Abstract
The Epsilon wave was first identified in 1977. Four decades of progress help people to better understand its pathological electrogenesis and diagnostic value. Currently, the Epsilon wave is on the list of the 2010 Task Force recommendations for the diagnosis of arrhythmogenic right ventricular dysplasia (ARVD). In this review, we provide the history of the first recording of the Epsilon wave in coronary artery disease and Uhl's anomaly, subsequently leading to the signal averaging technique to record late potentials. Based on our experience, we discuss some existing controversies. When we look back at the decades of progress of the Epsilon wave, we conclude that the Epsilon wave is only the tip of the iceberg of ECG abnormalities in ARVD.
1. INTRODUCTION
The Epsilon wave was originally observed in the epicardium of the myocardial border zone of a patient who had myocardial infarction that occurred 10 years previously and was complicated by episodes of ventricular arrhythmias. He had a normal left ventricular ejection fraction (Figure 1a; Fontaine et al., 1977). In this patient, a minor effort such as raising his arms to shave induced rapid ventricular tachycardia (VT) which ceased spontaneously when the motion was interrupted. The VT started again when this motion was resumed. If the patient felt palpitations and tried to abort the arrhythmia, the VT accelerated and was transformed into ventricular fibrillation (VF) with loss of consciousness necessitating cardiopulmonary resuscitation (CPR) and external defibrillation. After several attempts, physicians decided to send the patient to our hospital, Pitié‐Salpêtrière, in Paris, where the newly available technique of epicardial mapping could localize the “ectopic focus” and facilitate its extinction. The ablation procedure was performed on December 16, 1971. However, the Epsilon wave was only identified later. These low amplitude potentials were localized on a fibrous scar on the diaphragmatic aspect of the left ventricle found by a thorough examination of the magnetic tape recordings obtained during the operation. The equipment (Ampex SP300) had seven channels: three channels were used for standard leads I, II, III. There were the three bipolar leads of the tripolar roving probe and a voice channel. The exploring probe was designed to have the electrodes perpendicular to the hand‐held shaft to explore the diaphragmatic epicardium without having to move the apex upward, which is always poorly tolerated. This design was made after our group's myocardial mapping of the first case of Wolff–Parkinson–White syndrome performed in Europe in 1971 (Fontaine et al., 1972). The electrodes were made of platinum wire obtained from the epicardial pacing electrodes by Medtronic (model 5814). The three bipolar electrodes (Fontaine, 2010) 1.5 mm apart imbedded in epoxy resin were positioned on the summit of an equilateral triangle as proposed by Kaiser and Waldo (Figure 1b; Kaiser et al., 1969). With this arrangement, it was possible to record a large signal from the direction of epicardial activation.
Figure 1.
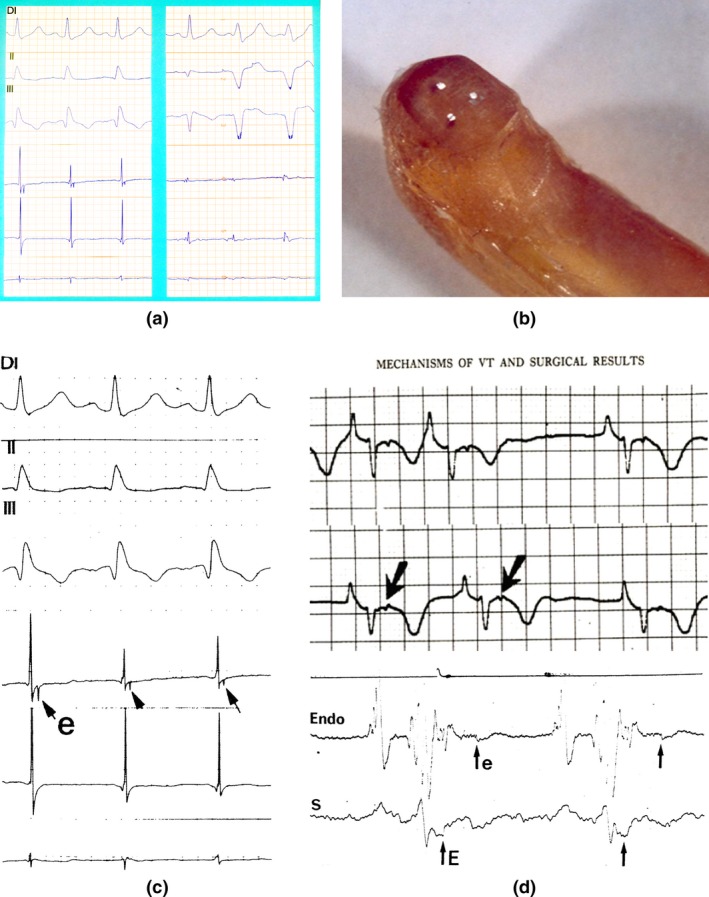
(a) Left: First recording of an Epsilon wave on the border zone of a diaphragmatic scar in a patient with an old myocardial infarction (insert arrow). Right: Epicardial potentials recorded on the scar tissue (insert arrow). (b) The electrodes are separated by 1.5 mm. Handmade instrument in Epoxy resin and platinum electrodes (With permission from Dr. Guy Fontaine, 2010). (c) First presentation of an epicardial Epsilon wave (With permission from Dr. Guy Fontaine (Fontaine et al., 1977)). (d) First presentation of an Epsilon wave on Holter‐ECG and on the endocardium (With permission from Dr. Guy Fontaine (Fontaine et al., 1977))
2. FIRST PUBLICATION OF EPSILON WAVES
The discovery of the Epsilon wave was completely unexpected because it occurred after the end of the QRS complex. Therefore, it was against the dogma of ECG electrogenesis. This strange phenomenon was frequently buried within the artifacts related to the movement of the three platinum electrodes. However, some recordings were quite reproducible from beat to beat when the electrodes were stable. From the first recording of an Epsilon wave, we selected examples where the potential was observed on one bipolar epicardial lead and not the others, because this showed that the phenomenon of a late epicardial activation was present at a very localized area, <1.5 mm. The first recordings of Epsilon waves were published in a French journal of Internal Medicine in 1973 (Fontaine, Frank, Bonnet, Cabrol, & Guiraudon, 1973). At that time the term of Epsilon wave did not exist. However, when one of us (GF) wrote a manuscript for a book chapter published in 1977 (Figure 1c,d), the smaller letter “ε” was added to this figure (Fontaine et al., 1977). When the phenomenon was well accepted and presented in an abstract form at one of the American Heart Association meetings, an example of what we called a “double potential” was published in 1984 (Fontaine, Guiraudon, & Frank, 1984).
