Abstract
Introduction
Atrial fibrillation (AF) is known as the most common arrhythmia and an independent risk factor for mortality. Recent studies suggest that AF is associated with morbidity and mortality in Takotsubo cardiomyopathy (TTC). However, a systematic review and meta‐analysis of the literature have not been done. We assessed the association between AF in patients with TTC and mortality by a systematic review of the literature and a meta‐analysis.
Methods
We comprehensively searched the databases of MEDLINE and EMBASE from inception to January 2018. Included studies were published prospective or retrospective cohort studies that compared all‐cause mortality in TTC with AF versus without AF. Data from each study were combined using the random‐effects, generic inverse variance method of DerSimonian and Laird to calculate risk ratios and 95% confidence intervals.
Results
Five studies from August 2008 to October 2017 were included in this meta‐analysis involving 2,321 subjects with TTC (243 with AF and 2,078 without AF). The presence of AF was associated with all‐cause mortality (pooled odds ratio = 2.19, 95% confidence interval: 1.57–3.06, p < 0.001, I 2 = 0%).
Conclusion
Atrial fibrillation increased all‐cause mortality by double among patients with TTC compared to without it. Our study suggests that the presence of AF in TTC is prognostic for all‐cause mortality.
Keywords: atrial fibrillation, mortality, Takotsubo cardiomyopathy
1. INTRODUCTION
Takotsubo cardiomyopathy (TTC) or acute stress‐induced cardiomyopathy is a phenotype of detrimental myocardial dysfunction following a major physical or emotional stress in the absence of significant coronary artery stenosis (Dawson, 2018). As its clinical manifestations include sudden onset of chest pain and shortness of breath, TTC is recognized as an acute coronary syndrome (ACS) mimicker. The prevalence of TTC is approximately 2%–3% in all patients presenting with suspected ACS (Redfors et al., 2015). TTC is associated with 1%–8% (Donohue & Movahed, 2005; Stiermaier et al., 2015) of all in‐hospital mortality and considerable risk of recurrence after recovery of myocardial function (Elesber et al., 2007). Several factors including older age, lower ejection fraction (EF), and ventricular arrhythmia have been shown to predict adverse outcomes in patients with TTC (Lyon et al., 2016).
Atrial fibrillation (AF) is a common arrhythmia in TTC patients with a prevalence of 18%–25% (El‐Battrawy, Lang, Ansari, Behnes, et al., 2017; Stiermaier, Santoro, et al., 2017). Some studies report that concomitant AF is a predictor of short‐term and long‐term mortality in TTC patients (El‐Battrawy, Lang, Ansari, Behnes, et al., 2017; Stiermaier, Santoro, et al., 2017). AF is also commonly known as a poor outcome predictor among patients with ACS (Angeli et al., 2012; Lin et al., 2011; Maagh et al., 2011; Poci, Hartford, Karlsson, Edvardsson, & Caidahl, 2012; Podolecki et al., 2012; Stamboul et al., 2015; Viliani et al., 2012). However, the association between AF and mortality in TTC patients is not well‐established. In this study, we performed a meta‐analysis to assess the association between the presence of AF and mortality in patients with TTC.
2. METHODS
2.1. Search strategy
Two investigators (NL and VK) independently searched for published studies indexed in MEDLINE and EMBASE databases from inception to January 2018 using a search strategy (Figure 1) that included the terms “atrial fibrillation,” “Takotsubo syndrome/cardiomyopathy,” “Apical ballooning syndrome,” “Broken heart syndrome” “stress cardiomyopathy” and “mortality.” Only English language publications were included. A manual search for additional pertinent studies and review articles using references from retrieved articles was also completed.
Figure 1.
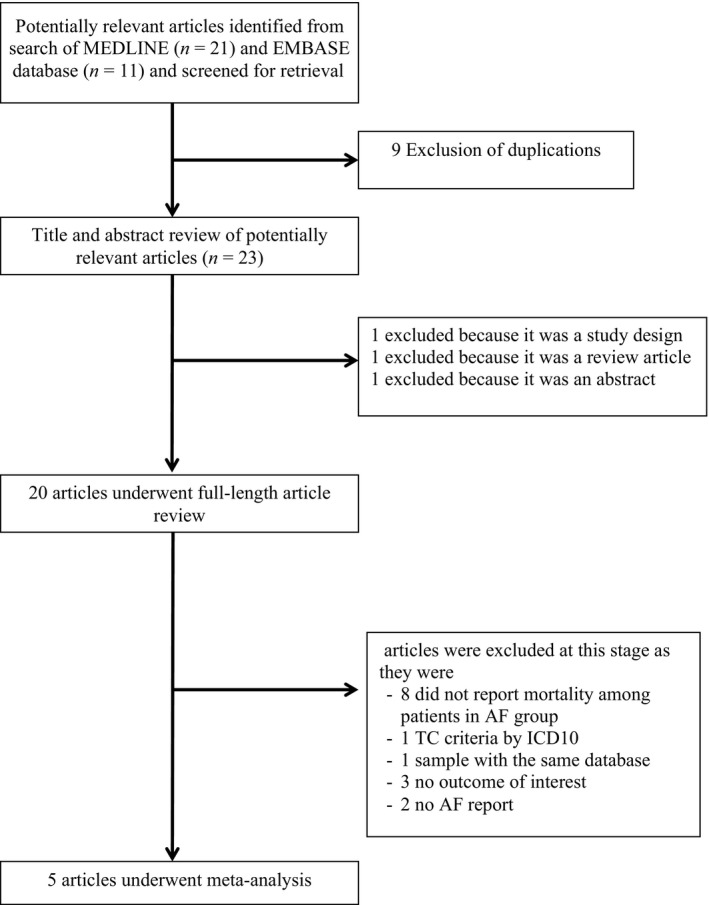
Search methodology and selection process
2.2. Study eligibility criteria
Two main criteria were assessed for inclusion of studies. First was the reporting of incidence of all‐cause mortality in patients with TTC who presented with and without AF. Second was reporting of relative risk, hazard ratio, odds ratio, incidence ratio, or standardized incidence ratio with 95% confidence intervals (or sufficient raw data for the calculation). Patients with TTC who did not have AF were used as controls. Study eligibility was independently determined by two investigators (NL and VK). Differences were resolved by mutual consensus. The Newcastle–Ottawa quality assessment scale was used to evaluate each study in three domains: recruitment and selection of the participants, similarity and comparability between the groups, and ascertainment of the outcome of interest among cohort studies (Stang, 2010).
