Abstract
Background
Implantable loop recorders (ILRs) allow continuous cardiac monitoring for 3–6 years and are a valuable tool for the investigation of syncopal episodes, palpitations, and atrial fibrillations as well as risk stratification after myocardial infarction. Although magnetic resonance imaging (MRI) in patients with ILRs has been shown to be safe, the impact of ILRs on cardiac MRI image quality has not been investigated yet. Thus, we tested the diagnostic value of cardiac MRI in patients with various types of ILRs.
Methods
Two patients with an ILR and a clinical indication to assess myocardial burden of scarring and fibrosis or stress‐induced myocardial ischemia underwent cardiac MRI. Device interrogation was performed prior to, immediately after, and 3 months after cardiac MRI.
Results
The post‐MRI follow‐ups revealed no change in programmed ILR parameters, sensing fidelity, and battery parameters. However, ILRs caused significant, uninterpretable hyperintensity artifacts in cardiac MRI.
Conclusions
Further clinical studies are warranted to investigate whether modified MRI techniques are helpful to eliminate imaging artifacts.
Keywords: implantable loop recorder, arrhythmia, cardiac magnetic resonance imaging, imaging artifacts
Implantable loop recorders (ILRs) are subcutaneously implanted devices that allow continuous cardiac monitoring for 3–6 years and are a valuable tool for the investigation of syncopal episodes, palpitations, and atrial fibrillation as well as risk stratification after myocardial infarction.1, 2, 3 Moreover, ILRs can provide long‐term electrocardiogram (ECG) monitoring in patients at risk for or with documented atrial fibrillation.4, 5 ILRs are currently available by Medtronic (Reveal XT/LINQ, Berlin, Germany), St. Jude Medical (SJM Confirm, Minneapolis, MN, USA), and Biotronik (BioMonitor, Sylmar, CA, USA).
Magnetic resonance imaging (MRI) is one of the fastest growing imaging tools in both hospital and nonhospital setting, with about 60 million magnetic imaging procedures performed worldwide each year.6 Cardiac MRI has become the gold standard imaging technique in the assessment of myocardial structure, myocardial anatomy, and ventricular function.7 Contrast‐enhanced cardiac MRI allows both perfusion imaging and late gadolinium enhancement (LGE) to assess both perfusion and myocardial scar location, size, and transmurality. The typical standard cardiac MRI protocol includes localizer scans, steady‐state free precession (SSFP) sequences, cine scans in short‐axis slabs as well as two‐, three‐, and four‐chamber views of the heart and black blood sequences such as half‐Fourier acquisition single‐shot turbo‐spin‐echo (HASTE) sequences. There is an estimated 50–75% probability that MRI will be indicated for a patient over the device lifetime.8 Due to the significant expansion of indications for both ILRs and MRIs, the number of patients that will present to the MRI scanner with an ILR is likely to grow rapidly. Thus, understanding the electromagnetic interactions and the impact of ILRs on cardiac MRI image quality is essential.
According to the definitions by the Food and Drug Administration (FDA) and the American Society for Testing and Materials International, currently available ILRs are labeled “MRI conditional.” Ex vivo evaluations and clinical MRI studies with the Reveal Plus (Medtronic, Inc.) have shown that MRI in patients with ILRs is safe and feasible.9, 10, 11 However, recording of artifacts mimicking an arrhythmia or asystole was common10, 12, 13 and patients may experience minor discomfort due to tissue heating or tugging at the implant site.14 Although ILRs are MRI conditionally safe, no clinical study to date has investigated effects of ILRs on cardiac MRI image quality.