3. FIRST EPSILON WAVES IN ARRHYTHMIC RIGHT VENTRICULAR DYSPLASIA
In patients with a normal surface ECG, despite obvious Epsilon waves detected on the epicardium, it was logical to think that these epicardial potentials were too small to be recorded on the skin surface, even on the precordial leads that are closest to the epicardium. It was also observed that these potentials were more frequently observed in patients with arrhythmogenic right ventricular dysplasia (ARVD) as opposed to patients operated for VT due to myocardial infarction. This was explained later when the histology of ARVD was understood, since in most cases the presence of fat and fibrosis was generally more pronounced on the epicardial layers (Fontaine et al., 1999; Marcus et al., 1982). This also explained that it took six more months to record this phenomenon from the endocardium. The example presented in the Kulbertus book (Fontaine et al., 1977) was made by a multipolar catheter using a bipolar recording between two electrodes encompassing a zone of approximately 6 cm. The recording demonstrated continuous activation all along the normal potential located in the QRS complex. It was therefore clear that the Epsilon wave which appeared to be independent of the QRS complex was in fact in a continuation of the area of normal activation (Fontaine et al., 1977).
If the Epsilon wave clearly recorded on the epicardium of some patients with ARVD was not visible on the surface ECG, it was thought that these electrical forces of delayed potentials were not of sufficient amplitude to be transmitted to the skin. This suggested that higher amplification was necessary to record them on the surface. A first attempt was made with an oscilloscope with maximal amplification. The signal was barely visible since it was buried inside skeletal muscle noise. To decrease this noise, a method used by neurophysiologists was the summation‐averaging technique. With the help of an engineer in computer science, it was possible to perform this specific function.
On 15 April 1975, a 43‐year‐old woman was operated for resistant VT. As soon as the pericardium was opened, a huge noncontractile transparent ball was seen in the right ventricle. It was possible to see the movement of the flow of blood inside this cavity. Someone suggested the name “Uhl's anomaly.” In that patient, epicardial mapping was able to demonstrate for the first time the circular pathway of VT. This was proof of the circus movement of VT observed on the infundibular area where a small layer of myocardium was still present forming a two‐dimensional structure. However, some areas in the zone of slow conduction showed continuous fragmentation of epicardial potentials. It was difficult to tell precisely which potential was really in the reentrant pathway. Therefore in the publication of this case (Fontaine et al., 1977), we labeled the recording of each test point as the epicardial potential with the assumption that in the future it would be possible to answer this question by a thorough analysis of the morphology of these potentials. We know that performing stimulation on each test point to obtain the “concealed entrainment” phenomenon would have provided the precise solution that was difficult to perform in the operating room due to time constraints. Because of impressive delayed potentials (sometimes triple potentials) also recorded in sinus rhythm, it was interesting to see if any abnormal activity could be recorded on a bipolar chest lead located in the precordial area. To do so, one of the first Holter recording machines (ICR) from the USA was used. This recording was difficult to analyze because of the small configuration of the QRS complex and the high amplitude of the P wave typical of Uhl's anomaly, due to major dilatation of the atrium. Nevertheless, it was possible to see the first recording of an Epsilon wave in a noncoronary artery disease patient that was distant from the end of the QRS complex (Figure 1c,d; Fontaine et al., 1977). Therefore, it was interesting to process a long recording of a bipolar chest lead to see if it was possible to extract the signal after summation‐averaging. The tape was processed by a very unique computer called the “Pacer 500.” This was the first computer able to process both numeric as well as analogic signals. In this patient, the latest epicardial signal was recorded at about 300 ms after the QRS complex. Subsequently, it was possible to draw the first recording of the late potential extracted by signal processing occurring at the same interval of 300 ms as on the epicardium (Fontaine et al., 1977). This coupling interval was shorter than the ventricular refractory period (250 ms). Therefore, this late potential was not transmitting reactivation of the ventricle at the time of the recording. However, this was an obvious possibility and could explain a reentrant phenomenon that was a controversial at that time.
Uhl's anomaly is a congenital disease with huge dilatation of the RV. Only a small rim of surviving flat layer of myocardium was observed at the base of the RV, especially in the infundibular area. In this condition, a reentrant pathway was identified by epicardial mapping. The mechanism of this strange anomaly reported for the first time by Henry Uhl from Johns Hopkins Hospital in a single pediatric case (Uhl, 1952). This patient died of heart failure. It was suspected that it was the result of major apoptosis as opposed to ARVD suggesting a less severe and long lasting phenomenon. A discussion of these two diseases has been reported (James, 1994). In 1966, the first demonstration of apoptosis in the human heart was reported in ARVD patients (Mallat et al., 1996).
4. ECG SIGNAL AVERAGING
The obvious technique to record the Epsilon waves was by signal averaging. However, it was observed that delayed potentials could be recorded by bipolar chest leads on the thorax of the patient with the adult form of Uhl's anomaly. The next step was to develop specific software to perform this technique with the same software as was used for His bundle detection (Berbari, Lazzara, El‐Sherif, & Scherlag, 1975). This historic document demonstrating the progressive increase in potentials by groups of 10 averaged beats was obtained on July 9, 1976. It showed the progressive increase in amplification with significant reduction of skeletal noise.
GF gave the name of “G” potential to the electrical activity recorded between the end of the atrial activity to the beginning of QRS. It was later observed that the G signal was quite large in continuity with the end of the P wave and was the result of a prolongation of the P wave not visible on the standard ECG.
The patient with typical ARVD and resistant VT was successfully operated by Dr. Guiraudon. The patient had a brother with the same arrhythmia that was controlled by drugs. It was this case that suggested that ARVD could be a genetic disease as indicated in a study of 24 cases in 1982 (Marcus et al., 1982).
The group of signals showed obvious Epsilon waves, which were reproducible during 60 ms. These waves were easy to see on the surface tracing. However, it was still possible to obtain a reproducible delayed activation 30 ms later that was visible only with the use of the signal averaging techniques. It was interesting to identify a technique to identify Epsilon waves on the ECG.
5. FONTAINE LEAD SYSTEM
Multiple positions of precordial electrodes were studied and finally the proposed “Fontaine Lead System (FLS)” was published in a book chapter (Fontaine, Frank, Fontaliran, Lascault, & Tonet, 1991; Figure 2a).
Figure 2.
