Abstract
Aim
The correct estimation of the VA origin as RVOT or LVOT results in reduced ablation duration reduced radiation exposure and decreased number of vascular access. In our study, we aimed to detect the predictive value of S‐R difference in V1‐V2 for differentiating the left from right ventricular outflow tract arrhythmias.
Methods
We included 123 patients with symptomatic frequent premature ventricular outflow tract contractions who underwent successful catheter ablation (70 male, 53 female; mean age 46.2 ± 13.9 years, 61 RVOT, 62 LVOT origins). S‐R difference in V1‐V2 was calculated with this formula on the 12‐lead surface ECG: (V1S + V2S) – (V1R + V2R). Conventional ablation was performed in 101 (82.1%) patients, CARTO electroanatomic mapping system was used in 22 (17.9%) patients.
Results
V1‐2 SRd was found to be significantly lower for LVOT origins than RVOT origins (p < .001). The cutoff value of V1‐2 SRd obtained by ROC curve analysis was 1.625 mV for prediction of RVOT origin (sensitivity: 95.1%, specificity: 85.5%, positive predictive value: 86.5%, negative predictive value: 94.5%). The area under the curve (AUC) was 0.929 (p < .001).
Conclusion
S‐R difference in V1‐V2 is a novel and simple electrocardiographic criterion for accurately differentiating RVOT from LVOT sites of ventricular arrhythmia origins. The use of this simple ECG measurement could improve the accuracy of OTVA localization, could be beneficial for decreasing ablation duration and radiation exposure. Further studies with larger patient population are needed to verify the results of this study.
Keywords: radiofrequency ablation, S‐R difference in V1‐V2, ventricular arrhythmias
1. INTRODUCTION
Outflow tract (OT) ventricular arrhythmias (OTVA) are usually considered a benign clinical condition. But cardiomyopathy induced or worsened by premature ventricular complexes (PVC) is a serious problem which can be reversed with ablation (Yarlagadda et al., 2005). Radiofrequency catheter ablation is accepted as safe and becoming a standard therapeutic strategy for idiopathic OTVAs (Ito et al., 2003; Kumagai et al., 2008; Takemoto et al., 2005). There are complex three dimensional anatomic relationships between the two outflow tract regions. So, it is challenging to recognize the origin of the OTVAs. Different electrocardiographic (ECG) algorithms have been proposed for differentiation of left ventricular (LV) outflow tract (LVOT) and right ventricular (RV) outflow tract RVOT origin of OTVAs (Bala et al., 2010; Ouyang et al., 2002; Yamada et al., 2008; Yang et al., 2007; Zhang et al., 2009). Their accuracy and usefulness remain limited especially when the transition in the precordial leads occurs in V3 (Yoshida et al., 2014).
The prediction of RVOT or LVOT origin based on ECG is of great value. Because the correct estimation of the VA origin as RVOT or LVOT results in reduced ablation duration, reduced radiation exposure, decreased number of vascular access. This information is also important for patient counseling, physician procedural planning, and logistics.
In our study, we aimed to detect the predictive value of S‐R difference in V1‐V2 (V1‐2 SRd) for differentiating the left from right ventricular outflow tract arrhythmias.
2. METHODS
2.1. Study protocol and study population
We included 123 patients with symptomatic frequent premature ventricular outflow tract contractions (PVCs) who underwent successful catheter ablation in a retrospective manner (53 male, 70 female; mean age 46.2 ± 13.9 years, 61 RVOT, 62 LVOT origins). Nineteen of these 123 patients underwent redo procedures, others were de novo ablation. No patient fulfilled Task Force criteria for arrhythmogenic right ventricular cardiomyopathy (McKenna et al., 1994). Patients with VAs originating from tricuspid annulus or mitral annulus were excluded from the study. We included all consecutive patients from January 2013 to May 2017 except of the patients with unsuccessful ablation or patients with aforementioned exclusion criteria. The Local Ethics Committee approved the study protocol and informed consent for the EPS and ablation procedure was taken from all patients.
The baseline characteristics of patients including age, sex, prior history of ablation, complete blood count, creatinine values, LV ejection fraction (LVEF) were recorded for all patients. LVEF were assessed using Simpson's equation in the apical 4‐chamber view.