METHODS
Two patients with an ILR (SJM Confirm and BioMonitor) and a clinical indication to assess myocardial burden of scarring and fibrosis or stress‐induced myocardial ischemia underwent cardiac MRI. The scans were performed with a 1.5 Tesla MRI scanner (Siemens Avanto, Siemens Healthcare Diagnostics, Erlangen, Germany) with a dedicated 32‐channel coil. ILR gain and sensitivity settings were set according to the manufacturer's instructions. Device interrogation was performed prior to, immediately after, and 3 months after cardiac MRI and patients were questioned about device movement or heating during the procedure. No changes were made to the ILR programmed settings prior to cardiac MRI.
RESULTS
Patient 1
A 60‐year‐old male was implanted with an ILR (SJM Confirm) for evaluation of paroxysmal atrial fibrillation. The patient was referred for a cardiac MRI 7 months after implantation to evaluate suspected stress‐induced myocardial ischemia. During the scan, substantial image artifacts were formed in the anterior and lateral left ventricular wall, as well as in the midventricular and basal septum (Figs. 1B–D). The two‐, three‐, and four‐chamber cine images were obscured by ring artifacts induced by the ILR on the left chest. Interestingly, susceptibility artifacts were also seen on HASTE sequences on every other slice (Fig. 1A). Due to the strong artifacts not only in the anterior wall but also in the septum and in long‐axis views, the originally planned stress perfusion study and LGE images were not acquired as no previous cardiac MRI images were available for comparison and perfusion as well as LGE image quality were presumed to be nondiagnostic.
Figure 1.
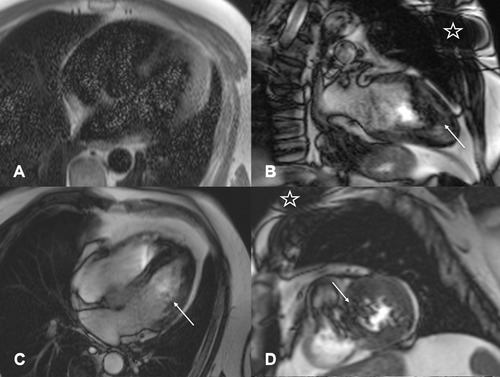
Cardiac MRI images in a patient with paroxysmal atrial fibrillation (patient 1). Long‐axis four‐chamber view HASTE sequence (A) with diffuse grainy hyperintense susceptibility artifacts which were detected in every second image slice. (B and C) Long‐axis two‐ and four‐chamber view and a midventricular short‐axis view (D) SSFP cine sequence with prominent dark rim artifacts affecting the apex, apical septum, and lateral left apical and midventricular myocardium (arrow; B–D). Star indicating the left pectoral location of the ILR. HASTE = half‐Fourier acquisition single‐shot turbo‐spin‐echo; SSFP = steady‐state free precession; ILR = implantable loop recorder.
Patient 2
A 55‐year‐old male with Anderson‐Fabry disease (AFD) was implanted with an ILR (BioMonitor, Biotronik). Three months after device implantation the patient was referred for a cardiac MRI to assess cardiac involvement and myocardial burden of scarring and fibrosis. The standard imaging protocol for assessment of cardiac involvement in AFD was performed in this patient. Due to the slightly different anatomy of this patient's torso and myocardial hypertrophy, rim artifacts and hyperintensity artifacts of the ILR were less problematic in this case (Figs. 2A–D). Notably, in this patient the scar burden is easily overestimated due to discrete hyperintensity artifacts on the turbo flash LGE images (Fig. 2C). However, on phase‐sensitive inversion recovery images, the same artifacts are more pronounced and hypointense. This was helpful in differentiating scar burden from artifacts (Fig. 2D).
Figure 2.
