Abstract
Background and aim
New‐onset atrial fibrillation (NOAF) has been associated with poor outcome in patients with acute coronary syndromes (ACS). Also, Syntax score (SS) is a scoring system that is derived from angiographic images and is associated with long‐term mortality and major adverse cardiac events. In this study, we aimed to assess the relationship between SS and NOAF with known predictors of atrial fibrillation.
Methods
In a prospective, single‐center, cross‐sectional study, 692 patients who were diagnosed with coronary artery disease for the first time were enrolled consecutively. NOAF was defined as atrial fibrillation, which was documented after hospital admission. SS was calculated by a computer software. Multivariable logistic regression analyzes were used to detect the relationship between variables and NOAF.
Results
New‐onset atrial fibrillation was detected in 82 patients (11.8%). Patients with NOAF had higher SS (22, interquartile range 18.3–25.1, vs. 12, interquartile range 7–19.5, p < 0.001). According to multivariable logistic regression analysis for NOAF, SS were independently and significantly associated (OR, 1.103; 95% confidence interval, 1.047–1.163; p < 0.001). Other independent predictors of NOAF were TIMI flow <3, C reactive protein, left ventricular ejection fraction, left atrial volume index and E/E′ ratio. The optimal cut‐off value for SS was 18 for the development of NOAF with 82% sensitivity and 68% specificity (area under the curve: 0.795, 95% confidence interval 0.749–0.841, p < 0.001).
Conclusion
Syntax score may be helpful to identify for patients who would develop atrial fibrillation in the setting of ACS.
Keywords: acute coronary syndrome, new‐onset atrial fibrillation, SYNTAX Score
1. INTRODUCTION
Atrial fibrillation (AF) is a commonly encountered arrhythmia in the setting of acute coronary syndromes (ACS) (Crenshaw et al., 1997; Kinjo et al., 2003; Mehta et al., 2003; Saczynski et al., 2009). AF in the setting of ACS is associated with poor clinical course and prognosis of the disease. Also, new‐onset atrial fibrillation (NOAF) in the setting of percutaneously treated ACS has been reported with increased in‐hospital and long‐term mortality (Rene et al., 2014; Ruwald et al., 2013). Especially, patients with NOAF, who were not diagnosed as AF before the ACS associated with higher mortality rates compared with those ACS patients who were admitted with history of atrial fibrillation (Kinjo et al., 2003; Køber et al., 2006; Lehto, Snapinn, Dickstein, Swedberg, & Nieminen, 2004; Pedersen, Bagger, Køber, & Torp‐Pedersen, 1999; Rathore et al., 2000). Therefore, it is crucial to determine the ACS patients who are at the increased risk to develop NOAF. Although the relationship between more severe multivessel disease and NOAF was shown in ACS (Crenshaw et al., 1997; Lau et al., 2009), this relationship was not evaluated with SYNTAX score. The SYNTAX score (SS) is a comprehensive angiographic scoring system that is derived just from the coronary anatomy and lesion characteristics (Sianos et al., 2005). It is a useful tool that provides additional information to known risk factors of long‐term mortality and major adverse cardiac events (MACE) (Magro et al., 2011). The main purpose of the present study is to determine the relationship between NOAF development and coronary artery disease (CAD) severity by using SS in ACS patients who were treated with percutaneously.
2. METHODS
This was a single‐centre, cross‐sectional study conducted between January 2015 and December 2017. A total of 692 patients with ACS who have not been diagnosed with any cardiac disease previously were enrolled consecutively. Patients who had known diseases, such as severe infection, autoimmune diseases, hyperthyroidism, chronic renal and liver diseases, and neoplastic diseases were excluded. Patients with a previous history of AF or atrial flutter were excluded as well. Additionally, patients were excluded if they were admitted at >24 hr from symptom onset. Each patient received standard pharmacologic treatment according to acute myocardial infarction (AMI) treatment guidelines (Amsterdam et al., 2014; O'Gara et al., 2013). Echocardiographic evaluation and baseline venous blood samples assessment were performed within 12–24 hr of symptom onset. Left ventricular ejection fraction (LVEF) was evaluated after the coronary intervention and was shown by using the modified Simpson’s method (Lang et al., 2005). LAVI was determined by the biplane area‐length method, using measurements at the apical 4‐ and 2‐chamber views at end‐systole and indexed by body surface area (Lester, Ryan, Schiller, & Foster, 1999). All patients were followed with in‐hospital continuous ECG monitoring for at least 48 hr and a 12‐lead ECG was obtained twice daily during the hospital stay. Additionally, when patients had symptoms suggesting a development of arrhythmia, such as palpitations or dyspnea, the rhythm was checked by 12‐lead ECG.
