Abstract
Background
Electrocardiography (ECG) may be an efficacious diagnostic and prognostic tool in hypertrophic cardiomyopathy (HCM). This study aimed to investigate association between deep T‐wave inversion (TWI) and apical HCM, and between fragmented QRS (fQRS) complex and myocardial fibrosis in patients with HCM.
Methods
Patients with documented HCM by cardiac magnetic resonance imaging (CMR) during 2005–2015 were studied. The 12‐lead ECG and CMR were performed on the same day. All patients underwent CMR for the assessment of cardiac structure, function, and late gadolinium enhancement (LGE). LGE was used to detect myocardial fibrosis.
Results
One hundred forty‐four HCM (mean age 66 ± 15.8 years, 60.4% male) were included. Twenty‐nine (20.14%) subjects had deep TWI, and apical HCM was found in 76 (52.78%). Deep TWI was associated with apical HCM with the Odds ratio (95%CI) of 5.82 (2.07, 16.04) and p < 0.001 in univariate analysis model. The association was still significant in multivariate analysis with adjusted Odds ratio (95%CI) of 9.86 (3.17, 30.66), p < 0.001. Forty‐seven (32.64%) subjects had fQRS complex, and myocardial fibrosis was detected in 101 (70.14%). fQRS complex was found to be associated with myocardial fibrosis in univariate analysis with the Odds ratio (95%CI) = 2.75 (1.16, 6.54), p = 0.019. However, the association cannot be demonstrated in the multivariate analysis.
Conclusion
Deep TWI is independently associated with apical HCM, but the relationship between fQRS complex and myocardial fibrosis did not survive multivariate analysis.
Keywords: apical hypertrophic cardiomyopathy, ECG, electrocardiographic predictors, myocardial fibrosis
1. INTRODUCTION
The prevalence of hypertrophic cardiomyopathy (HCM) is approximately 1:500 in the general population, and it is a leading cause of heart failure, atrial fibrillation, and sudden death—especially in population under 35 years of age (Gersh et al., 2011). The method most commonly used to diagnose HCM is echocardiography. HCM is characterized by wall thickness >15 mm. This condition can lead to cardiac arrhythmia, ischemic stroke, and myocardial infarction (Eriksson et al., 2002). Apical HCM may be difficult to detect by 12‐lead electrocardiogram (ECG), with a measurement error that is reported to range from 6.9% to 17.1% (Alfonso et al., 1990). Deep T‐wave inversion (TWI) from ECG may be a clue that can assist clinicians in diagnosing apical HCM (Alfonso et al., 1990). Accordingly, if deep TWI presents on ECG, the operator would be well‐advised to concentrate some added attention on the apical region to investigate for apical HCM.
Myocardial fibrosis in patients with HCM is associated with ventricular tachycardia and heart failure (Konno et al., 2015). Myocardial fibrosis is commonly identified using cardiac magnetic resonance (CMR) imaging; however, CMR has limited availability in developing countries and other low resource settings. In addition to the high cost of CMR, the images must be interpreted by a specialist. Fragmented QRS complex (fQRS) from a 12‐lead ECG was shown to be associated with myocardial fibrosis with a sensitivity and specificity of 40% and 80%, respectively (Konno et al., 2015). The ability to identify myocardial fibrosis and apical HCM by 12‐lead ECG would lower the cost of investigation and diagnosis and would give patients in low resource settings access to a diagnostic modality that can diagnose these conditions. Accordingly, the aims of this study were to investigate association between deep TWI and apical HCM, and between fQRS complex and myocardial fibrosis in patients with HCM.
2. METHODS
2.1. Population
This retrospective study included patients with a diagnosis of HCM that underwent both CMR and 12‐lead ECG at the Division of Cardiology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand during the 2005–2015 study period. Siriraj Hospital is Thailand’s largest national tertiary referral center. The protocol for this study was approved by the Siriraj Institutional Review Board.
Hypertrophic cardiomyopathy by CMR was defined as any wall segment thickness >15 mm that could not be solely explained by abnormal loading condition. Patients having one or more of the following were excluded: (a) ECG patterns that could adversely influence interpretation (Das et al., 2008; Guo, Li, Xu, Tang, & Li, 2012), such as Wolff‐Parkinson‐White syndrome; (b) myocarditis; (c) congenital heart disease; and/or, (d) ventricular paced rhythm.
2.2. Electrocardiography
All patients underwent 12‐lead ECG (GE MAC 1200; GE Marquette Medical Systems, Milwaukee, WI, USA) at rest in the supine position with the machine adjusted to the following settings: 40 Hz, 50 Hz, 25 mm/s, and 10 mm/mV.