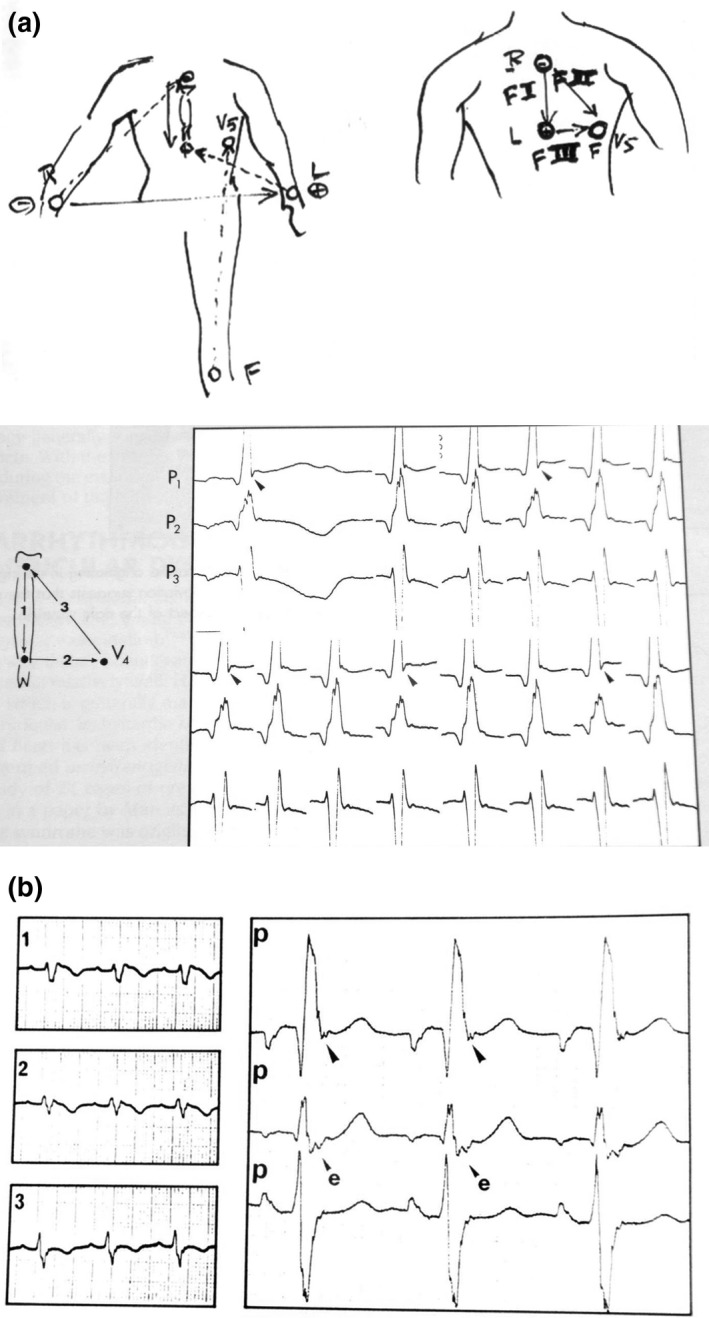
(a) Top, the first sketch presented the “F” leads (left) but later abandoned by modesty for the book illustration (right). Below, documents published in the Chatterjee and Parmley's book: Recording is performed at double speed and high amplification. There is obvious fragmentation of the top of the QRS complex. Note an intermittent small S wave. “P” means precordial lead. “F” leads are on the sketch. (b) The Fontaine lead shows an Epsilon wave located at the end of the QRS complex recorded with the same technique as before but compared with the regular V1–3 ECG tracing on the left
It was stated that records performed at double speed and high amplification would provide a more obvious fragmentation of the QRS complex. The comparison between recording from a standard ECG and the Fontaine Lead System was also noted (Figure 2b). This was observed in serial studies (Chiladakis, Zagli, Karantalis, & Alexopoulos, 2010; Fontaine et al., 1999; Gottschalk et al., 2014; Hurst, 1998; Kukla, Jastrzebski, & Kurdzielewicz, 2012; Marcus et al., 1982; Moreira et al., 2014; Wang et al., 2010), to assist in establishing a definitive diagnosis of ARVD by the Task Force Criteria (Marcus et al., 2010; McKenna et al., 1994).
6. COMPLEX EXPRESSION OF ECG ANOMALIES IDENTIFIED AS EPSILON WAVES
All of these ECG anomalies were the result of the special histologic structure of ARVD with surviving fibers embedded in fat and fibrous tissue mostly located in the RV (Fontaine et al., 1999; Mallat et al., 1996; Marcus et al., 1982; Mast et al., 2017; Zhang, Liu, Kowey, & Fontaine, 2014). Nevertheless, it was possible to correlate this phenomenon with prolongations of the QRS located in the right precordial leads. This led to the proposal of the QRS complex ≥110 ms in V1–V3 as a criterion for the diagnosis of ARVD. A more complex algorithm is the duration of V1, V2, or V3 longer than that in V6 by 25 ms. This indicates that the largest QRS complex duration of the three leads V1, V2, or V3 minus V6 that is greater or equal to 25 ms is a feature of ARVD. This formula is valid in complete as well as incomplete right bundle branch block (Figure 3a). In an example of incomplete RBBB, measurement of the QRS complexes in leads I, V2 and V6 shows a difference of 25 ms that is consistent with the diagnosis of ARVD. This was the case of a 72‐year‐old asymptomatic woman in whom an echocardiogram showed an anomaly of the RV and in whom magnetic resonance imaging (CMR) indicated a major presence of a thick layer of fat in the entire RV wall. In this case we observed an intraventricular conduction delay. However, it was consistent with ARVD due to an additional delayed activation in the RV myocardium that was observed in the right precordial leads. The term of “more than incomplete right bundle branch block” was proposed for this particular pattern. The same concept is applicable in patients with complete RBBB leading to the term “more than complete right bundle branch block” which is frequently observed in ARVD patients (Figure 3b; Fontaine et al., 2017). After almost 40 years of reviewing ECGs of patients with ARVD showing various forms of delayed potentials, it was concluded that any abnormality observed on the QRS complex on any lead of the ECG was the result of the disease process (Hulot, Jouven, Empana, Frank, & Fontaine, 2004; Sen‐Chowdhry et al., 2008). Note that the evolution of the ECG may sometimes provide clues due to myocarditis (Fontaine et al., 2017; Lopez‐Ayala et al., 2015; Saguner, Roland, Li, & Fontaine, 2017).
Figure 3.
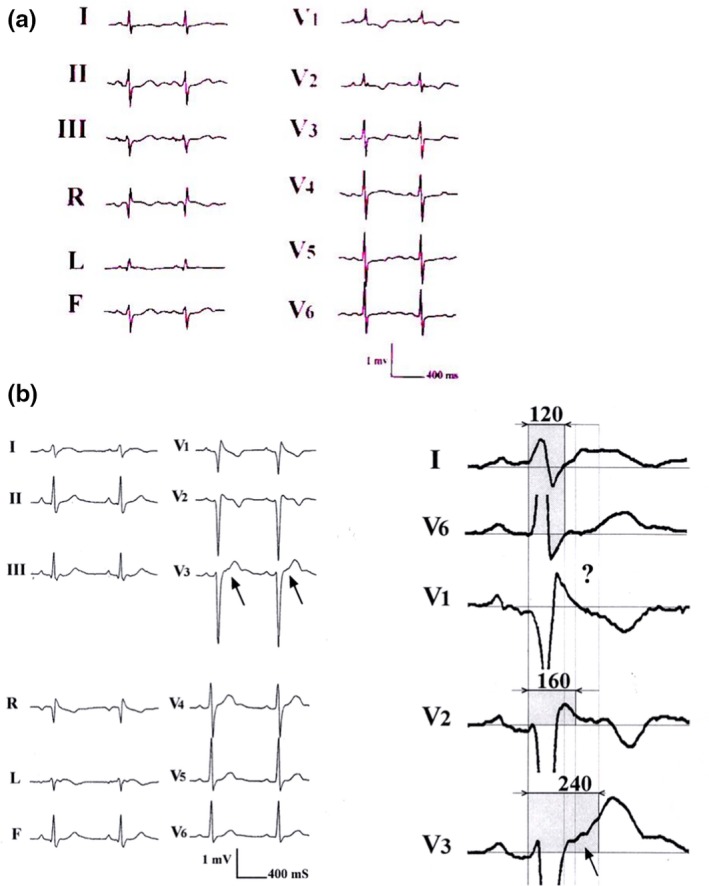
(a) An incomplete RBBB. (b) An example of more than incomplete right bundle branch block recorded in a young arrhythmogenic right ventricular dysplasia human with palpitations. The interesting aspect of this tracing was that the Epsilon wave is barely visible on a standard ECG. However, using the double amplitude recording it was possible to disclose late potentials up to 240 ms after the beginning of the QRS in some leads as opposed to lead I or V6 with a duration of 120 ms. The ‘?’ sign stresses the limit of Epsilon wave recognition on a single lead (With permission from Dr. Guy Fontaine (Fontaine et al., 2017))
7. UPDATED NAMES OF EPSILON WAVES
Initially, depolarization abnormalities occurring at the beginning, inside the QRS complex, or at the end of the QRS complex were classified as Epsilon waves. However, it was concluded that for practicing cardiologists, it was preferable to define an Epsilon wave as the recording of late potentials occurring after the end of the QRS that is clearly located on the ST segment. Therefore, it was necessary to apply new names for stressing the previous depolarization abnormalities inside the QRS complex.