Acute myocardial infarction was diagnosed when patients had characteristic symptoms, including elevation of the cardiac troponin‐T level (>0.01 ng/ml in any blood sample during admission) with serial ECG changes consisting of ST‐segment and T wave changes or new pathologic Q waves (O'Gara et al., 2013). ST‐segment elevation MI (STEMI) was defined as ST‐segment elevation ≥0.2 mV in two consecutive leads or new‐onset left bundle branch block detected on ECG. Non‐STEMI was defined as MI without ST‐segment elevation on ECG. Additionally, unstable angina is defined to be present in patients with ischemic symptoms suggestive of an ACS and no elevation in troponins, with or without electrocardiogram changes indicative of ischemia, according to the 2014 AHA/ACC guidelines (Amsterdam et al., 2014).
The presence of NOAF was observed during in‐hospital course. The diagnosis of AF was defined as the presence of the following criteria at least 30 s on a rhythm strip: (a) absence of P‐waves; (b) coarse or fine fibrillatory waves; and (c) irregular R–R intervals (Camm et al., 2010). NOAF was defined as AF, which was documented after hospital admission without a prior history. Atrial flutter assumed as AF.
All patients underwent coronary angiography by using the Judkins trans‐femoral technique. Fluoroscopic visualizations were evaluated by two expert cardiologists who were blinded to patients’ clinical specifications. In case of disagreement on visual evaluation, the decision of the third observer was obtained and the final decision was taken by a consensus. Each coronary lesion which constituted luminal obstruction ≥50% in vessels ≥1.5 mm was added to provide overall SS. We used online calculator version 2.11 to acquire overall SS (http://www.syntaxscore.com). In STEMI patients, an occluded culprit artery was scored as an occluded artery with <3‐months duration (Magro et al., 2011). The decisions of revascularizations were left to the discretion of physicians.
Sociodemographic data, medical history, and initial examination findings were acquired and recorded prospectively within 24 hr of admission. The study protocol was approved by the local ethical committee and informed consent was obtained from each patient.
Continuous variables were tested for normal distribution by the Kolmogorov‐Smirnov test. Data were expressed as mean ± standard deviation (SD) for normally distributed continuous variables, as median and inter‐quartile ranges for skew‐distributed continuous variables. Categorical variables were presented as numbers and percentages. Variables of both groups were compared with the chi‐squared, Mann–Whitney, and independent sample t tests when appropriate. The identification of the independent predictors of NOAF was assessed by using univariable and multivariable logistic regression analysis. The selection of covariates in multivariate models was based on both previous and empirical evaluations. Initially, significant baseline covariates that are known to affect parameters were included in the analyses. Also, factors that were significantly different for patients with and without NOAF were included in a univariate logistic regression model. The variables for which the nonadjusted p value was <0.10 in univariate logistic regression analysis were determined as a potential risk marker and included in the full model of backward multiple logistic regression. The following clinical variables were considered in the multivariable procedure: age, gender, history of diabetes mellitus, STEMI, TIMI flow grade <3, heart rate at admission, Killip class II–IV on admission, admission hemoglobin level, admission white blood cell count, CRP, LVEF, LAVI, E/E′ and SS. Receiver operating characteristic (ROC) analysis was used to determine the cut‐off level of SS to predict NOAF. Statistical analysis was performed by using SPSS 22.0 (SPSS Inc. Chicago, Illinois, USA), and a probability (p) value <0.05 (two‐tailed) was considered statistically significant.
3. RESULTS
A total of 692 consecutive patients with ACS were included in the analysis (mean age, 63 ± 13 years; 74% male). STEMI and non ST‐elevation acute coronary syndrome (NSTE‐ACS) were observed in 319 (46.1%) and 373 (53.9%) patients, respectively. Among all ACS patients who were diagnosed as first time CAD, NOAF was observed in 82 (11.8%) patients.
The baseline characteristics of patients with and without NOAF are showed in Table 1. There were noticeable significant clinical differences between the groups. Patients with NOAF were older (71, interquartile range 61–79, vs. 62, interquartile range 53–72, p < 0.001) and less likely to be male (61% vs. 75.7%, p = 0.004) than those without NOAF. Patients with NOAF also had lower TIMI flow grade (1.5% vs. 22%, p < 0.001), admission hemoglobin level (12.8, interquartile range 11.1–14 g/dl, vs. 13.8, interquartile range 12.4–14.9 g/dl, p < 0.001), left ventricular ejection fraction (37, interquartile range 30%–45%, vs. 50, interquartile range 43%–61%, p < 0.001), but higher left atrial volume index (LAVI, 32.39 ± 5.01 ml/m2, vs. 26.34 ± 4.94 ml/m2, p < 0.001), E/E′ ratio (16, interquartile range 14.5–17.2, vs. 12.6, interquartile range 10.5–14, p < 0.001), admission serum creatinine (0.94, interquartile range 0.74–1.16 g/dl, vs. 0.84, interquartile range 0.7–1 g/dl, p = 0.013), C‐reactive protein (CRP, 4.4, interquartile range 2.8–5.5, vs. 1.2, interquartile range 0.7–2.1, p < 0.001) and SS (22, interquartile range 18.3–25.1, vs. 12, interquartile range 7–19.5, p < 0.001) than those without NOAF (Figure 1). Additionally, patients with NOAF presented with higher heart rates (>100 beats/min, 26.8% vs. 14.1%, p = 0.003) and higher Killip class (42% vs. 4.6%, p < 0.001).
