Abstract
Background
The clinical utility of the Tp‐e interval and Tp‐e/QT ratio in the risk stratification of ventricular arrhythmic events is controversial. Therefore, we investigated the impact of CCC on these electrocardiographic indexes in the course of stable CAD.
Methods
Two hundred three consecutive patients with stable CAD who underwent coronary angiography and had documented total occlusion of one of the major coronary arteries were enrolled in this prospective cross‐sectional study. The Tp‐e interval and Tp‐e/QT ratio were measured by 12‐lead electrocardiogram.
Results
The Tp‐e interval, cTp‐e interval, Tp‐e/QT ratio, and cTp‐e/QT ratio were lower in the grade 3 CCC group compared with the others in all leads. Multivariate linear regression analyses was performed to identify the clinical factors affecting the cTp‐e interval and was indicated that age (β = 0.261, P < 0.001), male sex (β = 0.334, P < 0.001), poor Rentrop grade (β = –0.228, P < 0.001), and NLR (β = 0.137, P = 0.027) were independent predictors of a prolonged cTp‐e interval.
Conclusion
It could be concluded that the decreased dispersion of ventricular repolarization might contribute to the lower incidence of ventricular arrhythmias and SCD in CAD patients with a good CCC.
Keywords: coronary artery disease, coronary collateral circulation, Tp‐e interval, ventricular arrhythmogenesis
The coronary collateral circulation (CCC) constitutes an alternative blood source for ischemic myocardium in coronary artery disease (CAD). A well‐developed CCC can limit ischemic damage, reduce infarct size, and improve survival.1 Moreover, a well‐developed CCC is associated with a lower incidence of malignant arrhythmias and with reduced risks of all‐cause mortality and sudden cardiac death (SCD) in patients with stable CAD.2, 3
Increased dispersion of repolarization, which reflects the heterogeneity rather than the total duration of repolarization, is a known pathogenic factor in malignant ventricular arrhythmias.4 The Tp‐e interval is a novel index of the transmural dispersion of ventricular repolarization and is related to SCD risk.5 Myocardial repolarization can also be evaluated using the QT interval (QT) or QT dispersion (QTd). The Tp‐e/QT and cTp‐e/QT ratios can also be used as electrocardiographic indices of ventricular arrhythmogenesis.6
Neutrophil–lymphocyte ratio (NLR) determines systemic inflammatory response and has been proposed as a prognostic marker in CAD patients.7 The NLR shows the balance of the neutrophils (the active component of the inflammation), with the lymphocytes (the regulatory and protective component).7 Previous studies have indicated that higher levels of NLR are linked with adverse outcomes and increased cardiovascular mortality in patients with CAD.8, 9
There are few studies about the clinical utility of the Tp‐e interval and Tp‐e/QT ratio in the risk stratification of ventricular arrhythmic events. Therefore, we investigated the impact of CCC on these ventricular arrhythmia indexes in the course of stable CAD.
METHODS
Study Population
Between April 2008 and October 2013, 203 consecutive patients (134 men; mean age 63.6 ± 10.9 years) with stable CAD who underwent coronary angiography in our hospital and had documented total occlusion of one of the major coronary arteries were enrolled in this prospective cross‐sectional study.
Patients who had a myocardial infarction within the previous month, history of revascularization (coronary artery bypass graft operation or percutaneous coronary intervention), active angina, severe valvular or congenital heart disease, hepatic or renal dysfunction, asthma or reactive bronchospastic airway disease, thyroid disturbances, pericardial effusion, serum electrolyte imbalance, documented paroxysmal atrial fibrillation, an electronic cardiac pacemaker, sick sinus syndrome, bundle branch block, preexcitation syndromes, atrioventricular block, or technically inadequate coronary angiography were excluded from the study. Patients diagnosed with hematological disease, cancer, acute/chronic infective or inflammatory disease, or autoimmune disease were also excluded. All patients were in sinus rhythm at referral, and none were taking any medicine that could affect the Tp‐e interval.
The ejection fraction, presence of hypertension and diabetes mellitus, and their influences on the results were investigated. HT was diagnosed if the systolic blood pressure was ≥140 mm Hg or the diastolic blood pressure ≥ 90 mm Hg in at least two separate readings, or if the patient was taking any antihypertensive medication. DM was defined as a fasting blood glucose level ≥126 mg/dL on two separate occasions or if the patient was being treated with oral antihyperglycemic agents or insulin. Smoking status was defined as currently smoking. The local ethics committee approved the study protocol, and informed written consent was obtained from each patient.