2.2. Electrocardiographic assessment
The surface 12 lead ECGs were recorded during sinus rhythm and during the PVCs at 25 mm/s speed with chest and limb leads placed in a standard position in all patients. Special attention was paid to the correct positioning of V1 and V2. QRS duration was measured in the lead with widest QRS complex on the 12‐lead surface ECG (Zhang et al., 2009). S‐R difference in V1‐V2 was calculated with this formula on the 12‐lead surface ECG: V1‐2 SRd (mV): (V1S amplitude + V2S amplitude) – (V1R amplitude + V2R amplitude) (Figure 1). All measurements were performed by two electrophysiologists who were blinded to the final diagnosis and the site of origin in order to eliminate the interobserver variability and bias.
Figure 1.
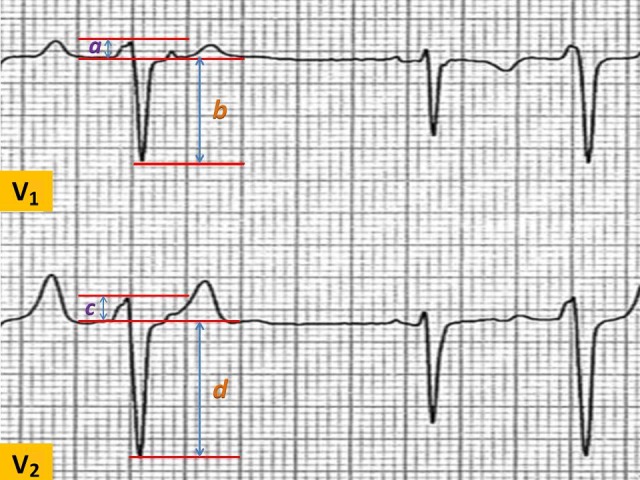
Calculation of the S‐R difference in V1‐V2. S‐R difference in V1‐V2 was calculated with this formula on the 12‐lead surface ECG: V1‐2 SRd (mV): [V1S amplitude (b) + V2S amplitude (d)] – [V1R amplitude (a) + V2R amplitude (c)]
2.3. Ablation protocol
All antiarrhythmic medications were discontinued at least five half lives before the ablation. We performed the ablation procedure with no sedation. Conventional ablation was performed in 101 (82.1%) patients using a Marin 7 Fr 4‐mm deflectable tip catheter (Medtronic Inc., Minneapolis, MN, USA), with an Atak II RF generator (Medtronic Inc). The operation was performed in temperature control mode, with a target temperature of 70°C and a power limit of 50 W in RVOT and the subvalvular LVOT, with a target temperature of 60°C and a power limit of 40 W in aortic root. VA ablation was performed with the CARTO (Biosense Webster, Diamond Bar, CA, USA) electroanatomic mapping system in 22 (17.9%) patients to guide ablation. In these patients, a 3.5‐mm irrigated‐tip catheter (NaviStar, Biosense Webster) was used for mapping and ablation. Target ablation sites were chosen with the combination of activation mapping and pace mapping as described previously (Arya et al., 2011). If radiofrequency application was successful, it was maintained for 60 s in RVOT and for 30–45 s in aortic root. If PVC persisted after a 30‐second application, radiofrequency delivery was stopped and another target was chosen for another ablation. Mapping and ablation of the LVOT area was performed with a retrograde aortic approach under 60 IU/kg heparin bolus. Further doses were administered if necessary in order to maintain an activated clotting time 250–350 s. Coronary angiography was performed in order to detect the anatomic proximity of the main coronary artery ostia to the ablation site. A distance >5 mm was accepted as safe to perform ablation. Acute procedural ablation success was defined as the absence of spontaneous or induced clinical PVCs at 30 min after the last radiofrequency energy application.
2.4. Statistical analyses
Statistical analyses were conducted using SPSS, version 14.0, (SPSS Inc. Chicago, IL, USA). Data are expressed as mean ± SD for continuous variables and percentage for categorical variables. The Shapiro‐Wilk test was used to test normality and a p value >.05 was defined as normally distributed data. Continuous variables that showed normal distribution were compared using the Student t test and ANOVA, whereas the Mann‐Whitney U test and Kruskal‐Wallis test were used for nonnormally distributed samples. Associations of the categorical variables between groups were tested using the chi‐square test. Statistical significance was defined as a p value <.05 for all comparisons. Pearson's and Spearman's correlation were used to examine the relationship between continuous variables. ROC analysis was made to determine cutoff value of V1‐2 SRd for differentiating the left from right ventricular outflow tract arrhythmias.
