Abstract
Background
Frequent ventricular ectopy on preimplantation Holter has been associated with attenuated benefit from cardiac resynchronization therapy (CRT). However, it is unclear whether ectopic burden measured post‐CRT implantation can be utilized to evaluate long‐term prognosis. We aimed to describe the association between post‐CRT implantation ectopic burden and subsequent risk of clinical outcomes.
Methods
At the 12‐month follow‐up visit, 24‐hour Holter recordings were performed in 698 CRT‐D patients from the MADIT‐CRT study. The mean number of ventricular premature complexes (VPCs/hour) was calculated. High ectopic burden was defined as >10 VPCs/hour and low burden as ≤10 VPCs/hour. Multivariate Cox proportional hazards models were utilized to assess the association between 12‐month ectopic burden and the risk of the end points of heart failure (HF) or death and ventricular tachyarrhythmias (VT/VF).
Results
At 12 months, 282 (40%) patients presented with low ectopic burden and 416 (60%) patients presented with high ectopic burden. The 3‐year risk of HF/death and VT/VF was lower in patients with a low burden (7% and 8%) and significantly higher (25% and 24%) in patients with high burden. In multivariate analyses, patients with a high ectopic burden had approximately threefold increased risk of both HF/death (HR=2.76 [1.62–4.70], p < .001) and VT/VF (HR=2.79 [1.69–4.58], p < .001).
Conclusion
In CRT‐D patients with mild heart failure, high ectopic burden at 12‐month follow‐up was associated with a high 3‐year risk of HF/death and VT/VF and threefold increased risk as compared to patients with low burden. Ectopic burden at 12 months may be a valuable approach for evaluating long‐term prognosis.
Keywords: appropriate therapy, cardiac resynchronization therapy, death, heart failure, premature beats, ventricular ectopy, ventricular tachycardia
1. INTRODUCTION
The beneficial effects of cardiac resynchronization therapy with defibrillator (CRT‐D) in heart failure (HF) patients with depressed left ventricular function and electromechanical delay have been widely established (Brignole et al., 2013). However, the efficacy of CRT‐D relies on the ability of the device to maintain a high level of biventricular (BIV) pacing, preferably above 97% (Hayes et al., 2011; Koplan et al., 2009; Ruwald et al., 2015). Effective BIV‐pacing capture can be affected by several potentially modifiable factors, including the duration of the programmed atrioventricular delay (AV‐delay), atrial and ventricular arrhythmias, including premature ectopic beats (Cheng, Landman, & Stadler, 2012; Ruwald, Mittal et al., 2014). Recently, it was shown that ectopic burden above 0.1% on 24‐hour Holter, measured before CRT‐D implantation, was associated with reduced overall BIV‐pacing percentage and significantly worse outcome, indicating that preimplantation Holter monitoring could be an effective tool for patients selection (Ruwald, Mittal et al., 2014).
Although methods for patient selection are priority, a number of patients do not derive the expected benefit from CRT‐D implantation (Abraham et al., 2002; Ge et al., 2014; Padeletti, Paoletti Perini, & Gronda, 2012). These patients may derive advantage from post‐implantation pharmacologic optimization, radiofrequency ablation, and/or modified device programming to achieve greater BIV‐capture and thus a better utilization of the CRT‐D device (Lakkireddy et al., 2012). Therefore, tools for evaluating CRT‐D efficacy post‐implantation are needed. Postimplantation 24‐hour Holter monitoring might prove to be an inexpensive, easy‐to‐use tool for identifying patients who might benefit from the above‐mentioned initiatives and for evaluating the projected long‐term prognosis. However, there is a paucity of studies investigating the clinical and predictive value of Holter monitoring in these patients.
We aimed to evaluate the association between 12‐month ventricular ectopic burden and the risk of HF/death and malignant ventricular tachyarrhythmias in CRT‐D patients with preexisting ischemic or nonischemic cardiomyopathy and mild HF symptoms from the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT‐CRT). We hypothesized that low ectopic burden would be associated with better outcome and high ectopic burden would be associated with impaired clinical benefit. Furthermore, as a sensitivity analyses, we aimed to evaluate whether ectopy burden at 12 months or change in ectopic burden from implantation to 12 months is a better predictor of outcome.
2. METHODS
2.1. MADIT‐CRT
The MADIT‐CRT trial was a major randomized device trial conducted from December 22, 2004 through June 22, 2009 involving 1820 patients randomized in a 3:2 fashion for primary prevention CRT‐D or ICD implantation. Inclusion criteria included depressed left ventricular (LV) function (LVEF<30%), prolonged QRS duration (>130 ms), ischemic (NYHA class I/II), or nonischemic (NYHA class II) cardiomyopathy and sinus rhythm at enrollment. Patients were excluded from randomization if they, within 3 months prior to enrollment, had undergone coronary bypass graft surgery, percutaneous intervention, or had experienced a myocardial infarction, or if they, within 1 month prior to enrollment, had experienced atrial fibrillation.