Coronary Angiography and Assessment of the CCC
Coronary angiography was done through the femoral artery using the Judkins technique for all patients. Two specialists who were blinded to the clinical details and results of the other investigations interpreted the coronary arteriographic data of each patient to assess the coronary artery lesion and CCC. If there was disagreement between the two cardiologists, the angiograms were evaluated by a third cardiologist and a consensus was reached. To classify the CCC, we used the Rentrop classification.10 The grades of collateral filling from the contralateral vessels were as follows: 0 = no filling of any collateral vessel; 1 = filling of side branches of the artery to be perfused by the collateral vessels without visualization of the epicardial segment; 2 = partial filling of the epicardial segment by collateral channels; and 3 = complete filling of the epicardial segment of the artery being dilated through collateral channels.10 When more than one occluded vessel was present, the one with the highest Rentrop grade was recorded. The study population was then classified according to their CCC grades into poor (Rentrop grades 0 or 1) and good (Rentrop grades 2 or 3) groups. The intra‐ and interobserver variability of the Rentrop classification was assessed using 40 subjects selected randomly from the study participants (20 patients each with poor and good CCC). The intraobserver variability was 2.8% and the interobserver variability 3.1%.
Biochemical and Hematological Parameters
Blood samples were taken after a 12‐hour fast on the day of admission. Kidney function, lipid profiles, and fasting glucose levels were analyzed for all patients (Architect c 16000, Abbott Diagnostics, Lake Forest, IL, USA). Hematological parameters were measured using an automatic blood counter (Cell Dyn 3700 Abbott Diagnostics).
Measuring the Tp‐e and QT Intervals from the 12‐Lead ECG
A 12‐lead electrocardiogram (ECG) was obtained at a paper speed of 50 mm/s with an amplification of 20 mm/mV. The heart rate was measured from the ECG data. Two cardiologists measured the Tp‐e and QT intervals manually from the ECG using calipers and a magnifying glass to decrease measurement errors. The average value of three measurements was calculated for each lead. The QT interval was measured from the beginning of the QRS complex to the end of the T wave and was corrected for heart rate using Bazett's formula (QTc = QT/√RR).11 The QTd was defined as the difference between the maximum and minimum QT intervals, and the QTc dispersion was defined as the difference between the maximum and minimum QTc intervals.11
The Tp‐e interval was defined as the interval from the peak of a T wave to the end of that T wave. The end of the T wave was described as the intersection of the tangent of the descending limb of the T wave with the isoelectric line (Fig. 1).12 The Tp‐e interval was measured from leads II, V2, and V5 and corrected for the heart rate (cTp‐e).13 When a U wave followed the T wave, the T‐wave offset was measured as the nadir between the T and U waves. If T‐wave deflections resulted in a biphasic or negative T wave, the QT interval was measured from the time of the final return to baseline. If the T‐wave amplitude was less than 1.5 mm in a lead, that lead was excluded from the analysis. The Tp‐e/QT and cTp‐e/QT ratios were calculated from these values. The intra‐ and interobserver variability of the cTp‐e interval was assessed using 40 randomly selected study participants (20 patients each with poor and good CCC). The intraobserver variability was 2.5%, and the interobserver variability was 2.6%.
Figure 1.
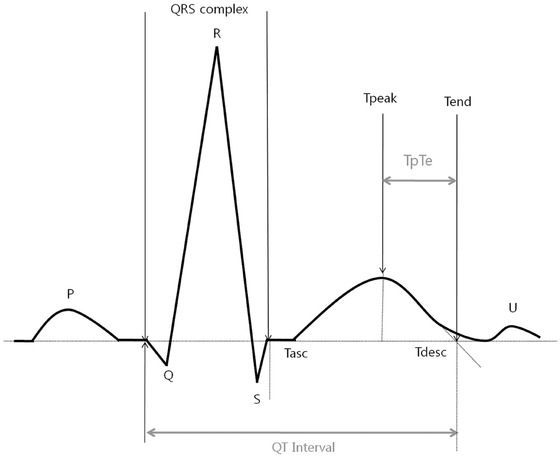
This figure is highlighting the electrocardiographic parameters measured when assessing the QT interval and the interval from the peak to the end of the T wave (Tp‐e interval).9
Statistical Analysis
The statistical analysis was performed using SPSS for Windows 17.0 (Chicago, IL, USA). Data were tested for normal distribution using the Kolmogorov–Smirnov test. Differences between the groups were evaluated using the independent‐samples t‐test and one‐way analysis of variance, with post hoc Tukey's test, where appropriate. Categorical variables were compared using the chi‐square test. Continuous variables were expressed as the mean ± standard deviation and categorical data as numbers and percentages. Pearson and Spearman correlation tests were used to evaluate the associations between the Tp‐e interval and the study parameters.t Linear multiple stepwise regression analysis was used to evaluate the association between a prolonged cTp‐e interval and independent variables that differed significantly in the univariate analyses (P < 0.1). A P value < 0.05 was considered statistically significant.