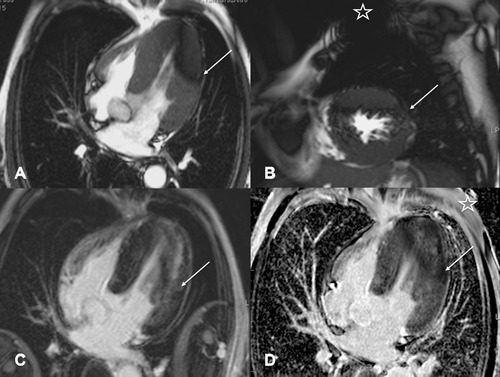
Cardiac MRI images in a patient with known history of Anderson‐Fabry disease and myocardial involvement with left ventricular hypertrophy (patient 2). Long‐axis four‐chamber view (A) and short‐axis (B) view in SSFP cine sequence with dark rim artifacts (arrow) affecting the apical to mid ventricular lateral left chamber wall. LGE 10 minutes after a double dose of gadobutrol i.v. (C and D) shows discrete hyperintense rim artifacts in the left lateral wall in Turbo‐FLASH (fast low angle shot) sequences with variable time to inversion (C; arrow) above a diffuse midmyocardial area of pathological LGE which is typical for cardiac involvement with Anderson‐Fabry disease. Phase‐sensitive inversion recovery sequence (D) shows darker rim like artifacts in the same region (arrow). Star indicates ILR on left chest wall (B and D). SSFP = steady‐state free precession; LGE = late gadolinium enhancement; ILR = implantable loop recorder.
During cardiac MRI, both patients were at sinus rhythm. Device interrogation was performed prior to, immediately after, and 3 months after cardiac MRI. At the postcardiac MRI follow‐ups, pre‐MRI programmed settings were unchanged, sensing fidelity of the patients real‐time ECG remained unchanged and battery parameters were stable. In contrast, a decrease in battery voltage from pre‐ to post‐MRI has been reported in both pacemakers and implantable cardioverter defibrillators (ICDs) after MRI.15, 16 During cardiac MRI, our patients reported no tugging or pulling sensation at the ILR implant area. Moreover, no patient reported tissue heating around the implant site.
DISCUSSION
Continuous prolonged monitoring with an ILR is a safe and efficient strategy and is currently the gold standard in the assessment of patients with unexplained, recurrent syncope. Moreover, ILRs were recently found to be an effective tool to detect atrial fibrillation in patients after cryptogenic stroke.17
Several publications reported that cardiac MRI with ICDs and cardiac pacemakers is safe.14, 18, 19 However, the diagnostic value of cardiac MRI in patients with cardiac implantable electronic devices (CIEDs) is often limited by imaging artifacts.20 With the increasing availability of MR‐conditional CIEDs, numerous studies have evaluated strategies to reduce imaging artifacts in cardiac MRI with these devices.14, 21, 22 However, although MRI in patients with ILRs has been shown to be safe,10, 11 the impact of ILRs on cardiac MRI image quality has not been investigated yet. Compared to pacemakers and ICDs, the overall imaging artifacts of ILRs should be minimal due to the smaller size and the lack of leads. However, as illustrated in our depicted cases, artifact size and extent were substantial in both patients.
Although all MRI sequences are affected by metal artifacts of devices,20, 23, 24 the extent of such artifacts depends on both the size and position of the device and the scanning conditions.20, 21 Thus, Rashid et al.22 reported that the distance of the device from the cardiac silhouette correlates inversely with the extent of artifacts present in the cardiac region. Accordingly, factors such as body mass index and thoracic anatomy, which can contribute to a greater distance between heart and device, affect the image quality. The main artifacts we face with implantable cardiac devices are caused by magnetic field inhomogeneity of the local magnetic field and susceptibility artifacts, caused by the paramagnetic properties of the device.20 Different MRI sequences are more sensitive to local magnetic field inhomogeneities and susceptibility artifacts than others. Gradient echo sequences are generally more prone to image artifacts compared to spin echo sequences, as they do not have a refocusing pulse.21 The standard sequence to assess cardiac function and wall motion is the SSFP sequence, which is a modified gradient echo sequence. Dark banding artifacts, typically seen on cine SSPF MRI sequences, are most prominent in the anterior segments and can make semiautomated volumetric measurements almost impossible to evaluate. However, artifacts are often much less prominent on two‐, three‐, and four‐chamber views, which allow a general visual assessment of cardiac function. Due to the inherent sequence design of cine SSFP imaging, reducing artifacts is difficult. Possible strategies include reducing the repetition time or using a frequency offset from the scanner center frequency.21 In most cases, a rough visual estimate of the ejection fraction and left ventricular function is often possible with long‐axis views of the heart even when automated volumetric assessment is not feasible (see online Supplementary Movies S1 and S2).