2.3. Cardiac magnetic resonance protocol
All patients underwent CMR on a Philips Gyroscan NT Intera 1.5 Tesla scanner (Philips Medical Systems, Best, the Netherlands) for assessment of cardiac structure, function, and late gadolinium enhancement (LGE) images after 10 min of intravenous administration of gadolinium‐DTPA (diethylenetriamine penta‐acetic acid) (0.15 mmol/kg).
The CMR parameters for functional images were 8 mm slice thickness, 70 degree flip angle, repetition time/echo time/number of excitations (TR/TE/NEX) = 3.7/1.8/2, 390 × 312 mm field of view, 256 × 240 matrix, and 1.52 × 1.3 reconstruction pixel. The parameters for LGE images were 3D segmented‐gradient‐echo inversion‐recovery sequence with 8 mm slice thickness, 15 degree flip angle, 1.5 SENSitivity Encoding (SENSE) factor, TR/TE = 4.1/1.25 ms, 303 × 384 mm field of view, 240 × 256 matrix, and 1.26 × 1.5 mm reconstruction pixel.
2.4. Analysis of ECG
The following ECG parameters were collected: QRS voltage, QRS duration, QRS axis, T‐wave axis, and QTc interval. The following criteria for left ventricular hypertrophy were assessed: Sokolow‐Lyon criteria (S in V1 + R in V5 or V6 >3.5 mV or R in V5 or V6 >2.6 mV) (Sokolow & Lyon, 1949), Cornell voltage criteria (R in aVL + S in V3 > 2.0 mV in female and >2.8 mV in male) (Casale et al., 1985), and Cornell product criteria (the product of QRS duration times the Cornell voltage combination with 0.6 mV added in women >244 mV × msec (Molloy, Okin, Devereux, & Kligfield, 1992).
Deep TWI was defined as more than 10 mV of negative T‐wave amplitude in at least one lead in any wall segment (Figure 1) (Elliott et al., 2014).
Figure 1.
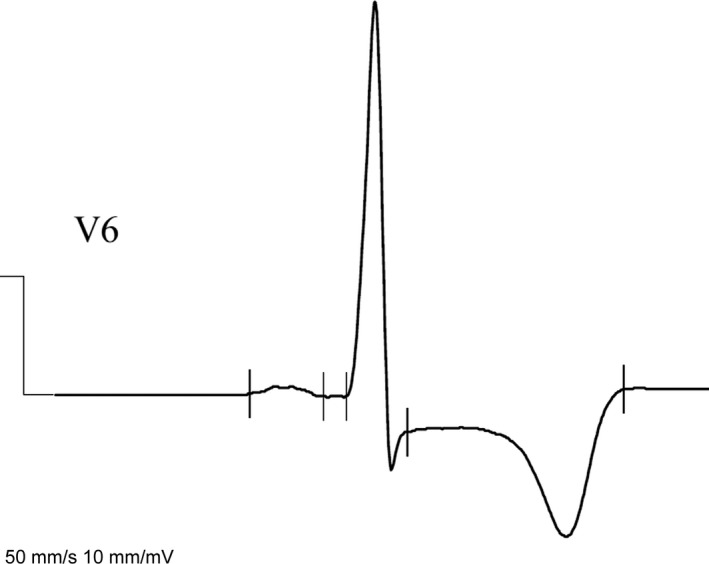
Deep T‐wave inversion (TWI) in lead V6 in patients with apical hypertrophic cardiomyopathy (HCM)
fQRS complex (Konno et al., 2015) was defined, as follows:
-
1. If QRS duration was <120 msec and at least one of the following findings was present (Figure 2):
-
2. If QRS duration was equal to or more than 120 msec and at least one of the following findings was present (Figure 3):
Figure 2.
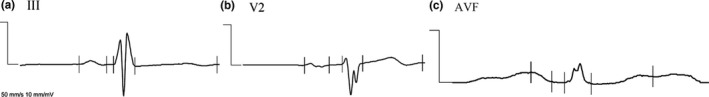
Fragmented QRS (fQRS) complex in patients with a QRS complex duration <120 msec: (a) additional R wave; (b) notching in nadir of S wave; and, (c) equal or more than one deflection in R prime
Figure 3.

Fragmented QRS (fQRS) complexes in patients with a QRS complex duration ≥120 msec: (a) RSR' with equal or more than 2 R′; (b) equal or more than two notches in R wave; and, (c) notch in upstroke of S wave
fQRS complexes in more than two leads in the same wall segment (V1–4 = anterior wall segment; V5, V6, and aVL = lateral wall segment; and, II, III, and aVF = inferior wall segment) were defined as fQRS complexes.