RESULTS
The study population consisted of 203 patients with stable CAD (75 with poor CCC, 128 with good CCC). Table 1 compares the clinical, laboratory, and angiographic characteristics of the patients according to collateral development. Both groups were similar in terms of age, gender, body mass index, left ventricular ejection fraction, and medication usage. In addition, there were no significant differences in the presence of hypertension, diabetes mellitus, smoking, or hypercholesterolemia between the groups. When compared to the patients with good CCC, the patients with poorly developed CCC had significantly higher neutrophil and lymphocyte counts and NLR.
Table 1.
Clinical Characteristics, Laboratory, and Echocardiographic Findings of the Study Population
| Poor CCC (n = 75) | Good CCC (n = 128) | P value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 63.1 ± 10.7 | 63.8 ± 11.1 | 0.638 |
| Men, n (%) | 55 (73.3) | 79 (61.7) | 0.093 |
| Body mass index (kg/m2) | 22.1 ± 1.98 | 21.8 ± 1.5 | 0.909 |
| Hypertension, n (%) | 38 (50.7) | 62 (48.4) | 0.761 |
| Diabetes, n (%) | 26 (34.7) | 48 (37.5) | 0.658 |
| Smoking, n (%) | 32 (42.7) | 63 (49.2) | 0.369 |
| Ejection fraction, % | 51.8 ± 7.4 | 51.1 ± 7.9 | 0.488 |
| Laboratory findings | |||
| Glucose (mg/dL) | 101.5 ± 17.8 | 101.8 ± 21.4 | 0.904 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 0.9 ± 0.3 | 0.690 |
| HDL(mg/dL) | 39.4 ± 11.4 | 38.1 ± 10.3 | 0.490 |
| LDL(mg/dL) | 78.2 ± 21.2 | 73.8 ± 23.4 | 0.189 |
| Triglyceride (mg/dL) | 99.6 ± 22.9 | 95.9 ± 25.9 | 0.312 |
| Hemoglobin (mg/dL) | 12.9 ± 2.1 | 13.0 ± 2.0 | 0.841 |
| Neutrophile,×109/L | 6.79 ± 2.98 | 4.96 ± 1.99 | <0.001 |
| Lymphocyte,×109/L | 2.08 ± 0.70 | 2.32 ± 0.78 | 0.032 |
| Neutrophile/lymphocyte ratio | 3.65 ± 1.99 | 2.42 ± 1.44 | <0.001 |
| Medication, n (%) | |||
| Acetylsalicylic acid | 59 (78.7) | 93 (72.7) | 0.343 |
| Beta‐blockers | 37 (49.3) | 68 (53.1) | 0.604 |
| ACE inhibitors/ARBs | 27 (36.0) | 46 (35.9) | 0.993 |
| Calcium channel blockers | 13 (17.3) | 23 (18.0) | 0.909 |
| Statins | 53 (70.7) | 86 (67.2) | 0.606 |
| Occluded artery, n (%) | |||
| LAD | 33 (44.0) | 53 (41.4) | 0.390 |
| CX | 18 (24.0) | 38 (29.7) | 0.384 |
| RCA | 34 (45.3) | 74 (57.8) | 0.088 |
CCC = coronary collateral circulation; HDL = high‐density lipoprotein; LDL = low‐density lipoprotein; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; LAD = left anterior descending; CX = circumflex; RCA = right coronary artery.
Table 2 presents the ECG findings according to the Rentrop grade. The heart rate, QT interval, QTd, QTc interval, and QTc dispersion were similar among the groups. The Tp‐e interval (Fig. 2), cTp‐e interval, Tp‐e/QT ratio, and cTp‐e/QT ratio were lower in the grade 3 CCC group compared with the others in all leads.