3. RESULTS
3.1. Comparison of baseline characteristics
A comparison of the baseline characteristics is shown in Table 1. Successful ablation was accomplished in all patients. OTVA was localized in the LVOT in 62 patients (50.4%), and in the RVOT in 61 patients (49.6%). Among patients with LVOT VA, in 43 patients the successful ablation site was localized in the aortic cusps, in 19 patients the successful ablation site was localized in subvalvular LVOT. Among patients with RVOT VA, in 60 patients the successful ablation site was localized in subvalvular RVOT, in one patient the successful ablation site was localized in the pulmonary artery. All clinical and demographic characteristics, laboratory, echocardiographic parameters were similar in the two groups (p > .05, for all). Patients with LVOT VA were significantly older (p < .05) as compared with RVOT VA patients. V1‐2 SRd was found to be significantly lower in LVOT origins than RVOT origins (p < .001).
Table 1.
Comparison of the baseline clinical, demographic, laboratory, electrocardiographic, and echocardiographic parameters
| RVOT n = 61 | LVOT n = 62 | p | |
|---|---|---|---|
| Age (years) | 43.1 ± 13.0 | 49.2 ± 14.2 | .015 |
| Gender (Male,%) | 24 (39.3) | 29 (46 .8) | .405 |
| Hemoglobin (g/dl) | 13.4 ± 2.0 | 13.3 ± 1.6 | .903 |
| Creatinine (mg/dl) | 0.70 ± 0.21 | 0.76 ± 0.28 | .256 |
| LV ejection fraction (%) | 60.7 ± 7.1 | 58.7 ± 6.3 | .128 |
| QRS duration (ms) | 139.4 ± 15.7 | 135.3 ± 20.1 | .203 |
| V1‐2 SRd (mV) | 2.46 ± 0.74 | 0.52 ± 1.13 | <.001 |
| Prior ablation history (n,%) | 5 (8.2) | 2 (3.2) | .273 |
| TICMP, n (%) | 4 (6.6) | 4 (6.5) | .981 |
| Patients treated with CARTO (n,%) | 7 (11.5) | 15 (24.2) | .066 |
Bold values indicate statistical significance. V1‐2 SRd, S‐R difference in V1‐V2, LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract; TICMP, tachycardia‐induced cardiomyopathy.
3.2. ROC curve analysis to determine predictive value of V1‐2 SRd for differentiating the left from right ventricular outflow tract arrhythmias
The cutoff value of V1‐2 SRd obtained by ROC curve analysis was 1.625 mV for prediction of RVOT origin (sensitivity: 95.1%, specificity: 85.5%, positive predictive value: 86.5%, negative predictive value: 94.5%). The area under the curve (AUC) was 0.929 (p < .001) (Figure 2). Scatterplots diagram of V1‐2 SRd for RVOT and LVOT origins was shown in Figure 3.
Figure 2.
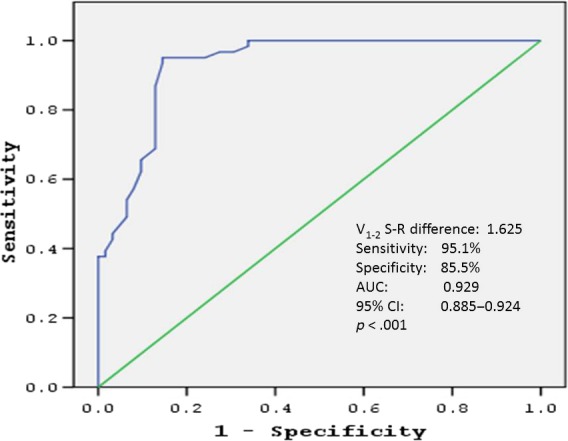
ROC curve analysis to determine predictive value of V1‐2 SRd for differentiating the left from right ventricular outflow tract arrhythmias
Figure 3.