2.3. Definition
Takotsubo cardiomyopathy was defined according to Mayo Clinic Criteria (Madhavan & Prasad, 2010) described below.
Transient hypokinesis, akinesis, or dyskinesis of the LV apical and/or midventricular or basal segments extending beyond a single epicardial vessel distribution territory.
Absence of significant obstructive coronary artery disease (CAD) explaining the extent of wall motion abnormalities and absence of acute plaque rupture on coronary angiography.
New electrocardiographic abnormalities (either ST segment elevation and/or T‐wave inversion) or modest elevation in cardiac troponin levels.
Absence of pheochromocytoma and myocarditis.
2.4. Data extraction
A standardized data collection form was used to obtain the following information from each study: title, name of first author, year of study, year of publication, country of origin, number of participants, demographic data of participants, method used to identify cases and controls, method used to diagnose the outcomes of interest (i.e., all‐cause mortality), average duration of follow‐up, adjusted and unadjusted risk ratios and their corresponding 95% confidence interval, and list of confounders that were adjusted for in multivariate analysis. To ensure accuracy, all investigators independently performed this data extraction process. Any data discrepancy was resolved by referring back to the original articles.
2.5. Statistical analysis
Meta‐analysis of the combined data was performed using a random‐effects, generic inverse variance method of DerSimonian and Laird (DerSimonian & Laird, 1986). The heterogeneity of effect size estimates across these studies was quantified using the I 2 statistic and Q statistic. For the Q statistic, substantial heterogeneity was defined as p < 0.10. The I 2 statistic ranges in value from 0% to 100% (I 2 < 25%, low heterogeneity; I 2 = 25%–50%, moderate heterogeneity; and I 2 > 50%, substantial heterogeneity) (Higgins, Thompson, Deeks, & Altman, 2003). A sequential exclusion strategy, as described by Patsopoulos and colleagues, was used to examine whether overall estimates were influenced by the substantial heterogeneity observed. We sequentially and cumulatively excluded studies that accounted for the largest share of heterogeneity until I 2 was less than 50%. We then examined whether relative risk estimates were consistent. In accordance with Cochrane, publication bias was assessed using funnel plot. Funnel plot asymmetry was further confirmed with Egger's test if there were more than 10 available studies (Sterne & Egger, 2001). All analysis was performed using Review manager version 5.3 and STATA version 14.1.
3. RESULTS
3.1. Description of included studies
Our search strategy yielded 32 potentially relevant articles (11 articles from EMBASE and 21 articles from MEDLINE). After exclusion of nine duplicated articles, 23 articles underwent title and abstract review. Three were excluded at this stage since one was an abstract, one was a description of study design and one was a review article, leaving 20 articles for full‐length article review. Fifteen studies were excluded. Eight studies were excluded because they did not report numbers of patients with AF among the death group. One was excluded due to the same database (El‐Battrawy, Lang, Ansari, Tulumen et al. ( 2017). Three were excluded because there was no outcome of interest. One was excluded because the criteria of TCC were defined by ICD10 (Mahmoud, Al‐Ani, Saad, Elgendy, & Elgendy, 2016). Lastly, two were excluded due to no AF report in the studies. Therefore, three retrospective and two prospective cohort studies with 243 AF and 2,078 without AF patients were included in this meta‐analysis. The clinical characteristics are described in Table 1.
Table 1.
The clinical characteristics and summary of included studies
| First author | Song | Dib | El‐Battrawy | Ghadri | Stiermaier |
| Country of origin | Korea | USA | Germany | Switzerland | Italy |
| Year | 2010 | 2008 | 2016 | 2016 | 2017 |
| Study type | Retrospective cohort | Retrospective cohort | Retrospective cohort | Prospective cohort | Prospective cohort |
| Participant description | Patients with a diagnosis of an ACS who underwent CAG at a tertiary‐care center in South Korea. | Patients who underwent CAG and left ventriculography in the Mayo Clinic Cardiac Catheterization database | Patient diagnosed with TT in the Takotsubo cardiomyopathy database | Patients included in the international Takotsubo Registry, an observational registry currently with 26 participating centers from nine countries | Patient included in international multicenter study, consecutively enrolled at three centers |
| Exclusion criteria | N/A | Diagnosed cardiomyopathy, structural heart disease, phaeochromocytoma, cocaine abuse, paced heart rhythm | Absence of angiogram or echocardiogram suggesting TT | Coexisting CAD, death during the acute phase before wall motion recovery | N/A |
| Total population | 87 | 37 | 114 | 1,750 | 387 |
| Female (%) | 73.56 | 100.00 | 83.33 | 89.77 | 89.70 |
| Mean age (years) | 63.53 | 69.00 | 67.03 | 66.42 | 74.00 |
| Total mortality (n) | 20 | 2 | 23 | 72 | 78 |
| Prevalence of AF (%) | 6.90 | 18.92 | 18.42 | 6.40 | 25.06 |
| Median duration of follow up (months) | 42 | N/A | 50.97 | N/A | 34.8 |
| Outcome definition | Long‐term mortality | In‐hospital mortality | 3‐year mortality rate | 1‐year mortality rate | All‐cause mortality |
| Conclusion by authors | Low rate in‐hospital mortality as well as long‐term cardiac mortality of TLVBS. The most common causes of deaths were associated with underlying noncardiac diseases | 24‐hr R‐R interval variation and prior history of arrhythmia increased risk of malignant arrhythmia in patients with ABS, and BB decreases the risk of the arrhythmia | The rate of in‐hospital mortality and long‐term events were higher among TT with AF | LVEF less than 45%, AF, and neurological disease predict mortality at 1‐year follow‐up among TT patients | AF associated with increased long‐term mortality rates |
| OR (CI) | 2.38 (0.96–5.89) | 4.29 (0.30–60.47) | 2.36 (1.15–4.83) | 2.70 (1.40–5.21) | 1.72 (1.00–2.96) |
| Confounder adjustment | None | None | None | Demographics, triggers, symptoms, labs, co‐morbidities, medication | Demographics, diabetes, clinical variables, LV functional parameters, presence of cardiogenic shock |
| Newcastle–Ottawa quality assessment | 8 | 7 | 9 | 9 | 9 |
Notes. ABS: apical ballooning syndrome; ACS: acute coronary syndrome; AF: atrial fibrillation; BB: beta blocker; CAG: coronary angiogram; CAD: coronary artery disease; CI: confidence Interval; LVEF: left ventricular ejection fraction; OR: odd Ratio; TLVBS: transient left ventricular ballooning syndrome; TT: Takotsubo cardiomyopathy.