Table 2.
Electrocardiographic Findings of the Study Population
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |||
|---|---|---|---|---|---|---|
| (n = 44) | (n = 31) | (n = 40) | (n = 88) | P value | ||
| Heart rate (beat/min) | 58.7 ± 14.5 | 60.5 ± 6.3 | 59.9 ± 5.3 | 60.1 ± 5.8 | 0.809 | |
| II | 366.7 ± 20.7 | 365.9 ± 20.4 | 363.9 ± 27.0 | 369.8 ± 19.3 | 0.495 | |
| QT interval (ms) | V2 | 375.2 ± 18.8 | 374.3 ± 14.2 | 377.7 ± 24.3 | 376.4 ± 15.3 | 0.863 |
| V5 | 358.5 ± 19.4 | 357.4 ± 13.7 | 361.3 ± 19.9 | 364.3 ± 14.0 | 0.118 | |
| QT dispersion of the 12 leads (ms) | 35.0 ± 9.3 | 36.1 ± 9.3 | 34.6 ± 8.9 | 34.7 ± 7.3 | 0.869 | |
| II | 372.4 ± 30.7 | 366.5 ± 23.4 | 362.7 ± 24.9 | 369.4 ± 26.4 | 0.348 | |
| QTc interval (ms) | V2 | 381.2 ± 31.8 | 375.1 ± 22.6 | 376.6 ± 23.9 | 375.8 ± 20.2 | 0.635 |
| V5 | 364.0 ± 29.7 | 358.1 ± 21.2 | 360.4 ± 19.6 | 363.9 ± 20.1 | 0.563 | |
| QTc dispersion of the 12 leads (ms) | 38.6 ± 9.5 | 39.1 ± 9.9 | 37.9 ± 9.5 | 37.9 ± 7.8 | 0.910 | |
| II | 74.5 ± 5.7 | 72.6 ± 8.4 | 74.5 ± 7.6 | 67.8 ± 5.8 | <0.001a | |
| cTp‐e interval (ms) | V2 | 78.7 ± 5.5 | 76.4 ± 8.3 | 79.2 ± 7.1 | 71.8 ± 5.8 | <0.001a |
| V5 | 70.4 ± 5.4 | 68.4 ± 9.2 | 70.1 ± 7.7 | 62.9 ± 5.8 | <0.001a | |
| II | 75.6 ± 7.0 | 72.7 ± 8.7 | 74.4 ± 8.3 | 67.7 ± 5.8 | <0.001a | |
| cTp‐e interval (ms) | V2 | 79.9 ± 6.9 | 76.5 ± 8.8 | 79.1 ± 8.1 | 71.7 ± 5.9 | <0.001a |
| V5 | 71.5 ± 6.6 | 68.4 ± 9.3 | 70.0 ± 8.6 | 62.9 ± 6.0 | <0.001a | |
| II | 0.19 ± 0.02 | 0.20 ± 0.02 | 0.21 ± 0.02 | 0.18 ± 0.02 | <0.001a | |
| Tp‐e/QT ratio | V2 | 0.21 ± 0.03 | 0.21 ± 0.02 | 0.21 ± 0.02 | 0.19 ± 0.02 | 0.001a |
| V5 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.18 ± 0.02 | <0.001a | |
| II | 0.20 ± 0.01 | 0.20 ± 0.02 | 0.21 ± 0.02 | 0.18 ± 0.01 | <0.001a | |
| cTp‐e/QT ratio | V2 | 0.21 ± 0.02 | 0.20 ± 0.02 | 0.21 ± 0.02 | 0.19 ± 0.02 | <0.001a |
| V5 | 0.20 ± 0.20 | 0.019 ± 0.03 | 0.20 ± 0.02 | 0.17 ± 0.02 | <0.001a |
QTc = corrected QT; cTp‐e = corrected Tp‐e.
Differences were found between the Grade 3 compared with the other groups, other binary comparisons were nonsignificant.
Figure 2.
