen saturation; T. Chol = total cholesterol; TG = triglyceride; TPG = transpulmonary pressure gradient.
The CPX variables are shown in Table 2. Although exercise duration did not differ between the two groups, peak VO2, %PPeak VO2, peak VO2/HR ratio, and ΔVO2/ΔWR were significantly lower in DCM with PH than in DCM without PH. VE/VCO2 slope was significantly higher in DCM with PH than in DCM without PH. Although resting HR and resting systolic BP did not differ significantly between the two groups, peak HR and peak systolic BP were significantly lower in DCM with PH than in DCM without PH.
Table 2.
Hemodynamic Parameters in Cardiopulmonary Exercise Testing
| DCM without PH | DCM with PH | ||
|---|---|---|---|
| (n = 75) | (n = 15) | P | |
| Exercise duration (min) | 7.5 ± 2.5 | 7.1 ± 3.9 | 0.671 |
| Peak VO2 (ml/kg/min) | 19.2 ± 4.8 | 11.3 ± 3.6 | <0.001 |
| %PPeak VO2 (%) | 72.0 ± 21.8 | 40.6 ± 12.8 | <0.001 |
| VE/VCO2 slope | 29.2 ± 7.4 | 38.6 ± 9.7 | 0.002 |
| Peak VO2/HR ratio | 10.1 ± 4.6 | 6.9 ± 3.4 | 0.005 |
| ΔVO2/ΔWR | 9.7 ± 3.8 | 5.5 ± 3.1 | <0.001 |
| Resting HR (bpm) | 85 ± 19 | 88 ± 17 | 0.60 |
| Peak HR (bpm) | 133 ± 29 | 117 ± 21 | 0.019 |
| Resting systolic BP (mmHg) | 121 ± 22 | 111 ± 30 | 0.212 |
| Peak systolic BP (mmHg) | 161 ± 36 | 131 ± 45 | 0.026 |
| Resting PETCO2 (mmHg) | 33.3 ± 6.4 | 30.4 ± 3.7 | 0.112 |
| EOV | 23 (31%) | 7 (47%) | 0.231 |
| Peak RER | 1.09 ± 0.08 | 1.07 ± 0.11 | 0.332 |
BP = blood pressure; HR = heart rate; EOV = exercise oscillatory ventilation; %PPeak VO2 = achieved percentage of predicted peak VO2; PETCO2 = end‐tidal carbon dioxide tension; RER = respiratory exchange ratio; ΔVO2/ΔWR = ratio of change in VO2 to change in work rate.
CPX Variables for Detecting PH
We performed an ROC curve analysis of the ability of peak VO2, %PPeak VO2, VE/VCO2 slope, and ΔVO2/ΔWR to detect mPAP ≥25 mmHg (Fig. 1). All four diagnostic models were significant for detecting mPAP ≥25 mmHg. A %PPeak VO2 cutoff value of 52.5% was the best predictor of mPAP ≥25 mmHg in the ROC analysis (area under the curve [AUC: 0.911]; 95% confidence interval [CI]: 0.846 to 0.977, P < 0.001). The sensitivity and specificity of using %PPeakVO2 to detect PH were 82.8% and 85.7%, respectively. The sensitivity and specificity with a peak VO2 cutoff value of 13.55 mL kg‐1 min‐1 were 90.6% and 71.4%, respectively (AUC: 0.904; 95% CI: 0.832 to 0.976, P < 0.001). A VE/VCO2 slope cutoff value of 31.01 had significant diagnostic value (AUC: 0.801; 95% CI: 0.681 to 0.920, P < 0.001), and a ΔVO2/ΔWR cutoff value of 7.79 also had significant diagnostic value (AUC: 0.841; 95% CI: 0.714 to 0.968, P < 0.001) for detecting PH.
Figure 1.
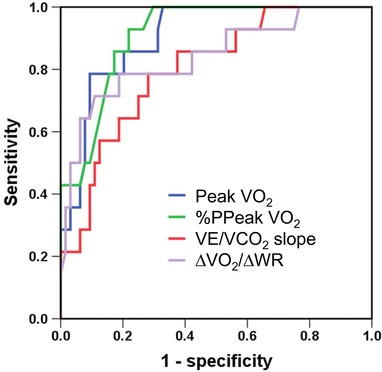
Receiver operating characteristic (ROC) curves showing the ability of cardiopulmonary exercise testing variables to detect pulmonary hypertension (PH). ROC curves of the abilities of peak VO2, %PPeak VO2, VE/VCO2 slope, and ΔVO2/ΔWR to detect PH (i.e., mPAP ≥25 mmHg). AUC = area under the curve; CI = confidence interval; %PPeak VO2 = achieved percentage of predicted peak VO2; WR = work rate.