Previously, Rashid et al.22 reported a modified LGE imaging technique to overcome imaging artifacts in patients with implanted ICDs. Other methods such as altering k‐space readout or chancing the phase encoding direction to reduce image artifacts are beyond the scope of this case report and are well described in the cited literature.21 Recent publications have also shown substantial reduction of image artifacts in LGE MRI with an increase of the inversion pulse frequency bandwidth from the typical 1 kHz to a wideband hyperbolic inversion with 3.8 kHz. Applying this strategy frequency shift artifacts caused by ICDs, which cause insufficient nulling of healthy myocardium by the inversion pulse, can be reduced.21, 22, 25 However, sequence modulation and choice of appropriate readout requires experience and expertise. When reading cMRI scans in routine clinical practice, it is most important to be aware of subtle artifacts as described in LGE sequences and to correlate areas of hyperintensity with additional image planes.
The cases presented here indicate that ILRs can cause significant, uninterpretable hyperintensity artifacts in cardiac MRI. Further clinical studies are warranted to investigate whether modified MRI techniques as described in patients with ICDs are helpful to eliminate imaging artifacts in patients with ILRs. Moreover, the development of smaller ILRs such as the Reveal LINQ might be a promising approach to overcome imaging artifacts encountered during cardiac MRI. However, cardiac MRIs with implanted Reveal LINQs are not feasible, as no local transmit coils on the chest should be used.
Supporting information
Supplementary Movie S1. Steady‐state free precession (SSFP) cine sequence of patient 1 showing the two‐chamber long‐axis view throughout the cardiac cycle. Note the substantial dark rim artifacts present in the anterior left chamber wall.
Supplementary Movie S2. Steady‐state free precession (SSFP) cine sequence of patient 1 showing a short‐axis slice of the mid‐ventricular left chamber throughout the cardiac cycle. Note the substantial dark rim artifacts present in the anterior and anteroseptal left chamber wall.
REFERENCES
- 1. Podoleanu C, DaCosta A, Defaye P, et al. Early use of an implantable loop recorder in syncope evaluation: A randomized study in the context of the French healthcare system (FRESH study). Arch Cardiovasc Dis 2014;107:546–552. [DOI] [PubMed] [Google Scholar]
- 2. Bloch Thomsen PE, Jons C, Raatikainen MJ, et al. Long‐term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: The Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation 2010;122:1258–1264. [DOI] [PubMed] [Google Scholar]
- 3. Houmsse M, Ishola A, Daoud EG. Clinical utility of implantable loop recorders. Postgrad Med 2014;126:30–37. [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Pokushalov E, Urban L, et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: Results of the XPECT trial. Circ Arrhythm Electrophysiol 2010;3:141–147. [DOI] [PubMed] [Google Scholar]
- 5. Gersak B, Pernat A, Robic B, et al. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:1059–1066. [DOI] [PubMed] [Google Scholar]
- 6. Sutton R, Kanal E, Wilkoff BL, et al. Safety of magnetic resonance imaging of patients with a new Medtronic EnRhythm MRI SureScan pacing system: Clinical study design. Trials 2008;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saeed M, Van TA, Krug R, et al. Cardiac MR imaging: Current status and future direction. Cardiovasc Diagn Ther 2015;5:290–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28:326–328. [DOI] [PubMed] [Google Scholar]
- 9. Shellock FG, Tkach JA, Ruggieri PM, et al. Cardiac pacemakers, ICDs, and loop recorder: evaluation of translational attraction using conventional ("long‐bore") and "short‐bore" 1.5‐ and 3.0‐Tesla MR systems. J Cardiovasc Magn Reson 2003;5:387–397. [DOI] [PubMed] [Google Scholar]