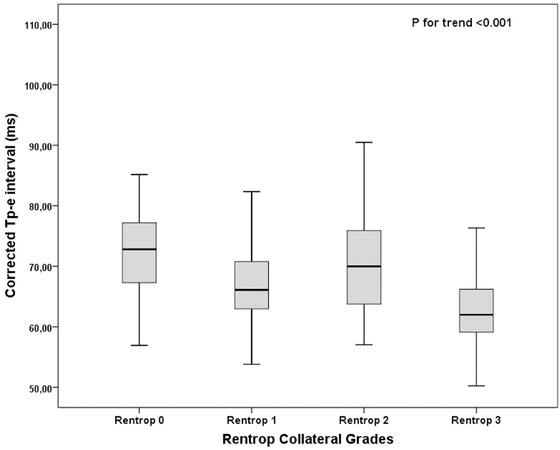
Relationship between corrected Tp‐e interval and Rentrop score (error bars: 95% confidence interval [CI]).
The correlations between the cTp‐e interval in lead V5 and the study parameters are presented in Table 3. There were significant correlations between the cTp‐e interval and age (P < 0.001), male sex (P < 0.001), neutrophil count (P = 0.038), NLR (P < 0.001), and poor Rentrop grade (P < 0.001).
Table 3.
Correlation of Study Parameters with cTp‐e Interval
| cTp–e interval | ||
|---|---|---|
| Parameters | R | P value |
| Age | 0.286 | <0.001 |
| Male sex | 0.447 | <0.001 |
| Body mass index | −0.063 | 0.375 |
| Hypertension | −0.111 | 0.116 |
| Diabetes | −0.009 | 0.897 |
| Ejection fraction | −0.059 | 0.402 |
| Smoking | −0.024 | 0.739 |
| Glucose | −0.112 | 0.111 |
| Creatinine | −0.104 | 0.141 |
| HDL | −0.054 | 0.447 |
| LDL | 0.055 | 0.435 |
| Triglyceride | 0.077 | 0.272 |
| Neutrophile | 0.146 | 0.038 |
| Lymphocyte | −0.053 | 0.457 |
| Neutrophile/lymphocyte ratio | 0.213 | <0.001 |
| Acetylsalicylic acid | 0.051 | 0.468 |
| Beta‐blockers | −0.072 | 0.310 |
| ACE inhibitors/ARBs | −0.071 | 0.313 |
| Calcium channel blockers | −0.040 | 0.566 |
| Statins | 0.037 | 0.601 |
| LAD | 0.079 | 0.264 |
| CX | −0.004 | 0.952 |
| RCA | −0.082 | 0.246 |
| Rentrop grade | −0.455 | <0.001 |
HDL = high‐density lipoprotein; LDL = low‐density lipoprotein; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; LAD = left anterior descending; CX = circumflex; RCA = right coronary artery.
Table 4 shows the results of the multivariate linear regression analyses performed to identify the clinical factors affecting the cTp‐e interval. These indicated that age (β = 0.261, P < 0.001), male sex (β = 0.334, P < 0.001), poor Rentrop grade (β = –0.228, P < 0.001), and NLR (β = 0.137, P = 0.027) were independent predictors of a prolonged cTp‐e interval.
Table 4.
Multivariate Analysis to Demonstrate Independent Predictors of Prolonged cTp‐e Interval
| β | P valuea | |
|---|---|---|
| Independent | Dependent variable: | |
| variables | cTp‐e interval | |
| Age | 0.261 | <0.001 |
| Male sex | 0.334 | <0.001 |
| Rentrop grade | −0.228 | <0.001 |
| Neutrophile/lymphocyte ratio | 0.137 | 0.027 |
Linear regression analyses using the stepwise method were used for the multivariate analysis of independent variables that were included if they were significantly different in the univariate analyses (P < 0.1).
DISCUSSION
This study evaluated the effects of the CCC on the Tp‐e interval and Tp‐e/QT ratio as indices of ventricular arrhythmogenesis in patients with CAD. Our results showed that: (i) the patients with good CCC had significantly lower neutrophil and lymphocyte counts and a NLR than the patients with a poorly developed CCC; (ii) the Tp‐e and cTp‐e intervals and Tp‐e/QT and cTp‐e/QT ratios were lower in the patients with grade 3 CCC; and (iii) age, male sex, NLR, and poor Rentrop grade were independent predictors of a prolonged cTp‐e interval in patients with CAD.
Although CAD is the condition mostly associated with SCD,14 no studies have clearly shown the effects of CCC on ventricular arrhythmias in CAD patients. Increased CCC is reported to reduce the vulnerability of the myocardium to ventricular fibrillation.15 Nagomoto et al. found that stopping the CCC flow to an occluded artery led to an increased frequency of ventricular tachycardia and ventricular premature beats in the ischemic tissue.16 Given the limited information, we assessed the relationship between the QT and Tp‐e intervals, as indices of ventricular arrhythmias, and the grade of CCC in patients with CAD who had at least one occluded coronary artery.