We suggested that we call these ECG depolarization abnormalities occurring at the beginning of the QRS complex as Presilon, or its top as Topsilon, and on its end as Postsilon. Yet, the Epsilon wave as currently defined in the 2010 TFC constitutes an independent wave aside from the QRS complex on the ST segment in V1–V3 (Figure 4a). However, this definition of Epsilon waves is a rare phenomenon that can only be seen in a minority of patients with advanced stages of ARVD (Platonov et al., 2016). Even if the typical pattern of the Epsilon wave was not present on some ECGs of suspected ARVD patients, it was clear that there was prolongation of the end of the QRS complexes. In addition, this prolongation was visible in the right precordial leads as compared to V5 or V6. This was logical because of the so‐called proximity effect. It was concluded that this phenomenon was basically the same as the phenomenon producing typical Epsilon waves. Based on our experience, “Postsilon” waves were obviously the most frequent anomaly observed in these patients. This approach is based on the histologic structure of dysplastic myocardium. The original Epsilon wave as used in the 2010 TFC was recorded on the epicardium with an interelectrode distance of 1.5 mm. A spectrum of coupling of these wave forms was obtained from a large amount of epicardial myocardium. Therefore, the classical Epsilon wave mentioned in the 2010 TFC occurs after the end of the QRS complex prior to the onset of the T wave. Our broader updated definition of Epsilon waves including QRS fragmentation and Presilon, Topsilon, and Postsilon waves in addition to the classical Epsilon wave and including all 12 ECG leads is not meant to replace the 2010 TFC definition. Furthermore, QRS fragmentation is not a novel concept in ARVD, but illustrates the broad spectrum of depolarization abnormalities in ARVD in all 12 leads, since ARVD is nowadays considered a biventricular disease with a continuum of disease stages. In this context, we believe that the 2010 TFC are very strict and more specific, but they are mainly able to capture full blown typical advanced ARVD cases, but can miss early stage disease. Furthermore, we would like to increase the awareness for depolarization abnormalities aside from the 2010 definition, which only restricts the term Epsilon wave to small amplitude signals after the end of the QRS prior to the onset of the T wave in leads V1–V3. This reduces the 12‐lead ECG's sensitivity to detect ARVD. A broader definition as used in the current review can increase sensitivity to detect structural heart disease, ARVD particularly, but one has to be aware that this is at the cost of a loss in specificity to diagnose ARVD. We did not assess our approach for its sensitivity and specificity as compared to a healthy control group in contrast to the 2010 TFC, which have done this in a rigorous scientific manner. Nevertheless, we should keep in mind that a diagnosis of ARVD is never made with 12‐lead ECG alone, and the clinical circumstances and pretest probability for ARVD play a very important role in the interpretation of the epsilon wave. Therefore, increasing its diagnostic sensitivity can improve diagnostic yield and help the clinician. Moreover, if the ECG diagnosis of ARVD is based solely on the Epsilon wave definition in the 2010 TFC, Epsilon waves in ARVD and particularly in the young <35 years of age are rarely observed, since this definition reflects advanced disease stages (Platonov et al.,2016). At earlier stages, depolarization abnormalities are often buried inside the QRS complex or can affect any of the 12 surface ECG leads including V5/V6 (Li, Saguner, Akdis, & Fontaine, 2018). Therefore, depolarization abnormalities observed in a young adult with a high pretest probability of ARVD is highly suggestive of ARVD, especially if this anomaly is larger in the right as compared with the left precordial leads.
Figure 4.
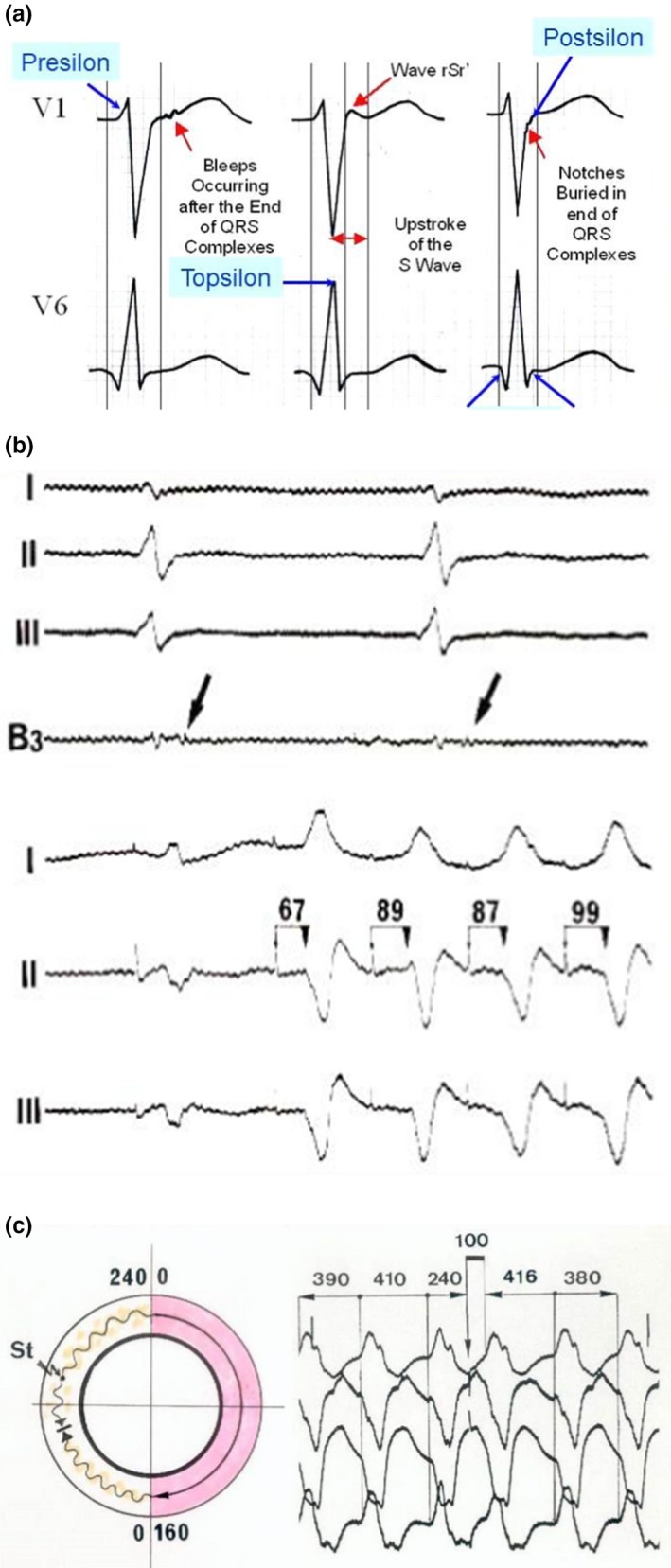
(a) The new names for identification of depolarization abnormalities (The original Epsilon wave was recorded on the epicardium with an interelectrode distance of 1.5 mm) (With permission from Dr Guoliang Li (Li et al., 2018)). (b) Example of stimulation in the zone of slow conduction of arrhythmogenic right ventricular dysplasia (ARVD). Upper tracings: Recording of late potentials on the epicardium. The stimulation in the same zone shows a delay between the stimulus artifact and the ventricular activation on the surface leads. This was possible because all electrophysiological parameters were stored on a magnetic tape during each procedure. Several years later, this phenomenon led to the concept of concealed entrainment presented on the tracing below. Entrainment by stimulation in the zone of slow conduction is obtained after a single stimulus. (c) An excellent demonstration of concealed entrainment with a single stimulus delivered in the zone of slow conduction. Note the typical ECG pattern of VT with LBBB pattern and superior axis in a patient with typical ARVD. There is a delay of 100 ms in between the stimulus and the ventricular response. Note that the morphology of the “entrained QRS” is exactly the same that the morphology observed during spontaneous VT and that this morphology is also followed by the same morphology with the same coupling beat to beat interval of 380–390 ms (With permission from Dr. Guy Fontaine (Fontaine, 2010))