2.5. CMR analysis
Left ventricular ejection fraction (LVEF) was calculated from left ventricular end‐systolic and end‐diastolic volume data. Left ventricular (LV) mass was calculated from the summation of subtraction of left ventricular end‐diastolic epicardial and endocardial area and slice thickness of each slide.
Patients having one of the following on CMR were diagnosed with apical HCM:
Apical LV thickness >15 mm that cannot be solely explained by abnormal loading condition or flow‐limiting coronary artery disease (Helmy et al., 2011); or,
Ratio of maximal apical part to maximal posterior part that is >1.5 (Eriksson et al., 2002) that cannot be solely explained by abnormal loading condition or flow‐limiting coronary artery disease.
Cardiac magnetic resonance LGE images were used to identify myocardial fibrosis; (Elliott et al., 2014; Konno et al., 2015). Myocardial fibrosis was diagnosed by the presence of the area with hyperintense signal which was evaluated the visual assessment of two cardiologists. Disagreements in interpretation were resolved by consensus. Visual assessment was shown to have excellent agreement with the quantitative technique for detention of myocardial fibrosis from LGE (Kappa = 0.963 and 0.952, respectively; p < 0.001) (Krittayaphong et al., 2007).
2.6. Statistical analysis
SPSS Statistics version 20 (SPSS, Inc., Chicago, IL, USA) was used to perform all data analyses. Categorical data are described as number and percentage, and continuous data are shown as mean ±standard deviation (SD). Comparisons of continuous variables were made using Student's t test for unpaired data, and comparisons of categorical variables were made using chi‐square test or Fisher's exact test. Multiple logistic regression analysis was employed to evaluate association between ECG and MRI findings. Univariate and multivariate analyses were performed to evaluate for factors independently associated with HCM and with myocardial fibrosis. The results of those analyses are reported as odds ratio (OR) and 95% confidence interval (CI). A p‐value <0.05 was considered statistically significant for all tests.
3. RESULTS
3.1. Population
One hundred and forty‐four subjects were included in this study. The average age of patients was 66.0 ± 15.8 years, and 87 (60.4%) were male. Table 1 summarizes CMR and ECG findings. Compared to nonapical HCM, apical HCM was more common in elderly (70.8 ± 12.5 vs. 60.6 ± 17.4 years, p < 0.001), had less LV mass (135.7 ± 49.4 vs. 170.8 ± 68.8 g, p = 0.001), and less likely to have myocardial fibrosis (56.6% vs. 85.3%, p < 0.001). LVH by Sokolow‐Lyon criteria was positive more (63.2% vs. 25.0%, p < 0.001) but Cornell voltage criteria was positive less (23.7% vs. 45.6%, p = 0.006) in patients with apical HCM compared to those with nonapical HCM. Patients with myocardial fibrosis had a greater LV mass compared to those without fibrosis (170.5 ± 59.8 vs. 109.6 ± 42.4 g, p < 0.001).
Table 1.
CMR and ECG finding
| Variables | |
|---|---|
| CMR findings | |
| HCM patterns | |
| Septal | 52 (36.1%) |
| Apical | 76 (52.8%) |
| Concentric | 16 (17.1%) |
| LVEDV/BSA (ml/m2) | 75.2 ± 21.9 |
| LVESV/BSA (ml/m2) | 21.5 ± 14.9 |
| Left ventricular mass (g) | 152.3 ± 61.7 |
| LVEF (%) | 72.5 ± 9.5 |
| Maximal wall thickness (mm) | 21.3 ± 5.0 |
| Maximal wall thickness ≥30 mm | 9 (6.3%) |
| Presence of LGE | 101 (70.1%) |
| ECG findings | |
| S in V1 (mV) | 12.6 ± 7.4 |
| R in V5 (mV) | 24.7 ± 12.3 |
| R in V6 (mV) | 23.0 ± 11.4 |
| R in aVL (mV) | 8.2 ± 6.6 |
| S in V3 (mV) | 13.4 ± 9.8 |
| QRS duration (msec) | 102.6 ± 15.7 |
| QRS product (Cornell product) (mV × msec) | 2,492 ± 140 |
| QTc interval (msec) | 457.8 ± 26.2 |
| QRS axis | 28.8 ± 43.2 |
| T‐wave axis | 59.7 ± 112.6 |
| LVH by Sokolow‐Lyon | 65 (45.1%) |
| LVH by Cornell voltage | 49 (34.0%) |
| LVH by Cornell product | 60 (41.7%) |
Values are shown as mean ± standard deviation or number and percentage.
A p‐value < 0.05 indicates statistical significance.