3.2. Quality assessment of included studies
The Newcastle–Ottawa scale (0 to 9) was used to evaluate included studies on three domains: selection, comparability, and outcomes. Higher scores represent higher study quality. All five studies received a score of 7 to 9, which reflected high quality of included studies. Detailed evaluation of each study is presented in a Supporting Information Table S1.
3.3. Meta‐analysis result
A total of five studies (two prospective, three retrospective) with 2,321 participants were included in the meta‐analysis. The prevalence of AF ranged from 6.4% to 25%. There was an association between AF and all‐cause mortality in patients with TTC (OR 2.19; 95%CI 1.57–3.06, p < 0.001) with no heterogeneity (I 2 = 0%) (Figure 2a). Subgroup analysis of four studies with long‐term mortality showed that the association remained significant and similar to overall analysis (OR 2.17; 95% CI 1.55–3.03, p < 0.001) with no heterogeneity (I 2 = 0%) as well (Figure 2a). Among five included studies, two studies reported multivariate adjusted OR (Ghadri, Cammann, & Napp, 2016; Stiermaier, Santoro, et al., 2017). We performed subgroup analyses among multivariate adjusted (Ghadri et al, 2016; Stiermaier, Santoro, et al., 2017) and unadjusted studies (Dib et al., 2008; El‐Battrawy, Lang, Ansari, Behnes, et al., 2017; Song et al., 2010). We found that both multivariate adjusted and non‐adjusted subgroup analyses showed substantial association between AF and risk of mortality in Takotsubo with similar OR (OR = 2.07 CI 1.34–3.20, p = 0.001, and OR = 2.43 CI 1.40–4.21, p = 0.002, respectively) and we found no significant different association between the two subgroups (p = 0.831, I 2 = 0%) (Figure 3). Low heterogeneity was found in multivariate adjusted (I 2 = 7%) and no heterogeneity (I 2 = 0%) was found in non‐adjusted analyses.
Figure 2.
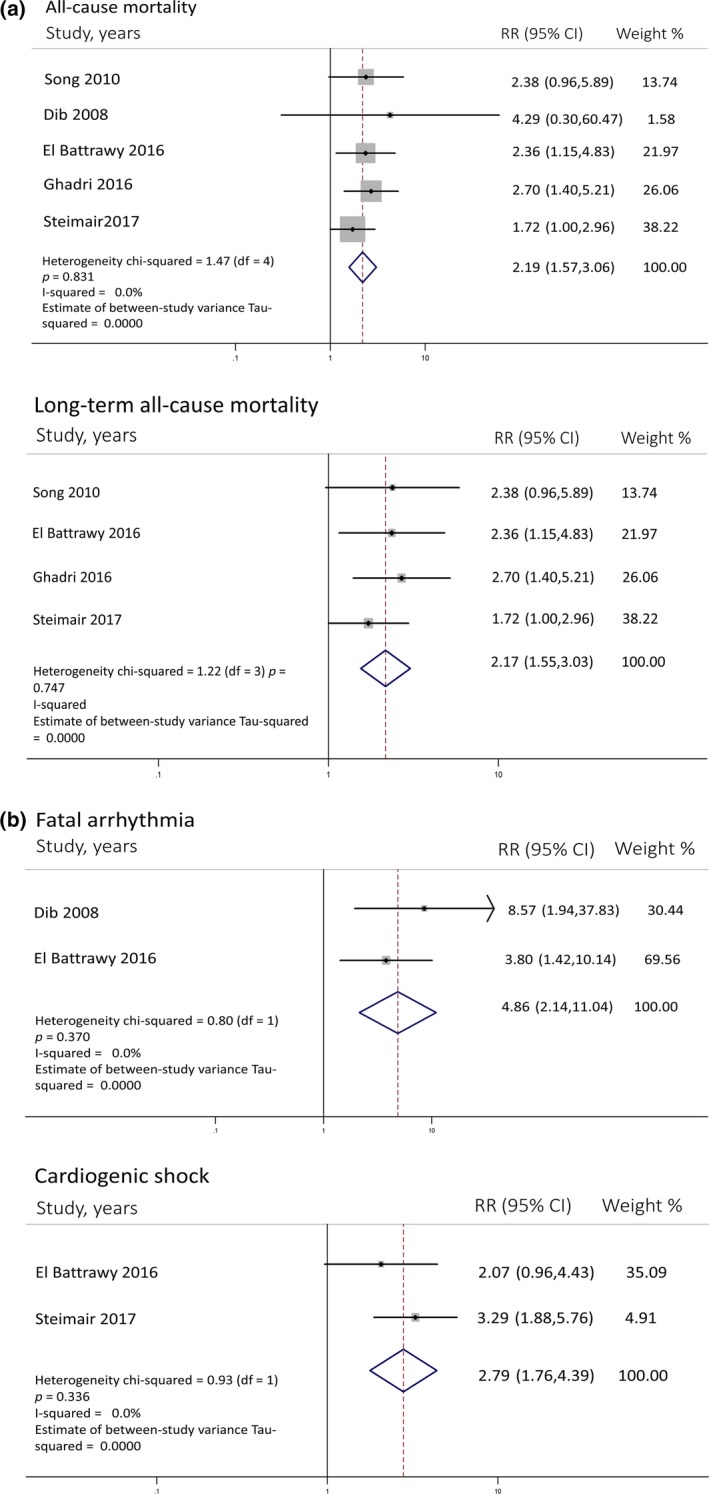
(a) Forest plot of studies comparing all‐cause mortality and long term overall mortality in patients with and without AF. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random‐effects model. A diamond data marker represents the overall adjusted odd ratio (OR) and 95% CI for the outcome of interest. (b) Forest plot of studies comparing fatal arrhythmia and cardiogenic shock in patients with and without AF. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random‐effects model. A diamond data marker represents the overall adjusted odd ratio (OR) and 95% CI for the outcome of interest. AF: atrial fibrillation
Figure 3.