Figure 4b shows an example of pacing in the zone of slow conduction in a patient with ARVD and epsilon waves. The stimulation in the same zone shows a delay between the stimulus artifact and ventricular activation on the surface leads (upper tracings). This observation was possible because all electrophysiological parameters were stored on magnetic tape during each procedure.
Several years later, this phenomenon led to the concept of concealed entrainment (Fontaine, Frank, Tonet, & Grosgogeat, 1989), whose potential in arrhythmias was widely recognized in a series of studies (Bogun, Bender, Li, & Hohnloser, 2000; Bogun et al., 1997; Morton, Sanders, Deen, Vohra, & Kalman, 2002). Concealed entrainment by stimulation in the zone of slow conduction can be obtained after a single stimulus (Figure 4c).
8. EMERGING METHODS OF RECORDING EPSILON WAVES
The phenomenon of the Epsilon wave is attractive but represents a small part of the modification of activation in the diseased myocardium. This is easily explained by the histology of the tissue affected by the dysplastic phenomenon (Fontaine, 2011; Fontaine & Chen, 2014; Fontaine et al., 1999; Marcus et al., 1982; Mast et al., 2017). The same phenomenon also occurs in CAD. This can be a new marker to determine disease progression in this entity as well as in ARVD (Mast et al., 2017). This is simpler to demonstrate in ARVD because abnormal conduction is related to the presence of fat and fibrosis as compared to fibrosis only in CAD. Also, it is suspected that the dysplastic phenomenon is probably more progressive in this disease because of a molecular mechanism. This concept opens a new field of interest concerning the evaluation of disease progression by repeated ECGs during patient follow‐up correlating with other phenotypic parameters (Fontaine, 2011; Messroghli et al., 2017; Peters, Trummel, & Koehler, 2008; Pinamonti et al., 2011; Te Riele et al., 2015).
However, a more precise analysis of the ECG signal requires a specific ECG machine with a larger band pass. Technically, this means a sampling band pass of 1K Hz giving a Shannon band pass of 500 Hz which is much higher above the filtering of 35 Hz in some ECG machines. Our preliminary testing by a novel 16‐lead high definition ECG machine indicates that these suggested parameters improve the recording of multielectric parameters (Li et al., 2018). In addition, the potential of the insertable loop recorder was recently suggested to record Epsilon waves (Fontaine et al., 2017). New models of ECG will significantly improve the development of recording methods, which in turn will help us better understand the mechanisms underlying ARVD (Asimaki et al., 2014; Caspi et al., 2013; Kim et al., 2013; Ma et al., 2013).
9. AN INTERESTING CASE REPORT TO CONCLUDE THIS REVIEW OF PROGRESS OF FOUR DECADES IN IDENTIFYING EPSILON WAVES
A 35‐year‐old human had an episode of VT at a rate of 220 bpm, recorded after a soccer game. He had extreme fatigue but no palpitations. He went to a nearby hospital in the USA where the arrhythmia was recorded in the emergency department. The VT had a right bundle branch block pattern (Figure 5a). A normal ECG was recorded after cardioversion. Because of this life‐threatening arrhythmia, an implantable cardioverter defibrillator (ICD) was suggested. However, the patient said that he was a French engineer, just married and wanted another opinion in France. After he came to our hospital of Pitié‐Salpêtrière in Paris, a SAECG indicated obvious delayed potentials (Figure 5b). Despite a normal 12‐lead ECG and VT with RBBB morphology, ARVD was suspected and finally confirmed by RV contrast angiography. This patient was diagnosed as having ARVD and am ICD was implanted. This case demonstrates that (a) VT of RBBB configuration can be present in ARVD since ARVD is often a biventricular disease. This may be the result of the dysplastic phenomenon involving the left ventricle which is frequent in this condition. It can be also the result of a focus of myocarditis involving the left ventricle (Fontaine et al., 2017; Lopez‐Ayala et al., 2015; Saguner et al., 2017), causing the deterioration of ARVD. (b) A normal ECG can be observed in an ARVD patient with life‐threatening arrhythmias. (c) The SAECG can show abnormal delayed activation despite a completely normal 12‐lead surface ECG. (d), There was an inversion of the usually positive P wave in inferior leads II, III, and aVF. This suggests that ARVD probably involved the atrium of this patient at the onset of the disease, which is in agreement with previous findings (Tonet et al., 1991; Wu et al., 2016). Abnormal behavior of the atrium can be a marker of ARVD in a young patient with a normal physical examination. This parameter can be added to the diagnostic criteria of ARVD used for screening a population at risk. Atrial arrhythmias may be the first sign of the disease (Camm et al., 2013; Wu et al., 2016). Abnormal behavior of the atrium can be a marker of ARVD in a young patien. This parameter can be added to the diagnostic criteria of ARVD used for screening a population at risk. Several different groups have reported atrial involvement and the presence of atrial arrhythmias and P wave prolongation in patients with ARVD (Kazmierczak, Kornacewicz‐Jach, & Wojtarowicz, 1999; Nogami et al., 1990; Platonov et al., 2011; Saguner et al., 2014; Takemura et al., 2008; Tonet et al., 1991). In fact, atrial arrhythmias may be the first sign of the disease.
Figure 5.