BSA: body surface area; CMR: cardiac magnetic resonance; ECG, electrocardiography; HCM: hypertrophic cardiomyopathy; LGE: late gadolinium enhancement; LVEDV: left ventricular end‐diastolic; LVEF: left ventricular ejection fraction; LVESV: left ventricular end‐systolic; LVH: left ventricular hypertrophy.
3.2. Deep negative T‐wave inversion and apical hypertrophic cardiomyopathy
The amplitude of deep negative TWI was within the range of 1.00–2.18 mV (mean 1.39 ± 0.34). Twenty‐nine (20.1%) subjects had deep TWI. A comparison of baseline characteristics between patients with and without deep TWI revealed significantly more male patients in the deep TWI group than in the no deep TWI group (79.3% vs. 55.7%; p = 0.020) (Table 2).
Table 2.
Baseline demographic and clinical characteristics of the study population
| Characteristics | All | Deep TWI | No deep TWI | p‐Value | fQRS | No fQRS | p‐Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 66.0 ± 15.8 | 64.8 ± 13.7 | 66.3 ± 16.3 | 0.664 | 63.0 ± 18.2 | 67.4 ± 14.4 | 0.149 |
| Male gender | 87 (60.4%) | 23 (79.3%) | 64 (55.7%) | 0.02 | 28 (59.6%) | 59 (60.8%) | 0.886 |
| Atrial fibrillation | 13 (9.0%) | 1 (3.4%) | 12 (10.4%) | 0.467 | 5 (10.6%) | 8 (8.8%) | 0.758 |
| CAD | 17 (11.8%) | 2 (6.9%) | 15 (13.0%) | 0.525 | 3 (6.4%) | 14 (14.4%) | 0.16 |
| Heart failure | 6 (4.2%) | 1 (3.4%) | 5 (4.3%) | 1 | 3 (6.4%) | 3 (3.3%) | 0.392 |
| Calcium channel blocker | 35 (24.3%) | 5 (17.2%) | 30 (26.1%) | 0.321 | 10 (21.3%) | 25 (25.8%) | 0.555 |
| Beta blocker | 75 (52.1%) | 18 (62.1%) | 57 (49.6%) | 0.228 | 28 (59.6%) | 47 (48.5%) | 0.21 |
| LVEF (%) | 72.5 ± 9.5 | 74.8 ± 6.1 | 71.9 ± 10.6 | 0.047 | 69.4 ± 9.7 | 73.9 ± 9.0 | 0.007 |
| LV mass (g) | 152.3 ± 61.7 | 164.0 ± 57.8 | 149.3 ± 62.6 | 0.254 | 167.9 ± 62.9 | 144.7 ± 60.0 | 0.034 |
Values are shown as mean ± standard deviation or number and percentage.
A p‐value < 0.05 indicates statistical significance (italic value).
CAD, coronary artery disease; fQRS, fragmented QRS complex; LV, left ventricular; LVEF, left ventricular ejection fraction; TWI, T‐wave inversion.
Deep TWI was found to be significantly associated with apical HCM in univariate analysis [odds ratio (OR): 5.815, 95% confidence interval (CI): 2.074–16.037; p < 0.001], and the association between deep TWI and apical HCM remained statistically significant in multivariate analysis (OR: 9.857, 95% CI: 3.169–30.656; p < 0.001) (Table 3). We use LVEF 50% and median LV mass of 144 g as a cut‐off for univariate and multivariate analysis. Deep TWI could detect apical HCM with a sensitivity of 31.2%, a specificity of 92.6%, a positive predictive value (PPV) of 82.8%, and a negative predictive value (NPV) of 54.8%. Figure 4 demonstrates a case of apical HCM with deep TWI.
Table 3.
Univariate and multivariate analysis for factors independently associated with apical hypertrophic cardiomyopathy by cardiac magnetic resonance (CMR) images
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age ≥65 years | 3.000 (1.484, 6.065) | 0.002 | 3.071 (1.334, 7.069) | 0.008 |
| Male gender | 1.010 (0.517, 1.971) | 0.977 | ||
| Atrial fibrillation | 5.585 (1.191, 26.182) | 0.016 | 5.286 (1.705, 25.996) | 0.04 |
| Coronary artery disease | 1.320 (0.473, 3.686) | 0.595 | ||
| Heart failure | 0.432 (0.077, 2.439) | 0.33 | ||
| Calcium channel blocker | 1.083 (0.505, 2.326) | 0.837 | ||
| Beta blocker | 0.937 (0.487, 1.804) | 0.845 | ||
| LVEF <50% | 0.892 (0.122, 6.511) | 0.91 | ||
| LV mass ≥144 g | 0.426 (0.218, 0.833) | 0.012 | 0.451 (0.207, 0.981) | 0.045 |
| T‐wave inversion | 5.815 (2.074, 16.307) | <0.001 | 9.857 (3.169, 30.656) | <0.001 |
A p‐value < 0.05 indicates statistical significance (italic value).