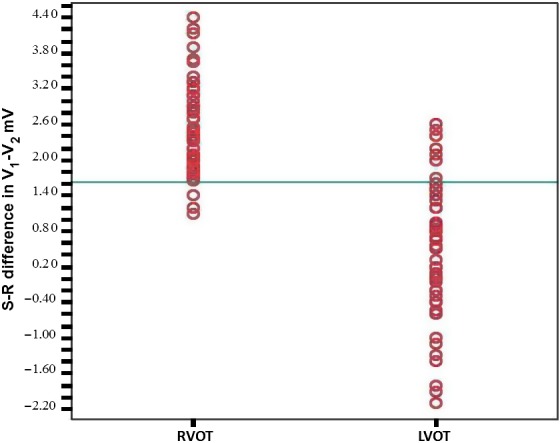
Scatter plot diagram V1‐2 SRd for RVOT and LVOT origins
4. DISCUSSION
We developed a novel ECG criterion, S‐R difference in V1‐V2, for differentiating the LVOT and RVOT origin by using 12 lead surface ECG measurements. To our knowledge, this is the first study that demonstrates the predictive value of S‐R difference in V1‐V2 for differentiating the left from right ventricular outflow tract arrhythmias. The main finding of the present study is that: [1] If we take the cutoff value as 1.625 mV, V1‐2 SRd was found to predict RVOT origin with 95.1% sensitivity and 85.5% specificity.
Outflow tract ventricular arrhythmias are considered idiopathic and benign VA, not associated with structural heart disease and cardiovascular risk factors. They are frequently observed in young women. OTVAs are known to mostly originate from RVOT in the literature (Buxton et al., 1983). However, contrary to the previously accepted profile, the present patient population of OTVAs from a single‐center experience had a mean age of 46.2 years. In this population, more than half of the patients had OTVA with LV origin. This higher rate of LVOT origin has clinical implications because it results in the mapping of more complex structures such as the aortic root or the distal coronary sinus. The correct estimation of the VA origin as RVOT or LVOT results in reduced ablation duration, reduced radiation exposure, decreased number of vascular access. So, predicting the probability of LVOT origin is important for patient counseling, operator procedural planning, and estimating the probability of complications. Localization of VA origin before ablation is important for increasing rates of successful ablation for minimizing the risk of complications.
In patients whose hearts are normally positioned, the RVOT is located anteriorly relative to the LVOT in the level of the aortic cusps. The pulmonary valves are positioned approximately 1–2 cm superior to the aortic valves (Asirvatham, 2009). Even though the posteroseptal part of the RVOT has a close contiguity to the anterior portion of the RCC, the RVOT and LVOT have no muscular fibers connecting them. So, impulse conduction can only occur through fibers connecting both ventricles transseptally. Regarding this anatomical background, V1 and V2 are the closest chest leads to LVOT and RVOT. So V1 and V2 are the best derivations to distinguish OTVAs. According to V1‐2, the RVOT is located anteriorly compared to LVOT. The closer a focus of an impulse is to a lead, the greater the degree of S wave in that lead. The R‐wave amplitude increases and the S‐wave amplitude decreases as the focus of the impulse gets far away from that lead. This contiguity can be thought to result in higher R‐wave amplitude and lower S‐wave amplitude in the frontal leads V1‐2 during OT‐VAs originating from the LVOT than those from the RVOT (Hutchinson & Garcia, 2013; Suleiman & Asirvatham, 2008a, 2008b). Because RVOT is closer to V1‐2 than LVOT. Even the most posterior part of the RVOT is located anteriorly to the most anterior part of the LVOT. Therefore, it would be reasonable to develop an ECG algorithm with using the R‐ and S‐wave amplitude in lead V1 or V2. S‐R difference in V1‐V2 is a quantitative measure of the proximity of the focus of the impulse to V1‐2 leads. In our study, V1‐2 SRd was found to be significantly higher in VAs originating from RVOT. With the cutoff value of 1.625 mV, V1‐2 SRd was found to predict RVOT origin with 95.1% sensitivity and 85.5% specificity. It is difficult to distinguish the RVOT‐originated VAs from an LVOT‐originated VAs with a 100% precision. Because the QRS morphology of OTVAs can be affected by several factors such as lead position, cardiac anatomy, cardiac rotation, aorta deformities, obesity, effect of medications, effect of breasts in women, ventricular hypertrophy, chest wall deformities, and preferential conduction (Betensky et al., 2011; Yamada et al., 2007; Yoshida et al., 2011).