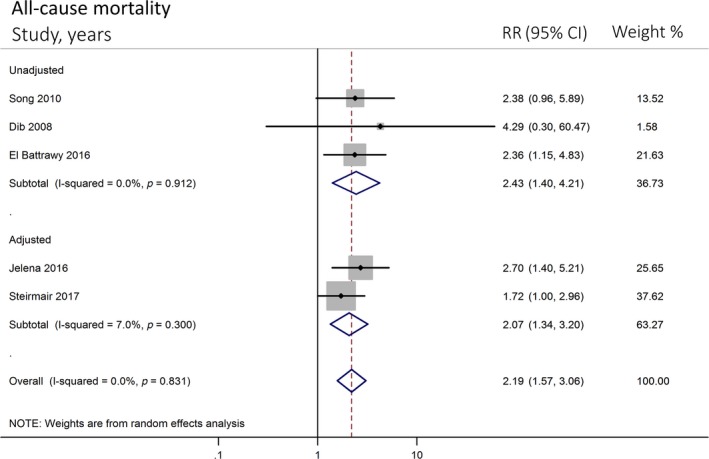
Forest plot of sub‐studies (adjusted and non‐adjusted subgroups) comparing all‐cause mortality in patients with and without AF. Horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random‐effects model. A diamond data marker represents the overall adjusted odd ratio (OR) and 95% CI for the outcome of interest. AF: atrial fibrillation
Moreover, to evaluate the effect of adjusted studies on overall outcome, we performed meta‐regression between multivariate adjusted and non‐adjusted studies. We found that there was no significant difference among adjusted and unadjusted studies in overall random effect pooled analysis (p = 0.675).
Also, our exploratory analyses regarding cardiogenic shock and fatal arrhythmia were performed and demonstrated a statistically substantial association with AF depicted in Figure 2b. Funnel plot did not suggest publication bias (Figure 4). Egger's test was not performed due to a low number of the studies.
Figure 4.
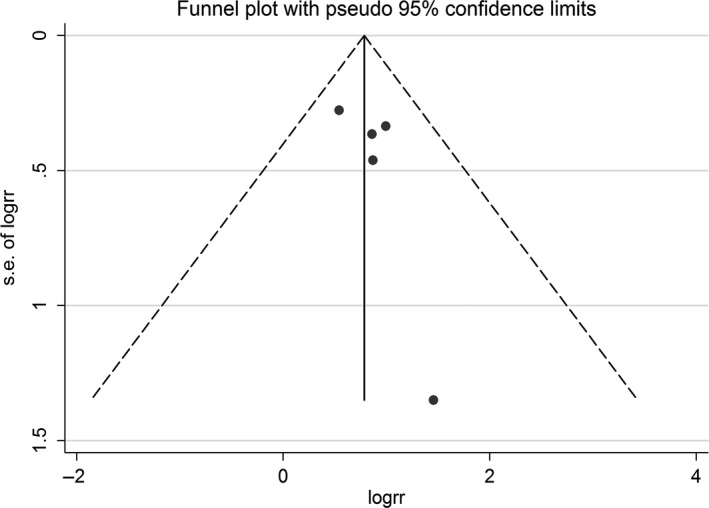
Funnel plot of AF and mortality. Circles represent published studies. AF: atrial fibrillation
4. DISCUSSION
Our meta‐analysis demonstrated that the presence of AF in patients with TTC was associated with increased risk of overall mortality. The prevalence of AF ranged from 6.4% to 25.06%. Additionally, our subgroup analysis remained statistically significant for studies with long‐term mortality. In our exploratory sub‐study, the presence of AF corresponded with high risk of cardiogenic shock and fatal arrhythmia.
There have not been well‐established studies conducted to examine AF prevalence in Takotsubo so far. Based on our included studies (Dib et al., 2008; El‐Battrawy, Lang, Ansari, Behnes, et al., 2017; Ghadri et al, 2016; Song et al., 2010; Stiermaier, Santoro, et al., 2017), the prevalence of AF in Takotsubo ranged from 6.40%–25.06%. Taking age into consideration, the discrepancy among these Takotsubo studies (Dib et al., 2008; El‐Battrawy, Lang, Ansari, Behnes, et al., 2017; Ghadri et al, 2016; Song et al., 2010; Stiermaier, Santoro, et al., 2017) are likely from the difference of mean age among each study (Table 1). On the other hand, from the GRACE study, McManus et al. (McManus et al., 2012) reported AF prevalence in ACS patients (Eagle et al., 2002; Investigators, 2001) at approximately 6.7%–8.4% and the mean age of the participants was 65.90 years old. Interestingly, two Takostubo studies (Ghadri et al, 2016; Song et al., 2010) that have similar age groups with the GRACE study reported that the prevalence ranged between 6.4%–6.9% which is similar to the prevalence reported in ACS. However, we believe further studies are required to determine TTC prevalence given the small number of participants.
Many risk factors such as age, sex, physical trigger, lower EF and high troponin level have been proposed as prognostic determinants in TTC (Templin et al., 2015). Recently, some studies also showed a relationship between AF and poor outcome among patients with stress cardiomyopathy (El‐Battrawy, Lang, Ansari, Behnes, et al., 2017; Ghadri et al, 2016; Konstantinos et al., 2017; Pant et al., 2013; Stiermaier, Santoro, et al., 2017). It has been known that AF is an independent adverse factor which increases morbidity and mortality (Andersson et al., 2013; Benjamin et al., 1998; Conen et al., 2011; Stewart, Hart, Hole, & McMurray, 2002) and is associated with detrimental consequences (Benjamin et al., 1998; Kannel, Abbott, Savage, & McNamara, 1982; Scardi & Mazzone, 2000; Stewart et al., 2002), especially in susceptible groups such as patients with myocardial infarction (Chen et al., 2013; Crenshaw et al., 1997; Eldar et al., 1998; Goldberg et al., 1990). There is a well‐performed meta‐analysis demonstrating increased risk of mortality in acute myocardial infarction published by Angeli et al. (2012). This study demonstrated that risk of mortality increased approximately two times in patients with AF with ACS. Surprisingly, our results suggested a similar risk in comparison to the aforementioned study even in a different population (our study did not include CAD). This implies that pre‐existing AF has significant impact on prognosis in both settings.