Patients were enrolled from 110 centers in the United States, Canada, and Europe, and extended follow‐up with event adjudication was conducted until September 10, 2010.
The protocol from the MADIT‐CRT trial has previously been published (Moss et al., 2005).
2.2. Study population
The current study population was limited to the 698 CRT‐D patients from the MADIT‐CRT trial who had all undergone both pre‐implantation and 12‐month 24‐hour Holter monitoring (Figure 1). Follow‐up was conducted in this analysis from 12 months after implantation, until end‐of‐follow‐up (September 10, 2010) or the occurrence of the end point of interest.
Figure 1.
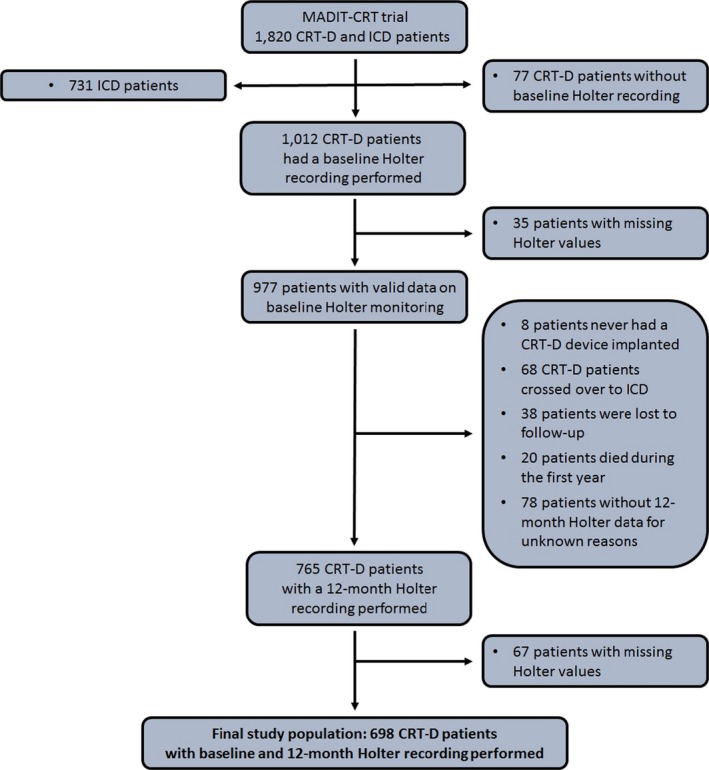
Study population flowchart. This flowchart depicts the selection of the 698 CRT‐D patients from the MADIT‐CRT trial that comprises the study population. Twenty‐four‐hour Holter recordings were performed. In the MADIT‐CRT study, Holter monitoring was limited to patients randomized to CRT‐D. CRT‐D, cardiac resynchronization therapy with defibrillator. ICD, implantable cardioverter defibrillator
2.3. Holter recordings and definition of ectopic burden
Mortara H12 12‐lead Holter recorders were used for all patients and data from the recordings were obtained by Mortara H‐Scribe scanning system (Mortara Instruments, Milwaukee, WI, USA). A blinded Rochester electrocardiographic core laboratory analyzed and adjudicated all Holter recordings, as previously described (Ruwald et al., 2014). The precise monitoring period and the presence of ventricular premature complexes (VPC) were quantified, and the mean number of beats per hour was calculated. All annotations of beats were performed by an experienced Holter technician. All paper copies of Holter records were reviewed by arrhythmologist (WZ) and cases with doubts regarding beat annotation or identification of arrhythmias were reexamined.
Based on previous reports (Bogun et al., 2007; Cairns, Connolly, Roberts, & Gent, 1997; Ruwald et al., 2014), we predefined low and high ectopic burden as the following: low ectopic burden ≤10 VPCs/hour and high ectopic burden >10 VPCs/hour.
In sensitivity analyses, we investigated the prognostic significance of changes in ectopic burden on outcome. Based on preimplantation and 12‐month postimplantation Holter recordings, we defined four groups of ectopic patterns:
Unchanged low burden, defined as patients who presented with both a low preimplantation and low 12‐month ectopic burden
Reduction in burden, defined as patients who presented with a high preimplantation ectopic burden and a low 12‐month ectopic burden
Increase in burden, defined as patients who presented with a low preimplantation ectopic burden and a high ectopic burden at 12‐month follow‐up
Unchanged high burden, defined as patients who presented with both a high preimplantation and high 12‐month ectopic burden.