- 10. Gimbel JR, Zarghami J, Machado C, et al. Safe scanning, but frequent artifacts mimicking bradycardia and tachycardia during magnetic resonance imaging (MRI) in patients with an implantable loop recorder (ILR). Ann Noninvasive Electrocardiol 2005;10:404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong JA, Yee R, Gula LJ, et al. Feasibility of magnetic resonance imaging in patients with an implantable loop recorder. Pacing Clin Electrophysiol 2008;31:333–337. [DOI] [PubMed] [Google Scholar]
- 12. Chrysostomakis SI, Simantirakis EN, Marketou ME, et al. Implantable loop recorder undersensing mimicking complete heart block. Europace 2002;4:211–213. [DOI] [PubMed] [Google Scholar]
- 13. Gimbel JR. Magnetic resonance imaging of implantable cardiac rhythm devices at 3.0 tesla. Pacing Clin Electrophysiol 2008;31:795–801. [DOI] [PubMed] [Google Scholar]
- 14. Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm 2009;6:138–143. [DOI] [PubMed] [Google Scholar]
- 15. Naehle CP, Strach K, Thomas D, et al. Magnetic resonance imaging at 1.5‐T in patients with implantable cardioverter‐defibrillators. J Am Coll Cardiol 2009;54:549–555. [DOI] [PubMed] [Google Scholar]
- 16. Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non‐pacemaker‐dependent patients: A prospective study with 115 examinations. Circulation 2006;114:1285–1292. [DOI] [PubMed] [Google Scholar]
- 17. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 18. Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable‐cardioverter defibrillators at 1.5 tesla. Circulation 2006;114:1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nordbeck P, Ertl G, Ritter O. Magnetic resonance imaging safety in pacemaker and implantable cardioverter defibrillator patients: How far have we come? Eur Heart J 2015;36:1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sasaki T, Hansford R, Zviman MM, et al. Quantitative assessment of artifacts on cardiac magnetic resonance imaging of patients with pacemakers and implantable cardioverter‐defibrillators. Circ Cardiovasc Imaging 2011;4:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferreira PF, Gatehouse PD, Mohiaddin RH, et al. Cardiovascular magnetic resonance artefacts. J Cardiovasc Magn Reson 2013;15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rashid S, Rapacchi S, Vaseghi M, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology 2014;270:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mesubi O, Ahmad G, Jeudy J, et al. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol 2014;37:1274–1283. [DOI] [PubMed] [Google Scholar]
- 24. Dickfeld T, Tian J, Ahmad G, et al. MRI‐Guided ventricular tachycardia ablation: integration of late gadolinium‐enhanced 3D scar in patients with implantable cardioverter‐defibrillators. Circ Arrhythm Electrophysiol 2011;4:172–184. [DOI] [PubMed] [Google Scholar]
- 25. Stevens SM, Tung R, Rashid S, et al. Device artifact reduction for magnetic resonance imaging of patients with implantable cardioverter‐defibrillators and ventricular tachycardia: Late gadolinium enhancement correlation with electroanatomic mapping. Heart Rhythm 2014;11:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie S1. Steady‐state free precession (SSFP) cine sequence of patient 1 showing the two‐chamber long‐axis view throughout the cardiac cycle. Note the substantial dark rim artifacts present in the anterior left chamber wall.
Supplementary Movie S2. Steady‐state free precession (SSFP) cine sequence of patient 1 showing a short‐axis slice of the mid‐ventricular left chamber throughout the cardiac cycle. Note the substantial dark rim artifacts present in the anterior and anteroseptal left chamber wall.