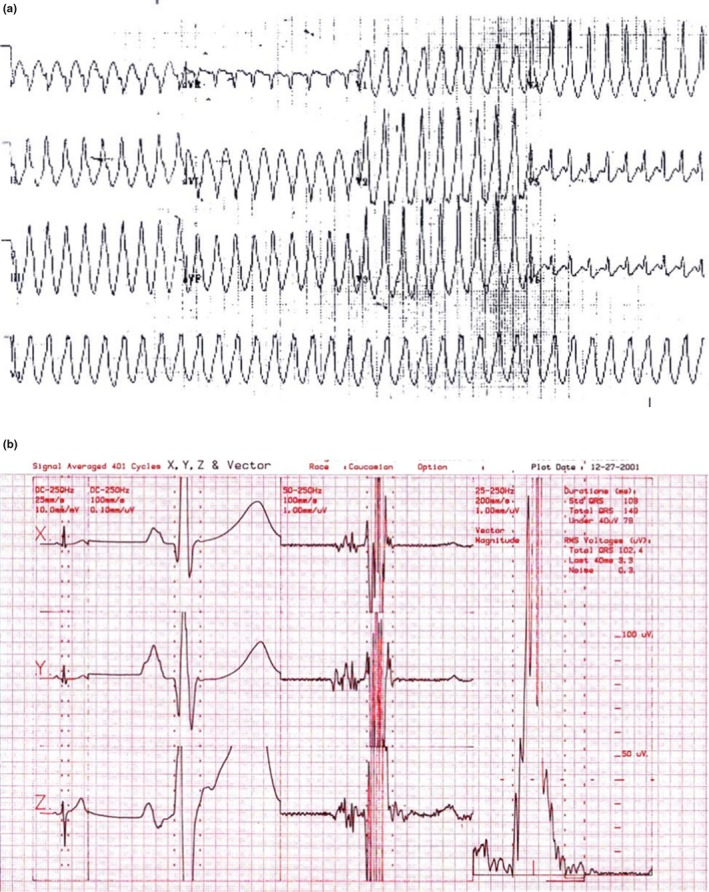
(a) Note the RBBB pattern during rapid ventricular tachycardia (VT) with inferior axis which is not frequent in arrhythmogenic right ventricular dysplasia (ARVD). The inferior axis suggests a VT origin at the base of the heart but not from the infundibulum (With permission from Dr. Guy Fontaine (Fontaine, 2010)). (b) The signal averaging ECG from a patient with ARVD. Note the end of the latest delayed potential (With permission from Dr. Guy Fontaine (Fontaine, 2010))
10. CONCLUSIONS
Abnormal delayed and fragmented activation is frequent in ARVD. A vast spectrum of entropy is observed encompassing a slight change in the slope of the rapid phase of the QRS complex to fragmentation extending over the entire depolarization process. Emerging methods of recording Epsilon waves and models of ARVD will significantly improve its sensitivity and specificity and promote its position as a major criterion of ARVD.
CONFLICT OF INTERESTS
Authors declare no conflict of interests for this article.
ACKNOWLEDGMENTS
We extend our gratitude to Dr. Frank I Marcus, University of Tucson, AZ, USA for editing this manuscript and Yvette Wingfield for her secretarial assistance. We also thank colleagues from all over the world who contributed to the ARVD work over the past four decades. Guo‐Liang Li was supported by funding from the National Natural Science Foundation of China (No. 81400258, 81370289) and the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University, China (No. XJTU1AF‐CRF‐2015‐007).
Li G, Saguner AM, Fontaine GH, Frank R. Epsilon waves: Milestones in the discovery and progress. Ann Noninvasive Electrocardiol. 2018;23:e12571 10.1111/anec.12571
REFERENCES
- Asimaki, A. , Kapoor, S. , Plovie, E. , Karin Arndt, A. , Adams, E. , Liu, Z. , … Saffitz, J. E. (2014). Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Science Translational Medicine, 6, 240ra74 10.1126/scitranslmed.3008008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari, E. J. , Lazzara, R. , El‐Sherif, N. , & Scherlag, B. J. (1975). Extracardiac recordings of His‐Purkinje activity during conduction disorders and junctional rhythms. Circulation, 51, 802–810. 10.1161/01.CIR.51.5.802 [DOI] [PubMed] [Google Scholar]
- Bogun, F. , Bahu, M. , Knight, B. P. , Weiss, R. , Paladino, W. , Harvey, M. , … Morady, F. (1997). Comparison of effective and ineffective target sites that demonstrate concealed entrainment in patients with coronary artery disease undergoing radiofrequency ablation of ventricular tachycardia. Circulation, 95, 183–190. 10.1161/01.CIR.95.1.183 [DOI] [PubMed] [Google Scholar]
- Bogun, F. , Bender, B. , Li, Y. G. , & Hohnloser, S. H. (2000). Ablation of atypical atrial flutter guided by the use of concealed entrainment in patients without prior cardiac surgery. Journal of Cardiovascular Electrophysiology, 11, 136–145. 10.1111/j.1540-8167.2000.tb00312.x [DOI] [PubMed] [Google Scholar]
- Camm, C. F. , James, C. A. , Tichnell, C. , Murray, B. , Bhonsale, A. , te Riele, A. S. , … Calkins, H. (2013). Prevalence of atrial arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm, 10, 1661–1668. 10.1016/j.hrthm.2013.08.032 [DOI] [PubMed] [Google Scholar]
- Caspi, O. , Huber, I. , Gepstein, A. , Arbel, G. , Maizels, L. , Boulos, M. , & Gepstein, L. (2013). Modeling of arrhythmogenic right ventricular cardiomyopathy with human induced pluripotent stem cells. Circulation Cardiovascular Genetics, 6, 557–568. 10.1161/CIRCGENETICS.113.000188 [DOI] [PubMed] [Google Scholar]
- Chiladakis, J. , Zagli, F. , Karantalis, V. , & Alexopoulos, D. (2010). New diagnosis of arrhythmogenic right ventricular cardiomyopathy in an octogenarian with the help of Fontaine electrocardiographic leads. Europace, 12, 1197–1198. 10.1093/europace/euq095 [DOI] [PubMed] [Google Scholar]
- Fontaine, G. H. (2010). La mort subite du jeune et de l’athlète (French) (pp. 388). Paris, France: SFEM Editions. [Google Scholar]
- Frank, R. (2018). Guy Fontaine MD PhD HDR. European Heart Journal 39, 2226–2227. 10.1093/eurheartj/ehy276 [DOI] [PubMed] [Google Scholar]
- Fontaine, G. H. (2011). The multiple facets of right ventricular cardiomyopathies. European Heart Journal, 32, 1049–1051. 10.1093/eurheartj/ehr088 [DOI] [PubMed] [Google Scholar]
- Fontaine, G. , & Chen, H. S. (2014). Arrhythmogenic right ventricular dysplasia back in force. American Journal of Cardiology, 113, 1735–1739. 10.1016/j.amjcard.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine, G. H. , Duthoit, G. , Li, G. L. , Andreoletti, L. , Gandjbakhch, E. , & Frank, R. (2017). Epsilon wave on an electronic loop in a case of arrhythmogenic right ventricular dysplasia with myocarditis: An updated definition of the Epsilon wave. Europace, 19, 1084–1090. 10.1093/europace/euw320 [DOI] [PubMed] [Google Scholar]
- Fontaine, G. , Fontaliran, F. , Hébert, J. L. , Chemla, D. , Zenati, O. , Lecarpentier, Y. , & Frank, R. (1999). Arrhythmogenic right ventricular dysplasia. Annual Review of Medicine, 50, 17–35. 10.1146/annurev.med.50.1.17 [DOI] [PubMed] [Google Scholar]
- Fontaine, G. , Frank, R. , Bonnet, M. , Cabrol, C. , & Guiraudon, G. (1973). [Experimental and clinical study of Wolff‐Parkinson‐White and myocardial ischemia syndromes by cartography of epicardial ventricular depolarization]. Coeur et Medecine Interne, 12, 105–113. [PubMed] [Google Scholar]
- Fontaine, G. , Frank, R. , Fontaliran, F. , Lascault, G. , & Tonet, J. (1991). Right ventricular tachycardias In Parmley W. W. & Chatterjee K. (Eds.), Cardiology (pp. 1–18). New York, NY: J.B. Lippincott. [Google Scholar]