2.4. Device implantation, programming, and interrogation
Boston Scientific CRT‐D devices were implanted using conventional transvenous implant methods and programmed according to a pre‐specified study protocol in DDD mode with a lower rate of 40 bpm and hysteresis off (Moss et al., 2005). It was recommended that AV‐delay be programmed according to the latest optimization techniques.
Devices were programmed with a ventricular tachycardia (VT) zone set at 180 beats per minute (bpm) with a detection time of 2.5 seconds (s), and a ventricular fibrillation (VF) zone set at 210 bpm with a 1.0 s detection time. All supraventricular tachycardia (SVT) discriminators and anti‐tachycardia pacing (ATP) were nominally programmed “on.” Sensitivity was set at the discretion of the implanting physician.
Patients were scheduled for follow‐up visits with device interrogation 1 month after implantation and thereafter every 3 months until the end of follow‐up. Interrogation discs were sent to an independent central core laboratory, where an arrhythmia adjudication committee adjudicated all arrhythmias and therapies according to predefined definitions of appropriate and inappropriate ICD therapy (Moss et al., 2005). Appropriate ICD therapy was defined as anti‐tachycardia pacing or ICD shock rendered for VT/VF.
2.5. End points
The primary end point of the current study was defined as nonfatal events constituted the secondary end point.
An independent HF and mortality adjudication committee reviewed all HF and death events according to prespecified classifications (Moss et al., 2005). A nonfatal HF event was defined as symptoms consistent with congestive HF with one of the following; 1. intravenous decongestive therapy for more than 2 hr without hospital admission, or 2. hospital admission with oral or intravenous decongestive therapy (Moss et al., 2005). Ventricular tachyarrhythmia events were defined as appropriate ICD therapy for VT/VF, as adjudicated by the independent arrhythmia adjudication committee. The physicians were encouraged to program the device to detect arrhythmias above or equal to 180 bpm; as a result, ventricular tachyarrhythmias <180 bpm were not considered in the current study.
2.6. Statistics
Baseline characteristics were compared between patients with a low and high ectopic burden at 12 months, using chi‐square test and Fisher's exact test for dichotomous variables and Kruskal–Wallis test for continuous variables.
Follow‐up started at 12 months, as a landmark analysis, and the cumulative risk of the end points over time was illustrated by the Kaplan–Meier method. Comparisons between groups were calculated using the log‐rank test.
Cox proportional hazard regression models were utilized to evaluate the relative risk of the end points between the groups, always starting follow‐up at 12 months. Separate models were fitted for each of the end points, with adjustment variables found by best subset regression, setting the limit for entry into the model at p < .05. For the end point of HF/death, the following variables entered the model: diabetes, left bundle branch block (LBBB) QRS morphology, male gender, hospitalizations 1 year prior to enrollment, glomerular filtration rate<60 ml/min/m2, and baseline LV end systolic volume indexed by body surface area (ml/m2). For the end point of VT/VF, we adjusted for prior ventricular arrhythmias, QRS duration, male gender, and baseline LV end systolic volume indexed by body surface area (ml/m2). Interactions were tested systematically and none were found. Logistic regression models were utilized to identify predictors of low ectopic burden at 12 months. Hazard ratios (HR) and odds ratios (OR) with their 95% confidence intervals (CI) and two‐sided p values are reported. A two‐tailed p value below .05 was considered statistically significant.
Analyses were performed using SAS statistical software 9.3 version (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Ectopic burden at 12 months
Out of 698 CRT‐D patients, 282 (40.4%) presented with low ectopic burden and 416 (59.6%) presented with high ectopic burden at 12 months. The mean follow‐up duration was 2.23 ± 0.98 years.
There were significant differences in baseline characteristics between patients with a low (≤10 VPCs/hour) and high ectopic burden (>10 VPCs/hour) at 12 months (Table 1). Patients with a low ectopic burden presented with younger age, more often had nonischemic cardiomyopathy and a lower utilization of diuretics, and were more commonly female.
Table 1.