Although the development of CCC is a chronic adaptation to myocardial ischemia, its exact mechanism is not known. Sufficient CCC reduces ischemic damage and infarct size and improves survival in CAD patients.3, 17, 18, 19 Nonfatal cardiovascular events are also less common in patients with a CCC in the setting of stable CAD.20, 21 The grade of CCC varies markedly among patients, and the heterogeneity of the degree of CCC in CAD patients is uncertain. In addition to growth factors, inflammatory cell mediators, endothelial chemokines, oxidative stress, smoking, endothelial dysfunction, DM, HT, and the severity and progression of coronary stenosis can affect the development of coronary collateralization.22, 23 Inflammation also plays a significant role in all stages of atherosclerosis, as well as in the development of CCC.24 Uysal et al. found that the NLR was significantly higher in patients with a poor CCC and concluded that a high NLR was associated with the development of CCC in stable CAD patients. In line with this, our patients with good CCC had a lower NLR. Although NLR was an independent predictor of a prolonged cTp‐e interval in our series, it is not clear whether this is a result of CCC formation, in which the NLR is involved, or a direct effect of the NLR. This needs to be clarified with further studies.
Amplification of the dispersion of ventricular repolarization is a predictor of ventricular arrhythmias.6 An increased QTd reflects inhomogeneous ventricular repolarization that might predispose to important ventricular arrhythmias. Changes in QTd associated with an acute myocardial infarction (MI) suggest that transient ischemic episodes in patients with stable CAD are important. Since most of the SCD in this population likely originates from ischemia‐related arrhythmias, it is obvious that the CCC has an important role in patients with CAD who have at least one totally occluded artery. Nevertheless, studies evaluating the QT dispersion in CAD patients have revealed contradictory results. Yılmaz et al. 25 found that the rest‐corrected QTd was increased in CAD patients. Meier et al. 26 also stated that myocardial ischemia leads to QT prolongation, which is inversely related to collateral function, indicating a protective mechanism of human coronary collaterals against cardiac death. Countering these studies, Tandogan et al. could not identify positive or corrective effects of the CCC on the corrected QTd, and they even noted higher corrected QTd values when a well‐developed CCC was present.27 In accordance with the latter study, we found that the presence of a CCC had no effect on the QTd.
Several studies have shown that the Tp‐e interval, as a sensitive index of arrhythmogenesis, corresponds to the dispersion of ventricular repolarization.4, 28, 29 Therefore, it has attracted increased attention in the last few decades.6 Panikkath et al. found that prolongation of the Tp‐e interval was independently associated with SCD in CAD patients, and this was particularly beneficial when the QTc interval was normal.30 Wang et al. found that Tp‐e prolongation was associated with more cardiac events in Brugada syndrome.31 Recently, Erikssen et al. reported that the Tp‐e interval, particularly the cTp‐e interval, was a strong predictor of mortality in acute MI patients.32 Although studies have reported the utility of the Tp‐e interval as an arrhythmogenic index in some cardiac conditions associated with an increased risk of malignant arrhythmia and SCD,33, 34 no study has evaluated the effect of the CCC on this ECG parameter in CAD patients.
Women with known CAD are at a lower risk of SCD than men.35 Korantzopoulos et al. found that women with CAD have a lower Tp‐e interval and a lower Tp‐e/QT ratio than men and postulated that the increased dispersion of ventricular repolarization contributes to the higher risk of ventricular arrhythmias and SCD in men with CAD.36 In line with these data, we evaluated the effect of the CCC on the Tp‐e interval in CAD patients and found that the CAD patients with a good CCC had shorter Tp‐e intervals than patients with a poor CCC. Furthermore, this decline was negatively correlated with the Rentrop grade. We also found that age, male sex, NLR, and Rentrop grade were independent predictors of a prolonged cTp‐e interval in patients with CAD. These results seem to support our hypothesis; nevertheless, since our study was a cross‐sectional design, prospective studies with longer follow‐up are needed to clarify the precise effects of the CCC on ventricular arrhythmias and SCD using the cTp‐e interval.