Tornvall, Collste, Ehrenborg, and Jarnbert‐Petterson (2016) compared mortality among patients with TTC, patients with CAD and patients without CAD and argued that the mortality among patients with TTC was significantly higher compared to population without CAD. Interestingly, the study showed similar mortality rate between TTC and CAD population. Unfortunately, since our recruited participants were only patients without CAD based on Mayo clinic criteria (Madhavan & Prasad, 2010), we were unable to examine differences in prevalence of AF and clinical significance of AF between TTC and ACS. It is worthwhile to investigate this further in the future.
The pathogenesis of AF in TTC as well as how it contributes to adverse outcomes has not been clearly elucidated. In general, AF pathogenesis typically involves electrical and structural remodeling of the left atrium (Allessie et al., 2001; Schotten, Verheule, Kirchhof, & Goette, 2011). There are many explanations for the mechanism of AF initiation in TTC. One of them is the emergence of left atrial dysfunction in TTC patients (Stiermaier, Graf, et al., 2017). Previous studies have suggested that left atrial abnormality may have a role in AF pathophysiology (Dodson et al., 2014; Sanfilippo et al., 1990; Vaziri, Larson, Benjamin, & Levy, 1994). Excessive sympathetic activity, which induces a massive release of catecholamine, is also a possible mechanism for AF development, given plausible pathophysiology of TTC (Abraham et al., 2009; Paur et al., 2012; Wittstein et al., 2005). Inflammatory mediators could facilitate the initiation of AF as well (Gutierrez & Van Wagoner, 2015; Harada, Van Wagoner, & Nattel, 2015; Issac, Dokainish, & Lakkis, 2007), suggested by a study showing increased levels of IL6 and IL10 among TTC patients (Santoro et al., 2016).
Despite the fact that TTC is a reversible condition, there has been emerging evidence supporting adverse outcomes among TTC with AF (El‐Battrawy, Lang, Ansari, Behnes, et al., 2017; Ghadri et al, 2016; Konstantinos et al., 2017; Pant et al., 2013; Stiermaier, Santoro, et al., 2017). AF could lead to adverse outcomes through possible physiologies including loss of atrial contraction, rapid ventricular rate, activation of neurohormonal vasoconstrictors, and mitral regurgitation (Gertz et al., 2011), implicating further deterioration of cardiac function. In addition, some studies suggested a noticeable association between AF and fatal arrhythmia. El‐Battrawy, Lang, Ansari, Tulumen, et al. (2017) portrayed that there was a high likelihood of the occurrence of fatal arrhythmias in patient with AF. Dip et al. noticed a higher prevalence of AF among patients with life‐threatening arrhythmia. Moreover, some studies reported that cardiac fibrosis and inflammation were prevalent in patients with TTC with AF (Dzeshka, Lip, Snezhitskiy, & Shantsila, 2015; Eitel et al., 2011). Lastly, thromboembolic phenomenon especially stroke may be precipitated by AF, which leads to further consequences.
In our subgroup analyses between multivariate adjusted and unadjusted studies. We found that both multivariate adjusted and non‐adjusted subgroup analyses showed substantial association between AF and risk of mortality in Takotsubo with similar OR. In meta‐regression, we found that there was no significant difference among adjusted and unadjusted studies in overall random effect pooled analysis. Low heterogeneity, similar risk ratio, and non‐significant meta‐regression among multivariate adjusted and non‐adjusted studies implies that participants’ characteristics were very similar among each study due to our specific Mayo clinic Takostubo inclusion criteria (Madhavan & Prasad, 2010). Therefore, these results confirmed accuracy and validity of our findings.
To the best of our knowledge, this is the first meta‐analysis conducted to determine the impact of AF on mortality in patients with TTC. We believe that the presence of AF can be helpful for risk stratification to predict morbidity and mortality in patients with TTC.
5. LIMITATION
We recognize there are limitations to our meta‐analysis. Studies with different methodology and population were included and might be potential sources of heterogeneity. First, since all included studies were observational in nature, the influence of residual confounders could not be completely excluded. Second, we did not further determine whether paroxysmal or persistent AF have different impacts on the outcome of interest due to insufficient data. Likewise, the presence of AF before, during or after hospitalization was not identified so that we could not analyze the relationship with mortality. Third, there were only five studies included in our study despite a relatively symmetrical funnel plot. Hence, there is a possibility of false negative result from the funnel plot. However, TTC is a very rare condition, which is still not well understood. In addition, our study did not demonstrate whether any ballooning patterns could affect the outcome with the presence of AF given lack of adequate data. Nonetheless, we used sensitivity analysis methods in the random‐effects model and found no difference between the imputed risk ratio and its 95% confidence interval.
6. CONCLUSION
In summary, the presence of AF in TTC is associated with higher all‐cause mortality, especially long‐term mortality. According to our study, further investigations should focus on the association between type of AF or AF onset and clinical outcomes in TTC.
CONFLICT OF INTEREST
None to declare.
AUTHOR CONTRIBUTION
NarutPrasitlumkum: Conception design, data interpretation, draft manuscript, corresponding; Veraprapas Kittipibul: Data acquisition, draft manuscript; NathLimpruttidham: Data acquisition; Pattara Rattanawong: Data acquisition, data interpretation statistical analysis; PakawatChongsathidkiet: Data acquisition; Thosaporn Boondarikpornpant: Data acquisition.
Supporting information
ACKNOWLEDGMENT
We would like to thanks Elysse Tom, MD., for critical reading.