CI, confidence interval; LVEF, left ventricular ejection fraction; LV, left ventricular; OR, odds ratio.
Figure 4.
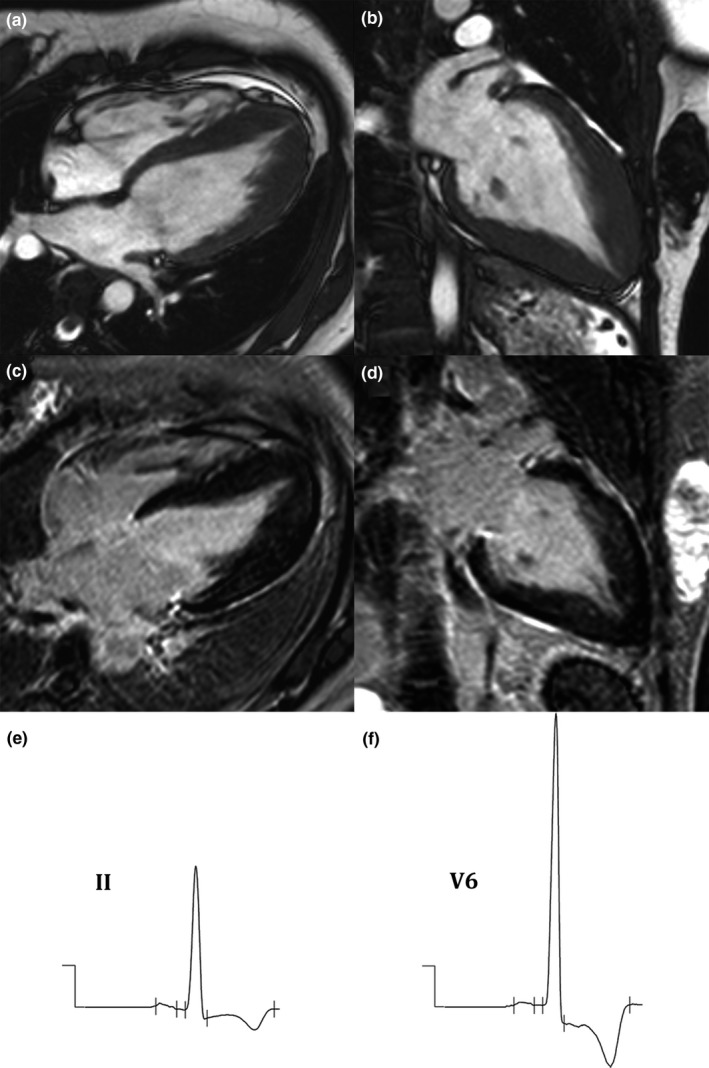
(a–d): Cardiac magnetic resonance (CMR) imaging of apical hypertrophic cardiomyopathy (HCM) shows apical hypertrophy in cine images in four‐chamber (a) and long‐axis (b) views, and late gadolinium enhancement (LGE) images in four‐chamber (c) and long‐axis (d) views. (e,f): Electrocardiogram (ECG) of apical HCM shows deep T‐wave inversion (TWI) in leads II (e) and V6 (f)
3.3. Fragmented QRS complex and cardiac fibrosis
Forty‐seven (32.64%) subjects had fQRS complex, and myocardial fibrosis was detected in 101 (70.14%). Patient characteristics were similar between groups (all p > 0.05) (Table 2). fQRS complex was found to be associated with myocardial fibrosis in univariate analysis (OR: 2.752, 95% CI: 1.157–6.544; p = 0.019). fQRS could detect myocardial fibrosis with a sensitivity of 38.6%, a specificity of 81.4%, a PPV of 83.6%, and, an NPV of 36.1%. However, fQRS complex failed to achieve statistical significance in multivariate analysis (Table 4). Figure 5 demonstrates a case of HCM with myocardial fibrosis and fQRS complexes.
Table 4.