Table 1.
The baseline characteristics of patients with and without new‐onset atrial fibrillation (NOAF)
| Characeristics | In‐hospital NOAF or flutter | ||
|---|---|---|---|
| No | Yes | p | |
| n = 610 | n = 82 | ||
| Sociodemographic factors | |||
| Age | 62 (53–72) | 71 (61–79) | <0.001 |
| Male (%) | 462 (75.7) | 50 (61) | 0.004 |
| Current smoker (%) | 282 (46.2) | 34 (41.5) | 0.416 |
| Diabetes mellitus (%) | 121 (19.8) | 34 (41.5) | <0.001 |
| Hypertensiona (%) | 462 (75.7) | 68 (82.9) | 0.149 |
| Dyslipidemiab (%) | 454 (74.4) | 64 (78) | 0.478 |
| Family history of CAD (%) | 304 (49.8) | 39 (47.6) | 0.699 |
| Clinical characteristics at admission | |||
| STEMI (%) | 253 (41.5) | 66 (80.5) | <0.001 |
| TIMI flow <3 (%) | 9 (1.5) | 18 (22) | <0.001 |
| Admission heart rate >100 (beats/min) (%) | 86 (14.1) | 22 (26.8) | 0.003 |
| Killip class II–IV (%) | 28 (4.6) | 35 (42) | <0.001 |
| Admission systolic blood pressure >100 mm Hg (%) | 542 (88.9) | 73 (89) | 0.963 |
| Syntax score | 12 (7.0–19.5) | 22 (18.3–25.1) | <0.001 |
| Laboratory tests | |||
| Admission hemoglobin, g/dl | 13.8 (12.4–14.9) | 12.8 (11.1–14) | <0.001 |
| Admission serum creatinine, g/dl | 0.84 (0.7–1) | 0.94 (0.74–1.16) | 0.013 |
| WBC, 103/ µl | 9.28 (7.43–11.63) | 10.09 (8.03–13.04) | 0.032 |
| Platelets,/mm3 | 214 (185–254) | 208 (184–256) | 0.745 |
| CRP, mg/dl | 1.2 (0.7–2.1) | 4.4 (2.87–5.5) | <0.001 |
| LDL‐C, mg/dl | 140 (104–165) | 130 (107–165) | 0.746 |
| HDL‐C, mg/dl | 43 (38.75–48) | 42 (38–46) | 0.535 |
| Total cholesterol, mg/dl | 199 (167–225) | 200 (174–244) | 0.280 |
| Triglyseride, mg/dl | 115 (78–165) | 102 (78–157) | 0.512 |
| Echocardiography | |||
| Ejection fraction (%) | 50 (43–61) | 37 (30–45) | <0.001 |
| LAVI(ml/m2) | 26.24 ± 4.94 | 32.39 ± 5.01 | <0.001 |
| E/E′ | 12.6 (10.5–14) | 16 (14.5–17.27) | <0.001 |
CAD: coronary artery disease; CRP: C reactive protein; HDL‐C: high‐density lipoprotein cholesterol; LAVI: left atrial volume index; LDL‐C: low‐density lipoprotein cholesterol; STEMI: ST‐elevation myocardial infarction; WBC: white blood cell.
Data are presented as mean ± standard deviation for normally distributed continuous data, as median and inter‐quartile ranges for skew‐distributed continuous data and percentage (%) for categorical variables.
Hypertension: history of hypertension diagnosed and/or treated by a physician, or blood pressure >140 mm Hg systolic or >90 mm Hg diastolic on at least two measurements
Dyslipidemia: history of dyslipidemia treatment, or total cholesterol ≥200 mg/dl, low density lipoprotein ≥130 mg/dl, and high density lipoprotein <40 mg/dl.
Figure 1.