Prasitlumkum N, Kittipibul V, Limpruttidham N, Rattanawong P, Chongsathidkiet P, Boondarikpornpant T. The presence of atrial fibrillation in Takotsubo cardiomyopathy is predictive of mortality: Systematic review and meta‐analysis. Ann Noninvasive Electrocardiol. 2019;24:e12566 10.1111/anec.12566
REFERENCES
- Abraham, J. , Mudd, J. O. , Kapur, N. K. , Klein, K. , Champion, H. C. , & Wittstein, I. S. (2009). Stress cardiomyopathy after intravenous administration of catecholamines and beta‐receptor agonists. Journal of the American College of Cardiology, 53(15), 1320–1325. 10.1016/j.jacc.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Allessie, M. A. , Boyden, P. A. , Camm, A. J. , Kleber, A. G. , Lab, M. J. , Legato, M. J. , … Waldo, A. L. (2001). Pathophysiology and prevention of atrial fibrillation. Circulation, 103(5), 769–777. 10.1161/01.CIR.103.5.769 [DOI] [PubMed] [Google Scholar]
- Andersson, T. , Magnuson, A. , Bryngelsson, I. L. , Frobert, O. , Henriksson, K. M. , Edvardsson, N. , & Poci, D. (2013). All‐cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: A swedish nationwide long‐term case‐control study. European Heart Journal, 34(14), 1061–1067. 10.1093/eurheartj/ehs469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli, F. , Reboldi, G. , Garofoli, M. , Ramundo, E. , Poltronieri, C. , Mazzotta, G. , … Verdecchia, P. (2012). Atrial fibrillation and mortality in patients with acute myocardial infarction: A systematic overview and meta‐analysis. Curr Cardiol Rep, 14(5), 601–610. 10.1007/s11886-012-0289-3. [DOI] [PubMed] [Google Scholar]
- Benjamin, E. J. , Wolf, P. A. , D'Agostino, R. B. , Silbershatz, H. , Kannel, W. B. , & Levy, D. (1998). Impact of atrial fibrillation on the risk of death: The framingham heart study. Circulation, 98(10), 946–952. 10.1161/01.CIR.98.10.946 [DOI] [PubMed] [Google Scholar]
- Chen, L. Y. , Sotoodehnia, N. , Buzkova, P. , Lopez, F. L. , Yee, L. M. , Heckbert, S. R. , … Alonso, A. (2013). Atrial fibrillation and the risk of sudden cardiac death: The atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med, 173(1), 29–35. 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conen, D. , Chae, C. U. , Glynn, R. J. , Tedrow, U. B. , Everett, B. M. , Buring, J. E. , & Albert, C. M. (2011). Risk of death and cardiovascular events in initially healthy women with new‐onset atrial fibrillation. JAMA, 305(20), 2080–2087. 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crenshaw, B. S. , Ward, S. R. , Granger, C. B. , Stebbins, A. L. , Topol, E. J. , & Califf, R. M. (1997). Atrial fibrillation in the setting of acute myocardial infarction: The gusto‐i experience. Global utilization of streptokinase and tpa for occluded coronary arteries. Journal of the American College of Cardiology, 30(2), 406–413. [DOI] [PubMed] [Google Scholar]
- Dawson, D. K. (2018). Acute stress‐induced (takotsubo) cardiomyopathy. Heart, 104(2), 96–102. 10.1136/heartjnl-2017-311579. [DOI] [PubMed] [Google Scholar]
- DerSimonian, R. , & Laird, N. (1986). Meta‐analysis in clinical trials. Controlled Clinical Trials, 7(3), 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Dib, C. , Prasad, A. , Friedman, P. A. , Ahmad, E. , Rihal, C. S. , Hammill, S. C. , & Asirvatham, S. J. (2008). Malignant arrhythmia in apical ballooning syndrome: Risk factors and outcomes. Indian Pacing Electrophysiol J, 8(3), 182–192. [PMC free article] [PubMed] [Google Scholar]
- Dodson, J. A. , Neilan, T. G. , Shah, R. V. , Farhad, H. , Blankstein, R. , Steigner, M. , … Kwong, R. Y. (2014). Left atrial passive emptying function determined by cardiac magnetic resonance predicts atrial fibrillation recurrence after pulmonary vein isolation. Circ Cardiovasc Imaging, 7(4), 586–592. 10.1161/CIRCIMAGING.113.001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue, D. , & Movahed, M. R. (2005). Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Failure Reviews, 10(4), 311–316. 10.1007/s10741-005-8555-8. [DOI] [PubMed] [Google Scholar]
- Dzeshka, M. S. , Lip, G. Y. , Snezhitskiy, V. , & Shantsila, E. (2015). Cardiac fibrosis in patients with atrial fibrillation: Mechanisms and clinical implications. Journal of the American College of Cardiology, 66(8), 943–959. 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- Eagle, K. A. , Goodman, S. G. , Avezum, A. , Budaj, A. , Sullivan, C. M. , Lopez‐Sendon, J. , & GRACE Investigators (2002). Practice variation and missed opportunities for reperfusion in st‐segment‐elevation myocardial infarction: Findings from the global registry of acute coronary events (grace). Lancet, 359(9304), 373–377. 10.1016/S0140-6736(02)07595-5. [DOI] [PubMed] [Google Scholar]
- Eitel, I. , von Knobelsdorff‐Brenkenhoff, F. , Bernhardt, P. , Carbone, I. , Muellerleile, K. , Aldrovandi, A. , … Friedrich, M. G. (2011). Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA, 306(3), 277–286. 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- El‐Battrawy, I. , Lang, S. , Ansari, U. , Behnes, M. , Hillenbrand, D. , Schramm, K. , … Akin, I. (2017). Impact of concomitant atrial fibrillation on the prognosis of takotsubo cardiomyopathy. Europace, 19(8), 1288–1292. 10.1093/europace/euw293. [DOI] [PubMed] [Google Scholar]
- El‐Battrawy, I. , Lang, S. , Ansari, U. , Tulumen, E. , Schramm, K. , Fastner, C. , … Akin, I. (2017). Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace, 20(5), 843–850. 10.1093/europace/eux073. [DOI] [PubMed] [Google Scholar]
- Eldar, M. , Canetti, M. , Rotstein, Z. , Boyko, V. , Gottlieb, S. , Kaplinsky, E. , & Behar, S. (1998). Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. Sprint and Thrombolytic Survey Groups. Circulation, 97(10), 965–970. [DOI] [PubMed] [Google Scholar]