- Fontaine, G. , Frank, R. , Tonet, J. , & Grosgogeat, Y. (1989). Identification of a zone of slow conduction appropriate for VT ablation: Theoretical and practical considerations. Pacing and Clinical Electrophysiology, 12, 262–267. 10.1111/j.1540-8159.1989.tb02656.x [DOI] [PubMed] [Google Scholar]
- Fontaine, G. , Guiraudon, G. , & Frank, R. (1984). Surgical management of ventricular tachycardia not related to myocardial ischemia In Josephson M. E. & Wellens H. J. J. (Eds.), Tachycardias: Mechanism, diagnosis, treatment (pp. 451–473). Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Fontaine, G. H. , Guiraudon, G. , Frank, R. , Vedel, J. , Grosgogeat, Y. , Cabrol, C. , & Facquet, J. (1977). Stimulation studies and epicardial mapping in ventricular tachycardia: Study of mechanisms and selection for surgery In Kulbertus H. (ed.), Re-entrant Arrhythmias. Mechanisms and Treatment (pp. 334–350). Lancaster, UK: MTP Pub. [Google Scholar]
- Fontaine, G. , Guiraudon, G. , Vachon, J. , Bernard, J. P. , Potier, J. C. , Grosgogeat, Y. , … Cabrol, C. (1972). [Kent's bundle section in a case of A‐B type Wolff‐Parkinson‐White syndrome. I. Preoperative electrophysiological investigations]. Archives des Maladies du Coeur et des Vaisseaux, 65, 905–924. [PubMed] [Google Scholar]
- Gottschalk, B. , Gysel, M. , Barbosa‐Barros, R. , De Sousa Rocha, R. P. , Pérez‐Riera, A. R. , Zhang, L. , … Baranchuk, A. (2014). The use of fontaine leads in the diagnosis of arrhythmogenic right ventricular dysplasia. Annals of Noninvasive Electrocardiology, 19, 279–284. 10.1111/anec.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot, J. S. , Jouven, X. , Empana, J. P. , Frank, R. , & Fontaine, G. (2004). Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation, 110, 1879–1884. 10.1161/01.CIR.0000143375.93288.82 [DOI] [PubMed] [Google Scholar]
- Hurst, J. W. (1998). Naming of the waves in the ECG, with a brief account of their genesis. Circulation, 98, 1937–1942. 10.1161/01.CIR.98.18.1937 [DOI] [PubMed] [Google Scholar]
- James, T. N. (1994). Normal and abnormal consequences of apoptosis in the human heart. From postnatal morphogenesis to paroxysmal arrhythmias. Circulation, 90, 556–573. 10.1161/01.CIR.90.1.556 [DOI] [PubMed] [Google Scholar]
- Kaiser, G. A. , Waldo, A. L. , Harris, P. D. , Bowman, F. O. Jr , Hoffman, B. F. , & Malm, J. R. (1969). New method to delineate myocardial damage at surgery. Circulation, 39, I83–I89. [DOI] [PubMed] [Google Scholar]
- Kazmierczak, J. , Kornacewicz‐Jach, Z. , & Wojtarowicz, A. (1999). Atrial epicardial pacing with long stimulus to P wave interval in a patient with arrhythmogenic right ventricular dysplasia complicated by right atrial thrombosis. Pacing and Clinical Electrophysiology, 22, 1111–1113. 10.1111/j.1540-8159.1999.tb00583.x [DOI] [PubMed] [Google Scholar]
- Kim, C. , Wong, J. , Wen, J. , Wang, S. , Wang, C. , Spiering, S. , … Chen, H. S. (2013). Studying arrhythmogenic right ventricular dysplasia with patient‐specific iPSCs. Nature, 494, 105–110. 10.1038/nature11799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukla, P. , Jastrzebski, M. , & Kurdzielewicz, W. (2012). [Higher right precordial leads and Fontaine leads: The better detection of QRS fragmentation and epsilon wave in arrhythmogenic right ventricular dysplasia‐cardiomyopathy]. Kardiologia Polska, 70, 958–959. [PubMed] [Google Scholar]
- Li, G. (2018). Guy Fontaine, a personal tribute. European Heart Journal, 39, 2228–2229. 10.1093/eurheartj/ehy277 [DOI] [PubMed] [Google Scholar]
- Li, G. L. , Saguner, A. M. , Akdis, D. , & Fontaine, G. H. (2018). Value of a novel 16‐lead high definition ECG machine to detect conduction abnormalities in structural heart disease. Pacing and Clinical Electrophysiology, 1–13. 10.1111/pace.13338 [DOI] [PubMed] [Google Scholar]
- Lopez‐Ayala, J. M. , Pastor‐Quirante, F. , Gonzalez‐Carrillo, J. , Lopez‐Cuenca, D. , Sanchez‐Munoz, J. J. , Oliva‐Sandoval, M. J. , & Gimeno, J. R. (2015). Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm, 12, 766–773. 10.1016/j.hrthm.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Ma, D. , Wei, H. , Lu, J. , Ho, S. , Zhang, G. , Sun, X. , … Liew, R. (2013). Generation of patient‐specific induced pluripotent stem cell‐derived cardiomyocytes as a cellular model of arrhythmogenic right ventricular cardiomyopathy. European Heart Journal, 34, 1122–1133. 10.1093/eurheartj/ehs226 [DOI] [PubMed] [Google Scholar]
- Mallat, Z. , Tedgui, A. , Fontaliran, F. , Frank, R. , Durigon, M. , & Fontaine, G. (1996). Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. New England Journal of Medicine, 335, 1190–1196. 10.1056/NEJM199610173351604 [DOI] [PubMed] [Google Scholar]
- Marcus, F. I. , Fontaine, G. H. , Guiraudon, G. , Frank, R. , Laurenceau, J. L. , Malergue, C. , & Grosgogeat, Y. (1982). Right ventricular dysplasia: A report of 24 adult cases. Circulation, 65, 384–398. 10.1161/01.CIR.65.2.384 [DOI] [PubMed] [Google Scholar]
- Marcus, F. I. , McKenna, W. J. , Sherrill, D. , Basso, C. , Bauce, B. , Bluemke, D. A. , … Zareba, W. (2010). Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation, 121, 1533–1541. 10.1161/CIRCULATIONAHA.108.840827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast, T. P. , James, C. A. , Calkins, H. , Teske, A. J. , Tichnell, C. , Murray, B. , … Cramer, M. J. (2017). Evaluation of structural progression in arrhythmogenic right ventricular dysplasia/cardiomyopathy. JAMA Cardiology, 2, 293–302. 10.1001/jamacardio.2016.5034 [DOI] [PubMed] [Google Scholar]