Univariate and multivariate analysis for factors independently associated with myocardial fibrosis by cardiac magnetic resonance (CMR) late gadolinium enhancement (LGE) images
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age ≥65 years | 0.284 (0.120, 0.674) | 0.003 | ||
| Male gender | 3.454 (1.642, 7.262) | 0.001 | 4.515 (1.852, 11.010) | 0.001 |
| Atrial fibrillation | 0.459 (0.145, 1.457) | 0.178 | ||
| Coronary artery disease | 2.146 (0.584, 7.888) | 0.241 | ||
| Heart failure | 2.188 (0.248, 19.302) | 0.471 | ||
| Calcium channel blocker | 0.762 (0.338, 1.717) | 0.511 | ||
| Beta blocker | 0.616 (0.298, 1.272) | 0.189 | ||
| LVEF < 50% | 0.693 (0.621, 0.774) | 0.186 | ||
| LV mass ≥ 144 g | 12.152 (4.670, 31.618) | <0.001 | 14.446 (5.207, 40.081) | <0.001 |
| Q wave | 1.112 (0.541, 2.285) | 0.619 | ||
| fQRS | 2.752 (1.157, 6.544) | 0.019 | ||
A p‐value < 0.05 indicates statistical significance (italic value).
CI, confidence interval; fQRS, fragmented QRS complex; LVEF, left ventricular ejection fraction; LV, left ventricular; OR, odds ratio.
Figure 5.
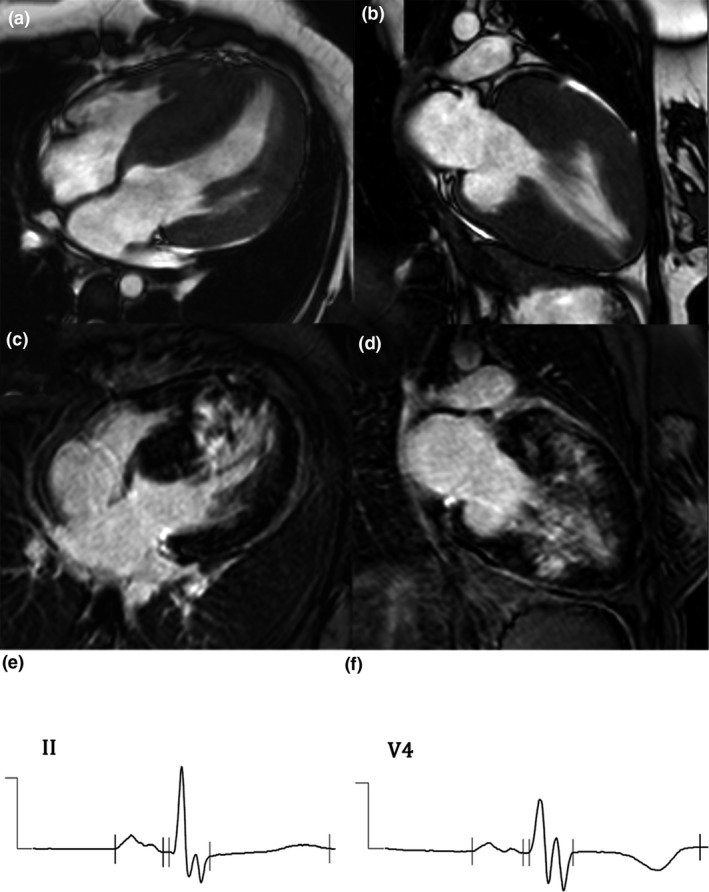
(a–d): Cardiac magnetic resonance (CMR) of hypertrophic cardiomyopathy (HCM) shows interventricular thickening in cine images in four‐chamber (a) and long‐axis (b) views, and myocardial fibrosis from late gadolinium enhancement (LGE) images in four‐chamber (c) and long‐axis (d) views. (e,f): Electrocardiogram (ECG) shows fragmented QRS (fQRS) complex in leads II (e) and V6 (f) in HCM with myocardial fibrosis
4. DISCUSSION
The results of this study revealed deep TWI to be independently associated with apical HCM. In contrast, fQRS was found to be associated with myocardial fibrosis in univariate analysis, but that association did not remain significant in multivariate analysis.
4.1. Deep negative T‐wave inversion and apical hypertrophic cardiomyopathy
Sakamoto, Tei, Murayama, Ichiyasu, and Hada (1976 initially described the association between deep TWI and apical HCM. They reported that three of nine cases with deep TWI had apical HCM. A study of this association by Yamaguchi et al. (1979) in Western population found that 47% of patients with deep TWI had apical HCM, while only 15% of patients without deep TWI had apical HCM.
A study by Flett et al. (2015) reported that 22 of 48 (45.83%) HCM patients had apical HCM, and that 16 (73%) of those 22 patients had deep TWI. In the present study, 76 (52.78%) subjects had apical HCM, but only 24 (31.58%) of those had deep TWI. Even though our prevalence was lower than that observed in the Western population, the association still remained significant. This indicates a strong association between deep TWI and apical HCM. LVEF was not significantly different between groups with and without apical HCM. The test sensitivity was relatively low (31.16%), but the specificity was high (92.6%). This finding suggests that the investigator should pay closer attention to the apical part if deep TWI presents on the patient's ECG.