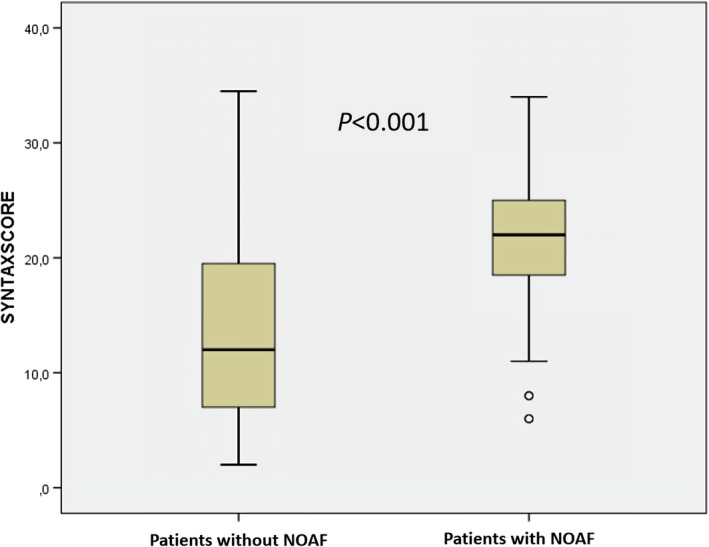
Patients with new‐onset atrial fibrillation (NOAF) had higher syntax score
Univariable logistic regression demonstrated a significant association between several variables and NOAF, including age, female gender, history of diabetes mellitus, STEMI, low TIMI flow grade, higher Killip class on admission, lower hemoglobin level, higher white blood cell count and CRP. Also, SS and echo parameters such as LVEF, E/E′ and LAVI were significantly associated with NOAF in univariable analysis. In a multivariable logistic regression model, a higher SS (odds ratio [OR], 1.101; 95% confidence interval [CI], 1.041–1.163; p < 0.001) emerged as independent predictor of NOAF. Other independent predictors of NOAF included TIMI flow <3, CRP levels, LVEF, LAVI and E/E′ ratio (Table 2). A second model of multivariable regression analysis was performed using dichotomized supramedian SS value instead of continuous SS values. A supramedian level of SS (or >13 SS) was also found to be independent predictor for NOAF (Table 2). ROC curve analysis was performed to detect the cut‐off value of SS. Figure 2 illustrates the results of ROC curve analysis for SS in the detection of NOAF. The optimal cut‐off value for SS was 18 with a sensitivity of 82% and a specificity of 68% (AUC: 0.795, 95% confidence interval 0.749–0.841, p < 0.001).
Table 2.
Univariable and multivariable logistic regression analyses with independent predictors of new‐onset atrial fibrillation (NOAF)
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | First model | Second model | |||
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Age, years | 1.061 (1.038–1.085) | <0.001 | ||||
| Male | 0.501 (0.309–0.810) | 0.005 | ||||
| Diabetes mellitus | 2.863 (1.767–4.637) | <0.001 | ||||
| STEMI | 5.821 (3.294–10.286) | <0.001 | 2.621 (1.046–6.562) | 0.040 | ||
| TIMI flow <3 | 18.781 (8.103–43.532) | <0.001 | 0.173 (0.033–0.902) | 0.037 | 0.163 (0.030–0.876) | 0.034 |
| Admission heart rate >100 (beats/min) | 2.234 (1.303–3.830) | 0.003 | ||||
| Killip class II–IV | 15.479 (8.675–27.619) | <0.001 | ||||
| Admission hemoglobin, g/dl | 0.797 (0.709–0.896) | <0.001 | ||||
| Admission serum creatinine, g/dl | 1.318 (0.840–2.068) | 0.230 | ||||
| WBC, 103/µl | 1.075 (1.014–1.139) | 0.016 | ||||
| CRP(mg/dl) | 1.821 (1.607–2.063) | <0.001 | 2.010. (1.659–2.436) | <0.001 | 2.104 (1.704–2.598) | <0.001 |
| Ejection fraction (%) | 0.905 (0.883–0.927) | <0.001 | 0.961 (0.924–1.000) | 0.050 | 0.956 (0.919–0.994) | 0.024 |
| LAVI(ml/m2) | 1.266 (1.201–1.334) | <0.001 | 1.228 (1.134–1.331) | <0.001 | 1.239 (1.140–1.346) | <0.001 |
| E/E′ | 1.630 (1.457–1.824) | <0.001 | 1.431 (1.223–1.662) | <0.001 | 1.234 (1.223–1.682) | <0.001 |
| SYNTAX score | 1.155 (1.115–1.198) | <0.001 | 1.101 (1.041–1.163) | 0.001 | ||
| Supramedian SS | 10.214 (5.019–20.788) | <0.001 | 5.234 (1.976–13.860) | 0.001 | ||
CRP: C reactive protein; LAVI: left atrial volume index; NOAF: new‐onset atrial fibrillation; SS: SYNTAX score; STEMI: ST‐elevation myocardial infarction; WBC: white blood cell.
A second model of multivariable regression analysis was performed for prediction of NOAF with using dichotomized supramedian SS value instead of continuous SS values.
Figure 2.
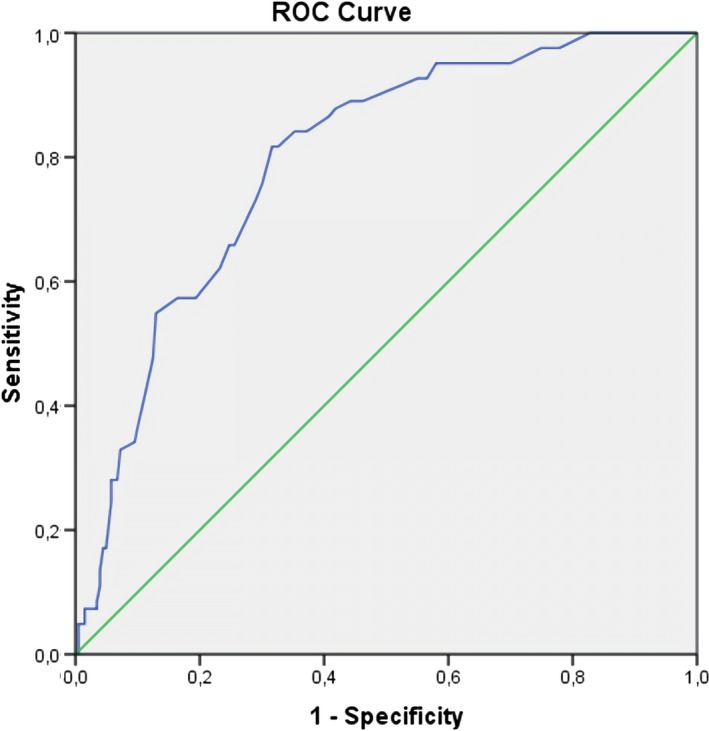
Receiver operating characteristic curve of SYNTAX score (SS) to predict new‐onset atrial fibrillation (NOAF). The area under curve was 0.795 (%95 CI, 0.749–0.841, p < 0.001). The optimal cut‐off level of SS was 18 with 82% sensitivity and 68% specificity. CI: confidence interval
4. DISCUSSION
In the present study, we aimed to investigate the association between development of NOAF and SS in patients with acute coronary syndrome who were treated percutaneously. Our study demonstrated that SS is an independent predictor of NOAF development during hospitalization.