- Elesber, A. A. , Prasad, A. , Lennon, R. J. , Wright, R. S. , Lerman, A. , & Rihal, C. S. (2007). Four‐year recurrence rate and prognosis of the apical ballooning syndrome. Journal of the American College of Cardiology, 50(5), 448–452. 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Gertz, Z. M. , Raina, A. , Saghy, L. , Zado, E. S. , Callans, D. J. , Marchlinski, F. E. , … Silvestry, F. E. (2011). Evidence of atrial functional mitral regurgitation due to atrial fibrillation: Reversal with arrhythmia control. Journal of the American College of Cardiology, 58(14), 1474–1481. 10.1016/j.jacc.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Ghadri, J. R., Cammann, V. L., Napp, L. C., … International Takotsubo (InterTAK) Registry (2016). Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome: Data from the international takotsubo registry. JAMA Cardiol, 1(3), 335–340. doi: 10.1001/jamacardio.2016.0225. [DOI] [PubMed] [Google Scholar]
- Goldberg, R. J. , Seeley, D. , Becker, R. C. , Brady, P. , Chen, Z. Y. , Osganian, V. , … Dalen, J. E. (1990). Impact of atrial fibrillation on the in‐hospital and long‐term survival of patients with acute myocardial infarction: A community‐wide perspective. American Heart Journal, 119(5), 996–1001. 10.1016/S0002-8703(05)80227-3 [DOI] [PubMed] [Google Scholar]
- GRACE Investigators . (2001). Rationale and design of the grace (global registry of acute coronary events) project: A multinational registry of patients hospitalized with acute coronary syndromes. American Heart Journal, 141(2), 190–199. [DOI] [PubMed] [Google Scholar]
- Gutierrez, A. , & Van Wagoner, D. R. (2015). Oxidant and inflammatory mechanisms and targeted therapy in atrial fibrillation: An update. Journal of Cardiovascular Pharmacology, 66(6), 523–529. 10.1097/FJC.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, M. , Van Wagoner, D. R. , & Nattel, S. (2015). Role of inflammation in atrial fibrillation pathophysiology and management. Circulation Journal, 79(3), 495–502. 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issac, T. T. , Dokainish, H. , & Lakkis, N. M. (2007). Role of inflammation in initiation and perpetuation of atrial fibrillation: A systematic review of the published data. Journal of the American College of Cardiology, 50(21), 2021–2028. 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- Kannel, W. B. , Abbott, R. D. , Savage, D. D. , & McNamara, P. M. (1982). Epidemiologic features of chronic atrial fibrillation: The framingham study. New England Journal of Medicine, 306(17), 1018–1022. 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- Konstantinos, G. , El‐Battrawy, I. , Schramm, K. , Uzair, A. , Hoffmann, U. , Martin, B. , & Ibrahim, A. (2017). Comparison and outcome analysis of patients with takotsubo cardiomyopathy triggered by emotional stress or physical stress. Front Psychol, 8, 527 10.3389/fpsyg.2017.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. J. , Liu, C. F. , Kung, C. T. , Sun, C. K. , Lin, Y. C. , Leu, S. , … Yip, H. K. (2011). The prognostic value of atrial fibrillation on 30‐day clinical outcome in patients with st‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int Heart J, 52(3), 153–158. [DOI] [PubMed] [Google Scholar]
- Lyon, A. R. , Bossone, E. , Schneider, B. , Sechtem, U. , Citro, R. , Underwood, S. R. , … Omerovic, E. (2016). Current state of knowledge on takotsubo syndrome: A position statement from the taskforce on takotsubo syndrome of the heart failure association of the european society of cardiology. European Journal of Heart Failure, 18(1), 8–27. 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- Maagh, P. , Butz, T. , Wickenbrock, I. , Prull, M. W. , Plehn, G. , Trappe, H. J. , & Meissner, A. (2011). New‐onset versus chronic atrial fibrillation in acute myocardial infarction: Differences in short‐ and long‐term follow‐up. Clin Res Cardiol, 100(2), 167–175. 10.1007/s00392-010-0227-6. [DOI] [PubMed] [Google Scholar]
- Madhavan, M. , & Prasad, A. (2010). Proposed mayo clinic criteria for the diagnosis of tako‐tsubo cardiomyopathy and long‐term prognosis. Herz, 35(4), 240–243. 10.1007/s00059-010-3339-x. [DOI] [PubMed] [Google Scholar]
- Mahmoud, A. N. , Al‐Ani, M. , Saad, M. , Elgendy, A. Y. , & Elgendy, I. Y. (2016). Development and validation of a simple integer risk score for prediction of in‐hospital mortality following takotsubo syndrome. Heart and Lung, 45(6), 510–514. 10.1016/j.hrtlng.2016.08.009. [DOI] [PubMed] [Google Scholar]
- McManus, D. D. , Huang, W. , Domakonda, K. V. , Ward, J. , Saczysnki, J. S. , Gore, J. M. , & Goldberg, R. J. (2012). Trends in atrial fibrillation in patients hospitalized with an acute coronary syndrome. American Journal of Medicine, 125(11), 1076–1084. 10.1016/j.amjmed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant, S. , Deshmukh, A. , Mehta, K. , Badheka, A. O. , Tuliani, T. , Patel, N. J. , … Paydak, H. (2013). Burden of arrhythmias in patients with takotsubo cardiomyopathy (apical ballooning syndrome). International Journal of Cardiology, 170(1), 64–68. 10.1016/j.ijcard.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Paur, H. , Wright, P. T. , Sikkel, M. B. , Tranter, M. H. , Mansfield, C. , O'Gara, P. , … Harding, S. E. (2012). High levels of circulating epinephrine trigger apical cardiodepression in a β2‐adrenergic receptor/gi‐dependent manner: A new model of takotsubo cardiomyopathy. Circulation, 126(6), 697–706. 10.1161/CIRCULATIONAHA.112.