Study Limitation
The cross‐sectional design of the study limited the follow‐up in terms of ventricular arrhythmias and SCD and was a main limitation of our study. Another limitation was the difficulty determining the nature of the CCC, which might have affected our results. Collaterals become visible angiographically only after they reach a diameter of 100 mm, and collaterals with smaller diameters cannot be observed angiographically. We can determine only a minor part of the collateral network, and it is impossible to observe intramural collaterals angiographically.37
CONCLUSION
We found that the cTp‐e interval and cTp‐e/QT ratio were lower in the patients with grade 3 CCC and that age, male sex, the NLR, and Rentrop grade were independent predictors of a prolonged cTp‐e interval in patients with CAD. Therefore, the decreased dispersion of ventricular repolarization might contribute to the lower incidence of ventricular arrhythmias and SCD in CAD patients with a good CCC.
Acknowledgments
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/4siXKy.
There is no support for this study.
Conflict of interest: None declared.
REFERENCES
- 1. Berry C, Balachandran KP, L'Allier PL, et al. Importance of collateral circulation in coronary heart disease. Eur Heart J 2007;28(3):278–291. [DOI] [PubMed] [Google Scholar]
- 2. Perez‐Castellano N, Garcia EJ, Abeytua M, et al. Influence of collateral circulation on in‐hospital death from anterior acute myocardial infarction. J Am Coll Cardiol 1998;31:512–518. [DOI] [PubMed] [Google Scholar]
- 3. Meier P, Gloekler S, Zbinden R, et al. Beneficial effect of recruitable collaterals: A 10‐year follow‐up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 2007;116:975–983. [DOI] [PubMed] [Google Scholar]
- 4. Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 2007;293:H2024–H2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp‐Te interval and its diagnostic value. J Electrocardiol 2008;41:575–580. [DOI] [PubMed] [Google Scholar]
- 6. Papadopoulos CE, Karvounis HI, Parharidis GE, et al. Preconditioning reduces QTc value in patients with first non‐ST‐segment elevation myocardial infarction (NSTEMI). Ann Noninvasive Electrocardiol 2003. Oct;8(4):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhutta H, Agha R, Wong J, et al. Neutrophil–lymphocyte ratio predicts medium‐term survival following elective major vascular surgery: A cross‐sectional study. Vasc Endovascular Surg 2011;45(3):227–231. [DOI] [PubMed] [Google Scholar]
- 8. Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012;225(2):456–460. [DOI] [PubMed] [Google Scholar]
- 9. Gupta P, Patel C, Patel H, et al. T(p‐e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 2008;41:567–564. [DOI] [PubMed] [Google Scholar]
- 10. Rentrop KP, Cohen M, Blanke H ,et al. Changes in collateral filling after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 1985;5(3):587–592. [DOI] [PubMed] [Google Scholar]
- 11. Day CP, McComb JM, Campbell RW. QT dispersion: An indication of arrhythmia risk in patients with long QT intervals. Br Heart J 1990;63:342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SM, Hwang GS, Park JS, et al. The pattern of Tpeak‐Tend and QT interval, and J wave during therapeutic hypothermia. J Electrocardiol 2014. Jan‐Feb;47(1):84–92. [DOI] [PubMed] [Google Scholar]
- 13.Castro Hevia J, Antzelevitch C, Tornes Barzaga F, et al. Tpeak‐Tend and Tpeak‐Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol 2006;47:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: Clinical and research implications. Prog Cardiovasc Dis 2008;51:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garza DA, White FC, Hall RE, et al. Effect of coronary collateral development on ventricular fibrillation threshold. Basic Res Cardiol 1974;69:371–378. [DOI] [PubMed] [Google Scholar]
- 16. Nagamoto Y, Fujita M, Furuno Y, et al. Myocardial blood flow,alternans of ST segment elevation, conduction delay, and ventricular arrhythmia during acute myocardial ischemia with and without retrograde blood flow in canine hearts. Jpn Circ J 1991;55:581–590. [DOI] [PubMed] [Google Scholar]