There have been a lot of studies aiming at localizing OTVAs. We compared our criteria to the V2S/V3R index (Yoshida et al., 2014) and the V2 transition ratio (Betensky et al., 2011) in our population. The cutoff value of V2S/V3R index obtained by ROC curve analysis was 1.45 for prediction of LVOT origin (sensitivity: 78.7%, specificity: 91.8%). The area under the curve (AUC) was 0.909 (p < .001). The cutoff value of V2 transition ratio obtained by ROC curve analysis was 0.41 for prediction of LVOT origin (sensitivity: 52.1%, specificity: 100.0%). The area under the curve (AUC) was 0.925 (p < .001).
There were some limitations in our study. As a single‐center study, our patient cohort might be different from that in other centers. The sample size is relatively small and our results need to be confirmed in future large multicenter prospective trials. Because of the retrospective nature of our study, we did not have the chance to investigate the confounding factors that affect QRS morphology of OTVAs such as lead position, cardiac anatomy, cardiac rotation, aorta deformities, obesity, effect of medications, effect of breasts in women, ventricular hypertrophy, chest wall deformities, and preferential conduction.
In conclusion, S‐R difference in V1‐V2 is a novel and simple electrocardiographic criterion for accurately differentiating RVOT from LVOT sites of ventricular arrhythmia origins. The use of this simple ECG measurement could improve the accuracy of OTVA localization, could be beneficial for decreasing ablation duration and radiation exposure. Further studies with larger patient population are needed to verify the results of this study.
CONFLICT OF INTEREST
The authors report no conflict of interest.
Kaypakli O, Koca H, Sahin DY, Karataş F,Ozbicer S, Koç M. S‐R difference in V1‐V2 is a novel criterion for differentiating the left from right ventricular outflow tract arrhythmias. Ann Noninvasive Electrocardiol. 2018;23:e12516 10.1111/anec.12516
REFERENCES
- Arya, A. , Huo, Y. , Frogner, F. , Wetzel, U. , Sommer, P. , Gaspar, T. , … Hindricks, G. (2011). Effect of limb lead electrodes location on ECG and localization of idiopathic outflow tract tachycardia: A prospective study. Journal of Cardiovascular Electrophysiology, 22, 886–891. [DOI] [PubMed] [Google Scholar]
- Asirvatham, S. J. (2009). Correlative anatomy for the invasive electrophysiologist: Outflow tract and supravalvar arrhythmia. Journal of Cardiovascular Electrophysiology, 20, 955–968. [DOI] [PubMed] [Google Scholar]
- Bala, R. , Garcia, F. C. , Hutchinson, M. D. , Gerstenfeld, E. P. , Dhruvakumar, S. , Dixit, S. , … Zado, E. (2010). Electrocardiographic and electrophysiologic features of ventricular arrhythmias originating from the right/left coronary cusp commissure. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 7, 312–322. [DOI] [PubMed] [Google Scholar]
- Betensky, B. P. , Park, R. E. , Marchlinski, F. E. , Hutchinson, M. D. , Garcia, F. C. , Dixit, S. , … Riley, M. P. (2011). The V(2) transition ratio: A new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. Journal of the American College of Cardiology, 57, 2255–2262. [DOI] [PubMed] [Google Scholar]
- Buxton, A. E. , Waxman, H. L. , Marchlinski, F. E. , Simson, M. B. , Cassidy, D. , & Josephson, M. E. (1983). Right ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation, 68, 917–927. [DOI] [PubMed] [Google Scholar]
- Hutchinson, M. D. , & Garcia, F. C. (2013). An organized approach to the localization, mapping, and ablation of outflow tract ventricular arrhythmias. Journal of Cardiovascular Electrophysiology, 24, 1189–1197. [DOI] [PubMed] [Google Scholar]
- Ito, S. , Tada, H. , Naito, S. , Kurosaki, K. , Ueda, M. , Hoshizaki, H. , … Nogami, A. (2003). Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. Journal of Cardiovascular Electrophysiology, 14, 1280–1286. [DOI] [PubMed] [Google Scholar]