We used binary logistic regression to assess the independent and combined abilities of CPX variables for detecting PH (Table 3). In the univariate analysis, peak VO2, %PPeak VO2, peak VO2/HR ratio, VE/VCO2 slope, ΔVO2/ΔWR, peak systolic BP, and rest PETCO2 were significant predictors of PH. In the multivariate analysis, %PPeak VO2 was the only significant independent predictor of PH (Wald 6.52, odds ratio 0.892, 95% CI 0.818 to 0.974; P = 0.011).
Table 3.
Binary Logistic Analysis for the Detection of Pulmonary Hypertension
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Analysis | Wald | OR | 95%CI | P | Wald | OR | 95%CI | P |
| Peak VO2 (mL/kg/min) | 15.1 | 0.672 | 0.550–0.821 | <0.001 | ||||
| %PPeak VO2 (%) | 14.0 | 0.902 | 0.854 –0.952 | <0.001 | 6.52 | 0.892 | 0.818–0.974 | 0.011 |
| Peak VO2/HR ratio | 7.13 | 0.744 | 0.600 –0.924 | 0.008 | ||||
| VE/VCO2 slope | 11.6 | 1.136 | 1.056 –1.222 | 0.001 | ||||
| ΔVO2/ΔWR | 13.9 | 0.609 | 0.470 –0.790 | <0.001 | ||||
| Peak HR (bpm) | 3.81 | 0.979 | 0.959 –1.000 | 0.051 | ||||
| Peak systolic BP (mmHg) | 6.62 | 0.976 | 0.958 –0.994 | 0.010 | ||||
| EOV | 0.52 | 1.023 | 0.726 –1.370 | 0.528 | ||||
| Rest PETCO2 (mmHg) | 6.99 | 0.862 | 0.772 –0.962 | 0.008 | ||||
| Peak W (watts) | 0.47 | 0.993 | 0.974 –1.013 | 0.490 | ||||
DISCUSSION
Here, we reported for the first time that reduced %PPeak VO2 was strongly associated with the presence of PH in patients with DCM. Other CPX variables, including peak VO2, VE/VCO2 slope, and ΔVO2/ΔWR, were also useful for detecting the presence of PH. Taken together, these results indicate that CPX variables could be important for diagnosing PH in patients with DCM.
Usefulness of Exercise Capacity for Detecting PH in DCM
Four CPX variables, namely peak VO2, %PPeak VO2, ΔVO2/ΔWR, and VE/VCO2 slope (see Fig. 1), were especially strong predictors of PH. Peak VO2 and %PPeakVO2 were superior to the other two as diagnostic markers of increased mPAP. In addition, %PPeak VO2 was the only significant independent predictor of PH by the multivariate logistic analysis (see Table 3).
Peak VO2 has traditionally been considered a “gold standard” for selecting candidates for cardiac transplantation.8 In contrast, %PPeakVO2 is age‐, gender‐, and weight‐adjusted and is based on directly measured peak VO2 during CPX. Furthermore, %PPeak VO2 provides important information that can be used for risk stratification of patients with HF.19 %PPeak VO2 and peak VO2 had similar abilities to detect PH by ROC analysis, but %PPeak VO2 was the only significant independent predictor of PH by the multivariate logistic analysis in our patients.
DCM patients range in age and tend to be younger than other HF patients; this was certainly the case in our DCM patients. The combination of reduced LV ejection fraction and relatively young age at onset suggests that DCM occurrence is at least partly influenced by genetic factors,20 although our study did not include genetic profiling to support this hypothesis. These patients are at risk of developing severe, refractory HF. From this perspective, %PPeak VO2 might be a more useful predictor of PH than other CPX variables, including peak VO2 in DCM because it is adjusted for age.
In patients with HF, the ventilatory parameters in CPX can reflect reactive PH.11 VE/VCO2 slope or EOV is important for diagnosing PH in hypertrophic cardiomyopathy,9 and HF, including with normal ejection fraction,10 or ischemia.11 We considered that the main reason for the difference between these studies.10, 11 and our study was different study populations. In addition, the mechanisms behind the occurrence of a steep VE/VCO2 slope and the presence of EOV are multifactorial. Abnormalities of ventilatory reflex control and pulmonary hemodynamics, as well as the presence of a low cardiac index, during exercise are all possible causes.16, 21, 22 In our study, VE/VCO2 slope (see Fig. 1), but not EOV (AUC: 0.625; 95% CI: 0.427 to 0.823, P = 0.211), was a significant parameter for detecting PH in the ROC analysis. Similarly, VE/VCO2 slope, but not EOV, was a significant predictor of PH in the univariate analysis (see Table 3). EOV was assessed by using the criteria previously reported by Leite et al.16 Defining EOV can be complex and difficult during exercise; further investigations are needed to determine whether it can be used to detect PH in DCM.