- 17. McMurtry MS, Lewin AM, Knudtson ML, et al. The clinical profile and outcomes associated with coronary collaterals in patients with coronary artery disease. Can J Cardiol 2011;27:581–588. [DOI] [PubMed] [Google Scholar]
- 18. Habib GB, Heibig J, Forman SA, et al. Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation 1991;83:739–746. [DOI] [PubMed] [Google Scholar]
- 19. Billinger M, Kloos P, Eberli FR, et al. Physiologically assessed coronary collateral flow and adverse cardiac ischemic events: A follow‐up study in 403 patients with coronary artery disease. J Am Coll Cardiol 2002;40:1545–50. [DOI] [PubMed] [Google Scholar]
- 20. Nathoe HM, Buskens E, Jansen EW, et al. Role of coronary collaterals in off‐pump and on‐pump coronary bypass surgery. Circulation 2004;110:1738–1742. [DOI] [PubMed] [Google Scholar]
- 21. Nathoe HM, Koerselman J, Buskens E, et al. Determinants and prognostic significance of collaterals in patients undergoing coronary revascularization. Am J Cardiol 2006;98:31–35. [DOI] [PubMed] [Google Scholar]
- 22. Kersten JR, Pagel PS, Chilian WM, et al. Multifactorial basis for coronary collateralization: A complex adaptive response to ischemia [review]. Cardiovasc Res 1999;43(1):44–57. [DOI] [PubMed] [Google Scholar]
- 23. Celik T, Celik M, Iyisoy A. Coronary collateral circulation. Turk Kardiyol Dern Ars 2010;38(7):505–514. [PubMed] [Google Scholar]
- 24. Imhof BA, Aurrand‐Lions M. Angiogenesis and inflammation face off. Nat Med 2006;12(2):171–172. [DOI] [PubMed] [Google Scholar]
- 25. Yilmaz R, Demirbag R, Gur M. The association of QT dispersion and QT dispersion ratio with extent and severity of coronary artery disease. Ann Noninvasive Electrocardiol 2006. Jan;11(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meier P, Gloekler S, de Marchi SF, et al. An indicator of sudden cardiac death during brief coronary occlusion: Electrocardiogram QT time and the role of collaterals. Eur Heart J 2010. May;31(10):1197–1204. [DOI] [PubMed] [Google Scholar]
- 27. Tandogan I, Aslan H, Aksoy Y, et al. Impact of coronary collateral circulation on QT dispersion in patients with coronary artery disease. Coron Artery Dis 2006. Nov;17(7):623–628. [DOI] [PubMed] [Google Scholar]
- 28. Antzelevitch C. T peak‐Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest 2001;31:555–557. [DOI] [PubMed] [Google Scholar]
- 29. Antzelevitch C. Heterogeneity and cardiac arrhythmias: An overview. Heart Rhythm 2007;4:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Panikkath R, Reinier K, Uy‐Evanado A, et al. Prolonged Tpeak‐Tend interval on the resting ECG is associated with increased risk of sudden cardiac death. Circ Arrhythm Electrophysiol 2001;4:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang JF, Shan QJ, Yang B, et al. [Tpeak‐Tend interval and risk of cardiac events in patients with Brugada syndrome]. Zhonghua Xin Xue Guan Bing Za Zhi 2007. Jul;35(7):629–32. [PubMed] [Google Scholar]
- 32. Erikssen G, Liestøl K, Gullestad L, et al. The terminal part of the QT interval (T peak to T end): A predictor of mortality after acute myocardial infarction. Ann Noninvasive Electrocardiol 2012. Apr;17(2):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamaguchi M, Shimizu M, Ino H, et al. T wave peak‐to‐end interval and QT dispersion in acquired long QT syndrome: A new index for arrhythmogenicity. Clin Sci (Lond) 2003;105:671–676. [DOI] [PubMed] [Google Scholar]
- 34. Shimizu M, Ino H, Okeie K, et al. T‐peak to T‐end interval may be a better predictor of high‐risk patients with hypertrophic cardiomyopathy associated with a cardiac troponin I mutation than QT dispersion. Clin Cardiol 2002;25:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kannel WB, Wilson PW, D'Agostino RB, et al. Sudden coronary death in women. Am Heart J 1998;136:205–212. [DOI] [PubMed] [Google Scholar]
- 36. Korantzopoulos P, Letsas KP, Christogiannis Z, et al. Gender effects on novel indexes of heterogeneity of repolarization in patients with stable coronary artery disease. Hellenic J Cardiol 2011. Jul‐Aug;52(4):311–315. [PubMed] [Google Scholar]
- 37. Hirai T, Fujita M, Nakajima H, et al. Importance of collateral circulation for prevention of left ventricular aneurysm formation in acute myocardial infarction. Circulation 1989;79:791–796. [DOI] [PubMed] [Google Scholar]