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poci, D. , Hartford, M. , Karlsson, T. , Edvardsson, N. , & Caidahl, K. (2012). Effect of new versus known versus no atrial fibrillation on 30‐day and 10‐year mortality in patients with acute coronary syndrome. American Journal of Cardiology, 110(2), 217–221. 10.1016/j.amjcard.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Podolecki, T. , Lenarczyk, R. , Kowalczyk, J. , Kurek, T. , Boidol, J. , Chodor, P. , … Kalarus, Z. (2012). Effect of type of atrial fibrillation on prognosis in acute myocardial infarction treated invasively. American Journal of Cardiology, 109(12), 1689–1693. 10.1016/j.amjcard.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Redfors, B. , Vedad, R. , Angeras, O. , Ramunddal, T. , Petursson, P. , Haraldsson, I. , … Omerovic, E. (2015). Mortality in takotsubo syndrome is similar to mortality in myocardial infarction – A report from the SWEDEHEART registry. International Journal of Cardiology, 185, 282–289. 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- Sanfilippo, A. J. , Abascal, V. M. , Sheehan, M. , Oertel, L. B. , Harrigan, P. , Hughes, R. A. , & Weyman, A. E. (1990). Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation, 82(3), 792–797. [DOI] [PubMed] [Google Scholar]
- Santoro, F. , Tarantino, N. , Ferraretti, A. , Ieva, R. , Musaico, F. , Guastafierro, F. , … Brunetti, N. D. (2016). Serum interleukin 6 and 10 levels in takotsubo cardiomyopathy: Increased admission levels may predict adverse events at follow‐up. Atherosclerosis, 254, 28–34. 10.1016/j.atherosclerosis.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Scardi, S. , & Mazzone, C. (2000). Impact of chronic atrial fibrillation on cardiovacular mortality. Ital Heart J Suppl, 1(9), 1117–1122. [PubMed] [Google Scholar]
- Schotten, U. , Verheule, S. , Kirchhof, P. , & Goette, A. (2011). Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiological Reviews, 91(1), 265–325. 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- Song, B. G. , Hahn, J. Y. , Cho, S. J. , Park, Y. H. , Choi, S. M. , Park, J. H. , … Gwon, H. C. (2010). Clinical characteristics, ballooning pattern, and long‐term prognosis of transient left ventricular ballooning syndrome. Heart and Lung, 39(3), 188–195. 10.1016/j.hrtlng.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Stamboul, K. , Zeller, M. , Fauchier, L. , Gudjoncik, A. , Buffet, P. , Garnier, F. , … Cottin, Y. (2015). Prognosis of silent atrial fibrillation after acute myocardial infarction at 1‐year follow‐up. Heart, 101(11), 864–869. 10.1136/heartjnl-2014-307253. [DOI] [PubMed] [Google Scholar]
- Stang, A. (2010). Critical evaluation of the newcastle‐ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. European Journal of Epidemiology, 25(9), 603–605. 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Sterne, J. A. , & Egger, M. (2001). Funnel plots for detecting bias in meta‐analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology, 54(10), 1046–1055. [DOI] [PubMed] [Google Scholar]
- Stewart, S. , Hart, C. L. , Hole, D. J. , & McMurray, J. J. (2002). A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the renfrew/paisley study. American Journal of Medicine, 113(5), 359–364. [DOI] [PubMed] [Google Scholar]
- Stiermaier, T. , Eitel, C. , Denef, S. , Desch, S. , Schuler, G. , Thiele, H. , & Eitel, I. (2015). Prevalence and clinical significance of life‐threatening arrhythmias in takotsubo cardiomyopathy. Journal of the American College of Cardiology, 65(19), 2148–2150. 10.1016/j.jacc.2015.02.062. [DOI] [PubMed] [Google Scholar]
- Stiermaier, T. , Graf, T. , Moller, C. , Eitel, C. , Ledwoch, J. , Desch, S. , … Eitel, I. (2017). Transient left atrial dysfunction is a feature of takotsubo syndrome. Journal of Cardiovascular Magnetic Resonance, 19(1), 15 10.1186/s12968-017-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiermaier, T. , Santoro, F. , Eitel, C. , Graf, T. , Moller, C. , Tarantino, N. , … Eitel, I. (2017). Prevalence and prognostic relevance of atrial fibrillation in patients with takotsubo syndrome. International Journal of Cardiology, 245, 156–161. 10.1016/j.ijcard.2017.07.053. [DOI] [PubMed] [Google Scholar]
- Templin, C. , Ghadri, J. R. , Diekmann, J. , Napp, L. C. , Bataiosu, D. R. , Jaguszewski, M. , … Luscher, T. F. (2015). Clinical features and outcomes of takotsubo (stress) cardiomyopathy. New England Journal of Medicine, 373(10), 929–938. 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- Tornvall, P. , Collste, O. , Ehrenborg, E. , & Jarnbert‐Petterson, H. (2016). A case‐control study of risk markers and mortality in takotsubo stress cardiomyopathy. Journal of the American College of Cardiology, 67(16), 1931–1936. 10.1016/j.jacc.2016.02.029. [DOI] [PubMed] [Google Scholar]
- Vaziri, S. M. , Larson, M. G. , Benjamin, E. J. , & Levy, D. (1994). Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation, 89(2), 724–730. [DOI] [PubMed] [Google Scholar]
- Viliani, D. , Vivas, D. , Chung, M. , Perez‐Vizcayno, M. J. , Hernandez‐Antolin, R. , Banuelos, C. , … Alfonso, F. (2012). Prognosis of different types of atrial fibrillation in the primary angioplasty era. Coronary Artery Disease, 23(8), 511–516. 10.1097/MCA.0b013e328358a5d2. [DOI] [PubMed] [Google Scholar]
- Wittstein, I. S. , Thiemann, D. R. , Lima, J. A. , Baughman, K. L. , Schulman, S. P. , Gerstenblith, G. , … Champion, H. C. (2005). Neurohumoral features of myocardial stunning due to sudden emotional stress. New England Journal of Medicine, 352(6), 539–548. 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