Previous studies have reported that the incidence of NOAF as 6%–7.7% among patients who received thrombolytic therapy or PCI (Kinjo et al., 2003; Wong et al., 2003). In our study, NOAF was observed as 11.8%. In agreement with previous studies (Crenshaw et al., 1997; Kinjo et al., 2003; Pizzetti et al., 2001) patients with NOAF were more likely to be older, female, have higher heart rate at admission and higher Killip class. They were also more likely to have a history of diabetes mellitus, low EF and TIMI flow <3.
Different studies have shown that the development of NOAF in the setting of ACS is a multifactorial process. Although the exact cause is unclear, possible contributing mechanisms have been demonstrated; including deterioration of LV systolic function, increased LV filling pressure, atrial ischemia or infarct, atrial stretching with elevated left atrial pressure (Aronson et al., 2011; Jons et al., 2010) and inflammation (Aronson et al., 2007; Hwang et al., 2011). In the present study, patients with NOAF showed more left atrial enlargement and LV diastolic dysfunction than those without NOAF. AMI generally leads to change in left ventricular filling properties, which may result in advanced diastolic dysfunction (Celik, Erdöl, Baykan, Kaplan, & Kasap, 2001). Additionally, diastolic dysfunction may cause an increased left atrial pressure (Geske, Sorajja, Nishimura, & Ommen, 2007) thus facilitates the emergence of atrial fibrillation. The ratio of transmitral doppler early filling velocity to tissue doppler early diastolic mitral annular velocity (E/E′) is an important indicator of diastolic dysfunction and increased filling pressures, which was demonstrated significantly higher among patients with NOAF. Furthermore, we assessed the left atrial volume index which is more accurate representation of the true left atrial size (Lester et al., 1999) and reflects subacute or chronic abnormal filling pressures (Tsang, Barnes, Gersh, Bailey, & Seward, 2002). As a result of the study analysis, LAVI values were higher in patients with NOAF. These results were consistent with the previous studies (Wi et al., 2016).
As is widely known, inflammation plays an important role in CAD and other manifestations of atherosclerosis (Hansson, 2005). Also, inflammation has been associated in various AF‐related pathological processes, including oxidative stress, fibrosis, and thrombogenesis (Guo, Lip, & Apostolakis, 2012; Van Wagoner, 2008). As a result, inflammation is a common factor in both clinical conditions including NOAF and CAD. We may say that new‐onset atrial fibrillation and more extensive coronary involvement are two clinical entities that appear to be the result of a common reason, increased inflammatory process. It is expected that the coexistence of both conditions will be more frequent with increasing inflammation. The inflammatory process in the atherosclerotic artery may lead to increased blood levels of inflammatory cytokines and other acute‐phase reactants. Therefore, elevated C‐reactive protein levels in patients with ACS likely reflect inflammation in the coronary artery (Liuzzo et al., 1996). We have demonstrated that increased C‐reactive protein level was an independent predictor of NOAF in our study population, which is consistent with previously reported studies (Aronson et al., 2007; Hwang et al., 2011).
A recent published report showed that higher SS is related to development of NOAF (Rencuzogullari et al., 2018). The results of this report were consistent with ours, but there are some differences; this study consisted only STEMI patients and planned retrospectively. Unlike this design, we enrolled appropriate patients both STEMI and NSTE‐ACS prospectively. We also excluded patients with previously known CAD due to investigate the pure effect of ACS on the development of NOAF.