Parameters obtained during submaximal exercise have an advantage over peak VO2 in that they can be obtained without maximum effort. In addition, measurement of peak VO2 depends on the subject's motivation and is easily influenced by the bias of the investigator. ΔVO2/ΔWR and VE/VCO2 slope are characterized by the time course of change in respiratory gas variables, reflecting the ability of cardiopulmonary function to adapt to increasing work rate. ΔVO2/ΔWR and VE/VCO2 slope may therefore, in some regards, be useful in addition to peak VO2 for assessing PH.
Clinical Implications
Exercise tolerance reflects a number of important prognostic factors, including cardiac function, oxygen‐carrying capacity, and autonomic nervous system balance.23, 24, 25 CPX is a diagnostic tool used to detect serial changes in exercise capacity. It is of particular benefit for assessing peak VO2 and VE/VCO2 slope in patients with chronic HF, because these parameters function as predictors of overall mortality or determinants of risk stratification in such individuals.26, 27, 28, 29, 30
The recognition of PH due to left heart disease has created the need for diagnostic tests to determine when a patient's PAP is elevated. CPX variables have already been evaluated by echocardiography to detect systolic PAP ≥40 mmHg in an HF population.10 Determination of systolic PAP echocardiographically by using the sum of the peak RV‐RA (right ventricular – right atrial) pressure gradient and the RA pressure has been established as reliable,31 but additional studies have questioned the accuracy of this relationship, particularly at higher pulmonary artery pressures.32, 33 In patients with very severe tricuspid regurgitation, the Doppler envelope may be truncated because of the early equalization of RV and RA pressures, and using a simplified Bernoulli equation may underestimate the RV‐RA gradient.34
Here, we examined resting hemodynamic variables, gold standard method, by cardiac catheterization. The use of CPX to diagnose secondary PH has already been demonstrated.9, 35 However, to our knowledge, this is the first study to have investigated the ability of crucial CPX variables to detect elevated PAP.
Currently, there is no specific therapy for PH due to left heart disease. All of our study patients who had DCM with PH used various combinations of diuretics and beta blockers. In addition, 80% of them were using spironolactone at the time of cardiac catheterization; we therefore considered that these patients were optimally medicated. Using CPX to detect PH may lead to early therapeutic interventions for DCM.
Study Limitations
This was a retrospective study in a single center and with a relatively small sample size. Moreover, hemodynamic diagnosis by challenge tests such as exercise and acute pulmonary vasoreactivity testing was not performed. Finally, the use of single time point measurements did not allow us to assess the time‐dependence of PAP in the Cox regression analysis and may have led us to underestimate the prognostic significance of PH.
CONCLUSIONS
%PPeak VO2 was strongly associated with the presence of PH in patients with DCM. Peak VO2, VE/VCO2 slope, and ΔVO2/ΔWR were also useful for detecting the presence of PH. Taken together, these findings indicate that CPX variables could be important for diagnosing PH in patients with DCM.
Disclosures
Takahisa Kondo and Akihiro Hirashiki are affiliated with a university department that receives funding from Actelion Pharmaceuticals Japan.
Acknowledgments
This work was supported in part by grants‐in‐aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology, Tokyo, Japan.