- Kumagai, K. , Fukuda, K. , Wakayama, Y. , Sugai, Y. , Hirose, M. , Yamaguchi, N. , … Shimokawa, H. (2008). Electrocardiographic characteristics of the variants of idiopathic left ventricular outflow tract ventricular tachyarrhythmias. Journal of Cardiovascular Electrophysiology, 19, 495–501. [DOI] [PubMed] [Google Scholar]
- McKenna, W. J. , Thiene, G. , Nava, A. , Fontaliran, F. , Blomstrom‐Lundqvist, C. , Fontaine, G. , & Camerini, F. (1994). Myocardial and pericardial disease of the european society of cardiology and of the scientific council on cardiomyopathies of the international society and federation of cardiology supported by the schoepfer association. Diagnosis of arrhythmogenic right ventricular dysplasia cardiomyopathy. British Heart journal, 71, 215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang, F. , Fotuhi, P. , Ho, S. Y. , Hebe, J. , Volkmer, M. , Goya, M. , … Kuck, K. H. (2002). Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: Electrocardiographic characterization for guiding catheter ablation. Journal of the American College of Cardiology, 39, 500–508. [DOI] [PubMed] [Google Scholar]
- Suleiman, M. , & Asirvatham, S. J. (2008a). Ablation above the semilunar valves: When, why, and how? Part I. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 5, 1485–1492. [DOI] [PubMed] [Google Scholar]
- Suleiman, M. , & Asirvatham, S. J. (2008b). Ablation above the semilunar valves: When, why, and how? Part II. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 5, 1625–1630. [DOI] [PubMed] [Google Scholar]
- Takemoto, M. , Yoshimura, H. , Ohba, Y. , Matsumoto, Y. , Yamamoto, U. , Mohri, M. , … Origuchi, H. (2005). Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. Journal of the American College of Cardiology, 45, 1259–1265. [DOI] [PubMed] [Google Scholar]
- Yamada, T. , Murakami, Y. , Yoshida, N. , Okada, T. , Shimizu, T. , Toyama, J. , … Hirai, M. (2007). Preferential conduction across the ventricular outflow septum in ventricular arrhythmias originating from the aortic sinus cusp. Journal of the American College of Cardiology, 50, 884–891. [DOI] [PubMed] [Google Scholar]
- Yamada, T. , Yoshida, N. , Murakami, Y. , Okada, T. , Muto, M. , Murohara, T. , … Kay, G. N. (2008). Electrocardiographic characteristics of ventricular arrhythmias originating from the junction of the left and right coronary sinuses of Valsalva in the aorta: The activation pattern as a rationale for the electrocardiographic characteristics. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 5, 184–192. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Saenz, L. C. , Varosy, P. D. , Badhwar, N. , Justin, H. , Kilicaslan, F. , … Scheinman, M. (2007). Using the initial vector from surface electrocardiogram to distinguish the site of outflow tract tachycardia. Pacing and Clinical Electrophysiology, 30, 891–898. [DOI] [PubMed] [Google Scholar]
- Yarlagadda, R. K. , Iwai, S. , Stein, K. M. , Markowitz, S. M. , Shah, B. K. , Cheung, J. W. , … Mittal, S. (2005). Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation, 112, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Yoshida, N. , Inden, Y. , Uchikawa, T. , Kamiya, H. , Kitamura, K. , Shimano, M. , … Murohara, T. (2011). Novel transitional zone index allows more accurate differentiation between idiopathic right ventricular outflow tract and aortic sinus cusp ventricular arrhythmias. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 8, 349–356. [DOI] [PubMed] [Google Scholar]
- Yoshida, N. , Yamada, T. , Mcelderry, H. T. , Inden, Y. , Shimano, M. , Murohara, T. , … Kay, G. (2014). A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: The V2S/V3R index. Journal of Cardiovascular Electrophysiology, 25, 747–753. [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Chen, M. , Yang, B. , Ju, W. , Chen, H. , Yu, J. , … Tse, H. F. (2009). Electrocardiographic algorithm to identify the optimal target ablation site for idiopathic right ventricular outflow tract ventricular premature contraction. Europace, 11, 1214–1220. [DOI] [PubMed] [Google Scholar]