In this prospective cross‐sectional study, we emphasized that higher SS is an independent predictor of NOAF development in patients with ACS who were treated percutaneously. Previous studies have examined the number of involved vessels while assessing coronary artery disease severity (Kinjo et al., 2003; Wi et al., 2016). In the present study, CAD severity was evaluated by SS which is derived from lesion numbers, characteristics, location, and complexity. An important angiographic finding from our study is that NOAF was predicted by more extensive coronary artery disease, but also there was no correlation between NOAF and number of diseased vessel (Table 3). According to the results, we can say that SS is a more valuable parameter than diseased vessels numbers in predicting NOAF. More frequent involvement of right coronary artery (RCA) in patients with NOAF implicates specified territories at risk, including the sinoatrial node, the atrioventricular node, and the atria. The emergence of atrial ischemia could lead to the development of NOAF (Alasady et al., 2011; Sinno et al., 2003). Furthermore, increased ischemic load with higher SS causes left ventricular systolic and diastolic dysfunction and could trigger a rhythm disturbance by increasing LV filling pressures. This hemodynamic mechanisms potentially more important at pathogenesis of NOAF and may explain our principal findings.
Table 3.
The angiographic characteristics of patients with and without new‐onset atrial fibrillation (NOAF)
| In‐hospital NOAF or flutter | |||
|---|---|---|---|
| No | Yes | p | |
| n = 610 | n = 82 | ||
| Infarct‐related artery (%) | |||
| LAD | 294 (48.2) | 37 (45.1) | 0.601 |
| LCx | 83 (13.6) | 5 (6.1) | 0.055 |
| RCA | 182 (29.8) | 38 (46.3) | 0.003 |
| Other | 51 (8.4) | 2 (2.4) | 0.058 |
| Number of diseased vessels (%) | |||
| 1 | 254 (41.6) | 16 (19.5) | <0.001 |
| 2 | 294 (48.2) | 54 (65.9) | 0.003 |
| 3 | 62 (10.2) | 12 (14.6) | 0.219 |
LAD: left anterior descending coronary artery; LCx: left circumflex coronary artery; RCA: right coronary artery.
Data are presented as number and percentage (%).
Our study has several limitations. It was a relatively small‐sized study managed by a single institution. Results need to be confirmed in other larger multicenter trials. Additionally, our AF screening strategy was based on routine clinical follow–up and patients symptoms after discharging of the coronary care unit. Therefore, it is likely to miss the asymptomatic or short duration AF attacks. Furthermore, we were unable to identify silent/asymptomatic paroxysmal AF episodes before admission. Finally, based on its cross‐sectional design, the present findings are inherently limited to explain the causal relation between NOAF and SS.
In conclusion, we know that there are many global and local hemodynamic and also neurohumoral factors that contribute to NOAF development in the process of acute coronary syndrome. Although we could not figure out the precise etiology of this rhythm disturbance, the higher SS was associated with NOAF. These patients tend to have worse outcomes including stroke and mortality. Taking into account other independent predictors, patients with elevated LV filling pressure as well as high SS should be followed more closely. It seems reasonable to consider a more aggressive approach to treatment and follow‐up strategies, including close rhythm monitoring after discharge from hospital.
CONFLICT OF INTEREST
The Author(s) declare(s) that there is no conflict of interest.
Cirakoglu OF, Aslan AO, Akyuz AR, et al. The value of syntax score to predict new‐onset atrial fibrillation in patients with acute coronary syndrome. Ann Noninvasive Electrocardiol. 2019;24:e12622 10.1111/anec.12622
REFERENCES
- Alasady, M. , Abhayaratna, W. P. , Leong, D. P. , Lim, H. S. , Abed, H. S. , Brooks, A. G. , … Sanders, P. (2011). Coronary artery disease affecting the atrial branches is an independent determinant of atrial fibrillation after myocardial infarction. Heart Rhythm, 8(7), 955–960. 10.1016/j.hrthm.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Amsterdam, E. A. , Wenger, N. K. , Brindis, R. G. , Casey, D. E. , Ganiats, T. G. , Holmes, D. R. , … Levine, G. N. (2014). 2014 AHA/ACC guideline for the management of patients with non–ST‐elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 64(24), e139–e228. [DOI] [PubMed] [Google Scholar]
- Aronson, D. , Boulos, M. , Suleiman, A. , Bidoosi, S. , Agmon, Y. , Kapeliovich, M. , … Suleiman, M. (2007). Relation of C‐reactive protein and new‐onset atrial fibrillation in patients with acute myocardial infarction. The American Journal of Cardiology, 100(5), 753–757. 10.1016/j.amjcard.2007.04.014 [DOI] [PubMed] [Google Scholar]