4.2. Fragmented QRS complex and cardiac fibrosis
Fragmented QRS complex was found to be significantly associated with myocardial fibrosis in univariate analysis, but that significant association did not hold up in multivariate analysis. This finding indicates that fQRS could indicate the presence of myocardial fibrosis, but dilution by other factors or a lack of statistical power caused by the small size of our study population could have rendered fQRS nonsignificant in multivariate analysis.
In the Konno et al. (2015) study, fQRS complex was most sensitive for detecting inferior segment fibrosis (sensitivity 51%), but it was not sensitive for detecting myocardial fibrosis in other segments (sensitivity for detecting anterior segment myocardial fibrosis: 38%; sensitivity for detecting lateral segment myocardial fibrosis: 30%). In our study, most patients had anterior segment fibrosis (55 subjects, 38.20%), compare to lateral segment fibrosis (26 subjects, 18.06%) and inferior segment fibrosis (29 subjects, 20.1%). This may explain the weaker association between fQRS complex and myocardial fibrosis in our study. Konno et al. (2015 also found the association between fQRS and myocardial fibrosis to vary according to the degree of LGE. Myocardial fibrosis has to be presented at a certain minimum amount in order to be reflected on a 12‐lead ECG as fQRS complex. This factor may influence and explain the disparity among study findings relative to the association between fQRS complex and myocardial fibrosis.
In our study, the pathologic Q wave did not associate with myocardial fibrosis (OR: 1.112, 95% CI: 0.541–2.285; p = 0.619). This finding indicates the higher sensitivity of fQRS for detecting myocardial fibrosis compared to pathologic Q waves. Konno et al. also reported the pathologic Q wave to be significantly associated with cardiac fibrosis in univariate analysis (OR: 0.23, 95% CI: 0.145–0.315; p = 0.015), but there was no association in multivariate analysis (OR: 0.11, 95% CI: 0.02–0.2; p = 0.22).
Male gender (OR: 5.060, 95% CI: 2.001–12.798; p = 0.001), use of beta blocker (OR: 5.060, 95% CI: 2.001–12.798; p = 0.025), and LV mass above median (OR: 17.634, 95% CI: 6.025–51.613); p < 0.001) were also significantly associated with higher rate of myocardial fibrosis.
The sensitivity of fQRS complex for detecting myocardial fibrosis was found to be modest (38.6%), but the specificity was relatively high (81.4%). Thus, more careful interpretation of CMR should be combined with increased suspicion of the presence of myocardial fibrosis if fQRS complex is presented on 12‐lead ECG.
4.3. Limitations
This study has some mentionable limitations. First, this was a retrospective study with a study population that was recruited from a single center. Second, the size of our study population was relatively small, which means that this study may have lacked the statistical power necessary to identify all significant differences and associations. Third, some ECG tracings had a shifting isoelectric line, which may have adversely affected our ability to identify deep TWI since we used the isoelectric line as a reference line. Fourth, fQRS complex and myocardial fibrosis may be caused by etiologies other than HCM, such as coronary artery disease and other cardiomyopathies. Although the imaging patterns of myocardial fibrosis may be different among different etiologies, it may be difficult in some cases to determine which etiology is responsible for the observed myocardial fibrosis.
5. CONCLUSION
Deep TWI is independently associated with apical HCM, but the relationship between fQRS complex and myocardial fibrosis, which was significant in univariate analysis, did not remain significant in multivariate analysis. These findings will enhance the ability of clinicians to diagnose and treat HCM in low resource settings that do not have access to more sophisticated diagnostic methods.
CONFLICT OF INTEREST
Both authors declare no personal or professional conflict of interests, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Khemajira Karaketklang of Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University for assistance with statistical analysis.