REFERENCES
- 1. Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012;126:975–990. [DOI] [PubMed] [Google Scholar]
- 2. Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–41. [DOI] [PubMed] [Google Scholar]
- 3. Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43–54. [DOI] [PubMed] [Google Scholar]
- 4. Fang JC, DeMarco T, Givertz MM, et al. World Health Organization Pulmonary Hypertension group 2: Pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012;31:913–933. [DOI] [PubMed] [Google Scholar]
- 5. Abramson SV, Burke JF, Kelly JJ, et al. Pulmonary hypertension predicts mortality and morbidity in patients with dilated cardiomyopathy. Ann Intern Med 1992;116:888–895. [DOI] [PubMed] [Google Scholar]
- 6. Hirashiki A, Kondo T, Adachi S, et al. Prognostic value of pulmonary hypertension in ambulatory patients with non‐ischemic dilated cardiomyopathy. Circ J 2014;78:1245–1253. [DOI] [PubMed] [Google Scholar]
- 7. Balady GJ, Arena R, Sietsema K, et al. Clinician's Guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 8. Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991;83:778–786. [DOI] [PubMed] [Google Scholar]
- 9. Arena R, Owens DS, Arevalo J, et al. Ventilatory efficiency and resting hemodynamics in hypertrophic cardiomyopathy. Med Sci Sports Exerc 2008;40:799–805. [DOI] [PubMed] [Google Scholar]
- 10. Guazzi M, Cahalin LP, Arena R. Cardiopulmonary exercise testing as a diagnostic tool for the detection of left‐sided pulmonary hypertension in heart failure. J Card Fail 2013;19:461–467. [DOI] [PubMed] [Google Scholar]
- 11. Lim HS, Theodosiou M. Exercise ventilatory parameters for the diagnosis of reactive pulmonary hypertension in patients with heart failure. J Card Fail 2014;20:650–657. [DOI] [PubMed] [Google Scholar]
- 12. Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005;46:e1–82. [DOI] [PubMed] [Google Scholar]
- 13. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br Heart J 1980;44:672–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 15. Bard RL, Gillespie BW, Clarke NS, et al. Determining the best ventilatory efficiency measure to predict mortality in patients with heart failure. J Heart Lung Transplant 2006;25:589–595. [DOI] [PubMed] [Google Scholar]
- 16. Leite JJ, Mansur AJ, de Freitas HF, et al. Periodic breathing during incremental exercise predicts mortality in patients with chronic heart failure evaluated for cardiac transplantation. J Am Coll Cardiol 2003;41:2175–2181. [DOI] [PubMed] [Google Scholar]
- 17. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 1973;85:546–562. [DOI] [PubMed] [Google Scholar]
- 18. Wasserman KHJ, Sue DY, Whipp BJ. Principles of Exercise Testing and Interpretation. Philadelphia, Lea & Febiger, 1986, pp.73. [Google Scholar]
- 19. Stelken AM, Younis LT, Jennison SH, et al. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol 1996;27:345–352. [DOI] [PubMed] [Google Scholar]
- 20. Ackerman MJ, Priori SG, Willems S, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 2011;8:1308–1339. [DOI] [PubMed] [Google Scholar]
- 21. Chua TP, Ponikowski P, Harrington D, et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997;29:1585–1590. [DOI] [PubMed] [Google Scholar]
- 22. Griffin BP, Shah PK, Ferguson J et al. Incremental prognostic value of exercise hemodynamic variables in chronic congestive heart failure secondary to coronary artery disease or to dilated cardiomyopathy. Am J Cardiol 1991;67:848–853. [DOI] [PubMed] [Google Scholar]
- 23. Myers J, Prakash M, Froelicher V et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 24. Wroblewski H, Kastrup J, Mortensen SA, et al. Abnormal baroreceptor‐mediated vasodilation of the peripheral circulation in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Circulation 1993;87(3):849–856. [DOI] [PubMed] [Google Scholar]
- 25. McBride BF, White CM. Anemia management in heart failure: A thick review of thin data. Pharmacotherapy 2004;24:757‐767. [DOI] [PubMed] [Google Scholar]
- 26. O'Neill JO, Young JB, Pothier CE, et al. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta‐blockers. Circulation 2005;111:2313–2318. [DOI] [PubMed] [Google Scholar]
- 27. Guazzi M, Myers J, Peberdy MA, et al. Echocardiography with Tissue Doppler Imaging and cardiopulmonary exercise testing in patients with heart failure: A correlative and prognostic analysis. Int J Cardiol 2010;143:323–329. [DOI] [PubMed] [Google Scholar]
- 28. Corra U, Mezzani A, Bosimini E, et al. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J 2002;143:418–426. [DOI] [PubMed] [Google Scholar]
- 29. Dickstein K, Cohen‐Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 30. Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:1977–2016. [DOI] [PubMed] [Google Scholar]
- 31. Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: A simultaneous Doppler‐catheterization study in 127 patients. J Am Coll Cardiol 1985;6:750–756. [DOI] [PubMed] [Google Scholar]
- 32. Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hinderliter AL, Willis PW 4th, Barst RJ, et al. Effects of long‐term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Primary Pulmonary Hypertension Study Group. Circulation 1997;95:1479–1486. [DOI] [PubMed] [Google Scholar]
- 34. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 86–88. [DOI] [PubMed] [Google Scholar]
- 35. Dumitrescu D, Oudiz RJ, Karpouzas G, et al. Developing pulmonary vasculopathy in systemic sclerosis, detected with non‐invasive cardiopulmonary exercise testing. PloS One 2010;5:e14293. [DOI] [PMC free article] [PubMed] [Google Scholar]