- Aronson, D. , Mutlak, D. , Bahouth, F. , Bishara, R. , Hammerman, H. , Lessick, J. , … Agmon, Y. (2011). Restrictive left ventricular filling pattern and risk of new‐onset atrial fibrillation after acute myocardial infarction. The American Journal of Cardiology, 107(12), 1738–1743. 10.1016/j.amjcard.2011.02.334 [DOI] [PubMed] [Google Scholar]
- Camm, A. J. , Kirchhof, P. , Lip, G. Y. H. , Schotten, U. , Savelieva, I. , Ernst, S. , … Zupan, I. (2010). Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the european society of cardiology (ESC). European Heart Journal, 31(19), 2369–2429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- Celik, S. , Erdöl, C. , Baykan, M. , Kaplan, S. , & Kasap, H. (2001). Relation between paroxysmal atrial fibrillation and left ventricular diastolic function in patients with acute myocardial infarction. The American Journal of Cardiology, 88(2), 160–162. 10.1016/S0002-9149(01)01611-3 [DOI] [PubMed] [Google Scholar]
- Crenshaw, B. S. , Ward, S. R. , Granger, C. B. , Stebbins, A. L. , Topol, E. J. , & Califf, R. M. (1997). Atrial fibrillation in the setting of acute myocardial infarction: The GUSTO‐I experience. Journal of the American College of Cardiology, 30(2), 406–413. [DOI] [PubMed] [Google Scholar]
- Geske, J. B. , Sorajja, P. , Nishimura, R. A. , & Ommen, S. R. (2007). Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: Correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation, 116(23), 2702–2708. 10.1161/CIRCULATIONAHA.107.698985 [DOI] [PubMed] [Google Scholar]
- Guo, Y. , Lip, G. Y. , & Apostolakis, S. (2012). Inflammation in atrial fibrillation. Journal of the American College of Cardiology, 60(22), 2263–2270. 10.1016/j.jacc.2012.04.063 [DOI] [PubMed] [Google Scholar]
- Hansson, G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine, 352(16), 1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- Hwang, H. J. , Ha, J. W. , Joung, B. , Choi, E. H. , Kim, J. , Ahn, M. S. , … Kim, S. S. (2011). Relation of inflammation and left atrial remodeling in atrial fibrillation occurring in early phase of acute myocardial infarction. International Journal of Cardiology, 146(1), 28–31. 10.1016/j.ijcard.2009.05.065 [DOI] [PubMed] [Google Scholar]
- Jons, C. , Moerch Joergensen, R. , Hassager, C. , Gang, U. J. , Dixen, U. , Johannesen, A. , … Bloch Thomsen, P. E. (2010). Diastolic dysfunction predicts new‐onset atrial fibrillation and cardiovascular events in patients with acute myocardial infarction and depressed left ventricular systolic function: A CARISMA substudy. European Journal of Echocardiography, 11(7), 602–607. 10.1093/ejechocard/jeq024 [DOI] [PubMed] [Google Scholar]
- Kinjo, K. , Sato, H. , Sato, H. , Ohnishi, Y. , Hishida, E. , Nakatani, D. , … Hori, M. (2003). Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. The American Journal of Cardiology, 92(10), 1150–1154. 10.1016/j.amjcard.2003.07.021 [DOI] [PubMed] [Google Scholar]
- Køber, L. , Swedberg, K. , McMurray, J. J. , Pfeffer, M. A. , Velazquez, E. J. , Diaz, R. , … Zannad, F. (2006). Previously known and newly diagnosed atrial fibrillation: A major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction☆. European Journal of Heart Failure, 8(6), 591–598. 10.1016/j.ejheart.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Bierig, M. , Devereux, R. B. , Flachskampf, F. A. , Foster, E. , Pellikka, P. A. , … Solomon, S. D. (2005). Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography, 18(12), 1440–1463. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Lau, D. H. , Huynh, L. T. , Chew, D. P. , Astley, C. M. , Soman, A. , & Sanders, P. (2009). Prognostic impact of types of atrial fibrillation in acute coronary syndromes. The American Journal of Cardiology, 104(10), 1317–1323. 10.1016/j.amjcard.2009.06.055 [DOI] [PubMed] [Google Scholar]
- Lehto, M. , Snapinn, S. , Dickstein, K. , Swedberg, K. , Nieminen, M. S. , & Optimaal Investigators (2004). Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: The OPTIMAAL experience. European Heart Journal, 26(4), 350–356. 10.1093/eurheartj/ehi064 [DOI] [PubMed] [Google Scholar]
- Lester, S. J. , Ryan, E. W. , Schiller, N. B. , & Foster, E. (1999). Best method in clinical practice and in research studies to determine left atrial size. The American Journal of Cardiology, 84(7), 829–832. 10.1016/S0002-9149(99)00446-4 [DOI] [PubMed] [Google Scholar]
- Liuzzo, G. , Biasucci, L. M. , Rebuzzi, A. G. , Gallimore, J. R. , Caligiuri, G. , Lanza, G. A. , … Maseri, A. (1996). Plasma protein acute‐phase response in unstable angina is not induced by ischemic injury. Circulation, 94(10), 2373–2380. 10.1161/01.CIR.94.10.2373 [DOI] [PubMed] [Google Scholar]