- McKenna, W. J. , Thiene, G. , Nava, A. , Fontaliran, F. , Blomstrom‐Lundqvist, C. , Fontaine, G. , & Camerini, F. (1994). Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. British Heart Journal, 71, 215–218. 10.1136/hrt.71.3.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messroghli, D. R. , Moon, J. C. , Ferreira, V. M. , Grosse‐Wortmann, L. , He, T. , Kellman, P. , … Friedrich, M. G. (2017). Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). Journal of Cardiovascular Magnetic Resonance, 19, 75 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, D. , Delgado, A. , Marmelo, B. , Correia, E. , Gama, P. , Pipa, J. , … Santos, O. (2014). Arrhythmogenic right ventricular cardiomyopathy: Contribution of different electrocardiographic techniques. Revista Portuguesa de Cardiologia, 33, 243. e1–7. [DOI] [PubMed] [Google Scholar]
- Morton, J. B. , Sanders, P. , Deen, V. , Vohra, J. K. , & Kalman, J. M. (2002). Sensitivity and specificity of concealed entrainment for the identification of a critical isthmus in the atrium: Relationship to rate, anatomic location and antidromic penetration. Journal of the American College of Cardiology, 39, 896–906. 10.1016/S0735-1097(02)01691-1 [DOI] [PubMed] [Google Scholar]
- Nogami, A. , Adachi, S. , Nitta, J. , Taniguchi, K. , Marumo, F. , Aonuma, K. , … Hiroe, M. (1990). Arrhythmogenic right ventricular dysplasia with sick sinus syndrome and atrioventricular conduction disturbance. Japanese Heart Journal, 31, 417–423. 10.1536/ihj.31.417 [DOI] [PubMed] [Google Scholar]
- Peters, S. , Trummel, M. , & Koehler, B. (2008). QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia‐cardiomyopathy. Heart Rhythm, 5, 1417–1421. 10.1016/j.hrthm.2008.07.012 [DOI] [PubMed] [Google Scholar]
- Pinamonti, B. , Dragos, A. M. , Pyxaras, S. A. , Merlo, M. , Pivetta, A. , Barbati, G. , … Sinagra, G. (2011). Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: Results from a 10‐year registry. European Heart Journal, 32, 1105–1113. 10.1093/eurheartj/ehr040 [DOI] [PubMed] [Google Scholar]
- Platonov, P. G. , Calkins, H. , Hauer, R. N. , Corrado, D. , Svendsen, J. H. , Wichter, T. , … Zareba, W. (2016). High interobserver variability in the assessment of epsilon waves: Implications for diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm, 13, 208–216. 10.1016/j.hrthm.2015.08.031 [DOI] [PubMed] [Google Scholar]
- Platonov, P. G. , Christensen, A. H. , Holmqvist, F. , Carlson, J. , Haunso, S. , & Svendsen, J. H. (2011). Abnormal atrial activation is common in patients with arrhythmogenic right ventricular cardiomyopathy. Journal of Electrocardiology, 44, 237–241. 10.1016/j.jelectrocard.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Saguner, A. M. , Ganahl, S. , Kraus, A. , Baldinger, S. H. , Medeiros‐Domingo, A. , Saguner, A. R. , … Duru, F. (2014). Clinical role of atrial arrhythmias in patients with arrhythmogenic right ventricular dysplasia. Circulation Journal, 78, 2854–2861. 10.1253/circj.CJ-14-0474 [DOI] [PubMed] [Google Scholar]
- Saguner, A. M. , Roland, F. , Li, G. L. , & Fontaine, G. H. (2017). Superimposed myocarditis leading to heart transplantation in a young patient with arrhythmogenic right ventricular dysplasia. European Heart Journal, 38, 1424. [Google Scholar]
- Sen‐Chowdhry, S. , Syrris, P. , Prasad, S. K. , Hughes, S. E. , Merrifield, R. , Ward, D. , … McKenna, W. J. (2008). Left‐dominant arrhythmogenic cardiomyopathy: An under‐recognized clinical entity. Journal of the American College of Cardiology, 52, 2175–2187. 10.1016/j.jacc.2008.09.019 [DOI] [PubMed] [Google Scholar]
- Takemura, N. , Kono, K. , Tadokoro, K. , Shinbo, G. , Ito, I. , Abe, C. , … Matsuoka, H. (2008). Right atrial abnormalities in a patient with arrhythmogenic right ventricular cardiomyopathy without ventricular tachycardia. Journal of Cardiology, 51, 205–209. 10.1016/j.jjcc.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Te Riele, A. S. J. M. , Marcus, F. I. , James, C. A. , Murray, B. A. , Tichnell, C. , Zimmerman, S. L. , … Calkins, H. (2015). The value of cardiac magnetic resonance imaging in evaluation of pediatric patients for arrhythmogenic right ventricular dysplasia/cardiomyopathy. Journal of the American College of Cardiology, 66, 873–874. 10.1016/j.jacc.2015.04.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonet, J. L. , Castro‐Miranda, R. , Iwa, T. , Poulain, F. , Frank, R. , & Fontaine, G. H. (1991). Frequency of supraventricular tachyarrhythmias in arrhythmogenic right ventricular dysplasia. American Journal of Cardiology, 67, 1153 10.1016/0002-9149(91)90886-P [DOI] [PubMed] [Google Scholar]
- Uhl, H. S. (1952). A previously undescribed congenital malformation of the heart: Almost total absence of the myocardium of the right ventricle. Bulletin of the Johns Hopkins Hospital, 91, 197–209. [PubMed] [Google Scholar]
- Wang, J. , Yang, B. , Chen, H. , Ju, W. , Chen, K. , Zhang, F. , … Chen, M. (2010). Epsilon waves detected by various electrocardiographic recording methods: In patients with arrhythmogenic right ventricular cardiomyopathy. Texas Heart Institute Journal, 37, 405–411. [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Guo, J. , Zheng, L. , Chen, G. , Ding, L. , Qiao, Y. , … Zhang, S. (2016). Atrial remodeling and atrial tachyarrhythmias in arrhythmogenic right ventricular cardiomyopathy. American Journal of Cardiology, 118, 750–753. 10.1016/j.amjcard.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Liu, L. , Kowey, P. R. , & Fontaine, G. H. (2014). The electrocardiographic manifestations of arrhythmogenic right ventricular dysplasia. Current Cardiology Reviews, 10, 237–245. 10.2174/1573403X10666140514102928 [DOI] [PMC free article] [PubMed] [Google Scholar]