Tangwiwat C, Kaolawanich Y, Krittayaphong R. Electrocardiographic predictors of myocardial fibrosis and apical hypertrophic cardiomyopathy. Ann Noninvasive Electrocardiol. 2019;24:e12612 10.1111/anec.12612
REFERENCES
- Alfonso, F. , Nihoyannopoulos, P. , Stewart, J. , Dickie, S. , Lemery, R. , & McKenna, W. J. (1990). Clinical significance of giant negative T waves in hypertrophic cardiomyopathy. Journal of the American College of Cardiology, 15(5), 965–971. 10.1016/0735-1097(90)90225-E [DOI] [PubMed] [Google Scholar]
- Casale, P. N. , Devereux, R. B. , Kligfield, P. , Eisenberg, R. R. , Miller, D. H. , Chaudhary, B. S. , & Phillips, M. C. (1985). Electrocardiographic detection of left ventricular hypertrophy: Development and prospective validation of improved criteria. Journal of the American College of Cardiology, 6(3), 572–580. 10.1016/S0735-1097(85)80115-7 [DOI] [PubMed] [Google Scholar]
- Das, M. K. , Suradi, H. , Maskoun, W. , Michael, M. A. , Shen, C. , Peng, J. , … Mahenthiran, J. (2008). Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circulation. Arrhythmia and Electrophysiology, 1(4), 258–268. 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- Elliott, P. M. , Anastasakis, A. , Borger, M. A. , Borggrefe, M. , Cecchi, F. , Charron, P. , … Watkins, H. (2014). 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). European Heart Journal, 35(39), 2733–2779. 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- Eriksson, M. J. , Sonnenberg, B. , Woo, A. , Rakowski, P. , Parker, T. G. , Wigle, E. D. , & Rakowski, H. (2002). Long‐term outcome in patients with apical hypertrophic cardiomyopathy. Journal of the American College of Cardiology, 39(4), 638–645. 10.1016/S0735-1097(01)01778-8 [DOI] [PubMed] [Google Scholar]
- Flett, A. S. , Maestrini, V. , Milliken, D. , Fontana, M. , Treibel, T. A. , Harb, R. , … Moon, J. C. (2015). Diagnosis of apical hypertrophic cardiomyopathy: T‐wave inversion and relative but not absolute apical left ventricular hypertrophy. International Journal of Cardiology, 183, 143–148. 10.1016/j.ijcard.2015.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersh, B. J. , Maron, B. J. , Bonow, R. O. , Dearani, J. A. , Fifer, M. A. , Link, M. S. , … Society of Thoracic Surgeons . (2011). 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation, 124(24), e783–e831. 10.1161/CIR.0b013e318223e2bd. [DOI] [PubMed] [Google Scholar]
- Guo, R. , Li, Y. , Xu, Y. , Tang, K. , & Li, W. (2012). Significance of fragmented QRS complexes for identifying culprit lesions in patients with non‐ST‐elevation myocardial infarction: A single‐center, retrospective analysis of 183 cases. BMC Cardiovascular Disorders, 12, 44 10.1186/1471-2261-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy, S. M. , Maauof, G. F. , Shaaban, A. A. , Elmaghraby, A. M. , Anilkumar, S. , Shawky, A. H. , & Hajar, R. (2011). Hypertrophic Cardiomyopathy: Prevalence, Hypertrophy Patterns, and Their Clinical and ECG Findings in a Hospital at Qatar. Heart Views, 12(4), 143–149 . 10.4103/1995-705X.90900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno, T. , Hayashi, K. , Fujino, N. , Oka, R. , Nomura, A. , Nagata, Y. , … Yamagishi, M. (2015). Electrocardiographic QRS fragmentation as a marker for myocardial fibrosis in hypertrophic cardiomyopathy. Journal of Cardiovascular Electrophysiology, 26(10), 1081–1087. 10.1111/jce.12742. [DOI] [PubMed] [Google Scholar]
- Krittayaphong, R. , Saiviroonporn, P. , Boonyasirinant, T. , Nakyen, S. , Thanapiboonpol, P. , & Udompunturak, S. (2007). Accuracy of visual assessment in the detection and quantification of myocardial scar by delayed enhancement magnetic resonance imaging. Journal of the Medical Association of Thailand, 90(Suppl 2), 1–8. [PubMed] [Google Scholar]
- Molloy, T. J. , Okin, P. M. , Devereux, R. B. , & Kligfield, P. (1992). Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage‐duration product. Journal of the American College of Cardiology, 20(5), 1180–1186. 10.1016/0735-1097(92)90376-X [DOI] [PubMed] [Google Scholar]
- Sakamoto, T. , Tei, C. , Murayama, M. , Ichiyasu, H. , & Hada, Y. (1976). Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle. Echocardiographic and ultrasono‐cardiotomographic study. Japanese Heart Journal, 17(5), 611–629. 10.1536/ihj.17.611 [DOI] [PubMed] [Google Scholar]
- Sokolow, M. , & Lyon, T. (1949). The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. American Heart Journal, 37, 161–186. 10.1016/0002-8703(49)90562-1 [DOI] [PubMed] [Google Scholar]
- Yamaguchi, H. , Ishimura, T. , Nishiyama, S. , Nagasaki, F. , Nakanishi, S. , Takatsu, F. , … Machii, K. (1979). Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): Ventriculographic and echocardiographic features in 30 patients. American Journal of Cardiology, 44(3), 401–412. 10.1016/0002-9149(79)90388-6 [DOI] [PubMed] [Google Scholar]