- Magro, M. , Nauta, S. , Simsek, C. , Onuma, Y. , Garg, S. , van der Heide, E. , … Serruys, P. W. (2011). Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST‐elevation myocardial infarction: The MI SYNTAXscore study. American Heart Journal, 161(4), 771–781. 10.1016/j.ahj.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Mehta, R. H. , Dabbous, O. H. , Granger, C. B. , Kuznetsova, P. , Kline‐Rogers, E. M. , Anderson Jr, F. A. , … GRACE Investigators (2003). Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. The American Journal of Cardiology, 92(9), 1031–1036. 10.1016/j.amjcard.2003.06.001 [DOI] [PubMed] [Google Scholar]
- O'Gara, P. T. , Kushner, F. G. , Ascheim, D. D. , Casey, D. E. , Chung, M. K. , De Lemos, J. A. , … Granger, C. B. (2013). 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 61(4), e78–e140. 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Pedersen, O. D. , Bagger, H. , Køber, L. , & Torp‐Pedersen, C. (1999). The occurrence and prognostic significance of atrial fibrillation/‐flutter following acute myocardial infarction. European Heart Journal, 20(10), 748–754. 10.1053/euhj.1998.1352 [DOI] [PubMed] [Google Scholar]
- Pizzetti, F. , Turazza, F. M. , Franzosi, M. G. , Barlera, S. , Ledda, A. , Maggioni, A. P. , … Tognoni, G. (2001). Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: The GISSI‐3 data. Heart, 86(5), 527–532. 10.1136/heart.86.5.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore, S. S. , Berger, A. K. , Weinfurt, K. P. , Schulman, K. A. , Oetgen, W. J. , Gersh, B. J. , & Solomon, A. J. (2000). Acute myocardial infarction complicated by atrial fibrillation in the elderly: Prevalence and outcomes. Circulation, 101(9), 969–974. 10.1161/01.CIR.101.9.969 [DOI] [PubMed] [Google Scholar]
- Rencuzogullari, I. , Çağdaş, M. , Karakoyun, S. , Yesin, M. , Gürsoy, M. O. , Artaç, İ. , … Tanboga, I. H. (2018). Propensity score matching analysis of the impact of Syntax score and Syntax score II on new onset atrial fibrillation development in patients with ST segment elevation myocardial infarction. Annals of Noninvasive Electrocardiology, 23(2), e12504 10.1111/anec.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rene, A. G. , Généreux, P. , Ezekowitz, M. , Kirtane, A. J. , Xu, K. , Mehran, R. , … Stone, G. W. (2014). Impact of atrial fibrillation in patients with ST‐elevation myocardial infarction treated with percutaneous coronary intervention (from the HORIZONS‐AMI [Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction] trial). The American Journal of Cardiology, 113(2), 236–242. 10.1016/j.amjcard.2013.09.016 [DOI] [PubMed] [Google Scholar]
- Ruwald, A. C. H. , Thomsen, P. E. B. , Gang, U. , Jørgensen, R. M. , Huikuri, H. V. , & Jons, C. (2013). New‐onset atrial fibrillation predicts malignant arrhythmias in post–myocardial infarction patients—A Cardiac Arrhythmias and RIsk Stratification after acute Myocardial infarction (CARISMA) substudy. American Heart Journal, 166(5), 855–863. 10.1016/j.ahj.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Saczynski, J. S. , McManus, D. , Zhou, Z. , Spencer, F. , Yarzebski, J. , Lessard, D. , … Goldberg, R. J. (2009). Trends in atrial fibrillation complicating acute myocardial infarction. The American Journal of Cardiology, 104(2), 169–174. 10.1016/j.amjcard.2009.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sianos, G. , Morel, M. A. , Kappetein, A. P. , Morice, M. C. , Colombo, A. , Dawkins, K. , … Serruys, P. W. (2005). The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention, 1(2), 219–227. [PubMed] [Google Scholar]
- Sinno, H. , Derakhchan, K. , Libersan, D. , Merhi, Y. , Leung, T. K. , & Nattel, S. (2003). Atrial ischemia promotes atrial fibrillation in dogs. Circulation, 107(14), 1930–1936. 10.1161/01.CIR.0000058743.15215.03 [DOI] [PubMed] [Google Scholar]
- Tsang, T. S. , Barnes, M. E. , Gersh, B. J. , Bailey, K. R. , & Seward, J. B. (2002). Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. The American Journal of Cardiology, 90(12), 1284–1289. 10.1016/S0002-9149(02)02864-3 [DOI] [PubMed] [Google Scholar]
- Van Wagoner, D. R. (2008). Oxidative stress and inflammation in atrial fibrillation: Role in pathogenesis and potential as a therapeutic target. Journal of Cardiovascular Pharmacology, 52(4), 306–313. 10.1097/FJC.0b013e31817f9398 [DOI] [PubMed] [Google Scholar]
- Wi, J. , Shin, D. H. , Kim, J. S. , Kim, B. K. , Ko, Y. G. , Choi, D. , … Jang, Y. (2016). Transient new‐onset atrial fibrillation is associated with poor clinical outcomes in patients with acute myocardial infarction. Circulation Journal, 80(7), 1615–1623. 10.1253/circj.CJ-15-1250 [DOI] [PubMed] [Google Scholar]
- Wong, C. K. , White, H. D. , Wilcox, R. G. , Criger, D. A. , Califf, R. M. , Topol, E. J. , & Ohman, E. M. (2003). Significance of atrial fibrillation during acute myocardial infarction, and its current management: Insights from the GUSTO‐3 trial. Cardiac Electrophysiology Review, 7(3), 201–207. 10.1023/B:CEPR.0000012382.81986.47 [DOI] [PubMed] [Google Scholar]