Clinical characteristics in CRT‐D patients with a low or a high burden of ventricluar ectopy at 12‐month follow‐up
| Clinical Characteristics | Low burden N = 282 | High burden N = 416 |
|---|---|---|
| Female | 103 (37) | 75 (18)a |
| Age (years) | 62.4 ± 10.9 | 65.5 ± 10.2a |
| Left bundle branch block | 226 (80) | 273 (66)a |
| QRS duration at baseline (ms) | 162.0 ± 19.1 | 156.1 ± 19.0a |
| Heart rate (beats per minute) | 67.2 ± 10.9 | 67.6 ± 10.5 |
| Ectopic burden (VPCs/hour) (median with interquartile ranges) | 2.2 [0.5–5.0] | 68.0 [25.5–190.1] |
| BIV‐pacing ≥97% (average at the end of follow‐up) | 229 (83) | 218 (53) |
| Glomerular filtration rate (ml/min/1.73 m2) | 71.3 ± 21.3 | 69.5 ± 20.6 |
| Ischemic cardiomyopathy | 119 (42) | 250 (60)a |
| Prior myocardial infarction | 90 (32) | 190 (47)a |
| Diabetes | 83 (29) | 132 (32) |
| Hypertension | 172 (61) | 264 (64) |
| Past atrial arrhythmias | 28 (10) | 49 (12) |
| Past ventricular arrhythmias | 15 (5) | 31 (8) |
| Hospitalizations within the year prior to implantation | 124 (44) | 202 (49) |
| NYHA > II within 3 months prior to enrollment | 31 (11) | 42 (10) |
| Pharmacotherapy at baseline | ||
| Antiarrhythmic drugs including amiodarone and sotalol | 20 (7) | 36 (9) |
| Amiodarone | 19 (7) | 26 (6) |
| ACE inhibitor or ARB | 273 (97) | 398 (96) |
| Beta‐blocker | 269 (95) | 387 (93) |
| Digitalis | 68 (24) | 115 (28) |
| Diuretic | 180 (64) | 298 (72)a |
| Echocardiographic findings at baseline | ||
| Left ventricular ejection fraction (%) | 24.2 ± 5.2 | 23.9 ± 5.0 |
| LVEDV indexed by BSA (ml/m2) | 120.2 ± 24.9 | 125.5 ± 26.4a |
| LVESV Indexed by BSA (ml/m2) | 85.1 ± 20.4 | 89.6 ± 21.3a |
| LAV indexed by BSA (ml/m2) | 44.3 ± 9.4 | 47.1 ± 10.2a |
| Echocardiographic change from baseline to 1‐year follow‐up | ||
| Left ventricular ejection fraction increase (% points) | 12.9 ± 5.4 | 10.0 ± 5.0a |
| LVEDV % reduction (%) | 24.6 ± 11.7 | 18.6 ± 10.8a |
| LVESV % reduction (%) | 37.8 ± 14.9 | 29.4 ± 15.0a |
| LAV % reduction (%) | 31.6 ± 12.0 | 26.0 ± 12.2a |
| CRT‐D programming | ||
| Programmed AV delay (msec) | 114.1 ± 34.8 | 123.8 ± 38.6a |
| Cutoff rate of lowest VT zone (bpm) | 177.3 ± 8.0 | 176.6 ± 8.3 |
CRT‐D, cardiac resynchronization therapy with defibrillator; LVEDV, left ventricular end diastolic volume; BSA, body surface area; LVESV, left ventricular end systolic volume; LAV, left atrial volume; ACE, angiotensin‐converting enzyme; ARB, angiotensin‐II‐receptor blocker.
Values are presented as mean with standard deviations or as crude numbers with percentages in parenthesis.
p < .05.
Furthermore, they presented with echocardiographic smaller ventricular and atrial volumes, more often had LBBB QRS morphology, had longer QRS duration, and experienced the greatest level of reverse remodeling from baseline to 1‐year follow‐up. No other differences in comorbidities and pharmacotherapy were evident between the groups.
3.2. Association between postimplantation ectopic burden and the risk of VT/VF, HF/death, and death
Patients with a low ectopic burden at 12‐month postimplantation had a very low risk of HF/death and VT/VF, with 3‐year cumulative incidences of 7%and 8%, respectively, for the end points, significantly lower than for patients with high 12‐month ectopic burden whose 3‐year cumulative incidences were 25% and 24%, respectively (Figure 2). In multivariate Cox proportional hazard regression analyses, high ectopic burden was associated with a significant 2.8‐fold increased risk of HF/death and VT/VF, as compared to patients with low ectopic burden (Table 2). No differential effect was found between patients with ischemic or nonischemic cardiomyopathy (p interaction=.071), or between LBBB and non‐LBBB patients (p interaction=.287). To assess whether ectopic burden added additional prognostic value to echocardiographic measurements, we conducted a sensitivity analysis, adjusting for LVESV percentage change from baseline to 12‐month follow‐up, and found a significant association between the end points of HF/death and VT/VF and both LVESV percentage change (HF/death: HR = 1.03 [1.02–1.05], p < .001; VT/VF: HR = 1.02 [1.01–1.03], p = .003) and high ectopic burden (HR = 1.87 [1.02–3.45], p = .044; VT/VF: HR = 2.52 [1.37–4.63], p < .003).
Figure 2.
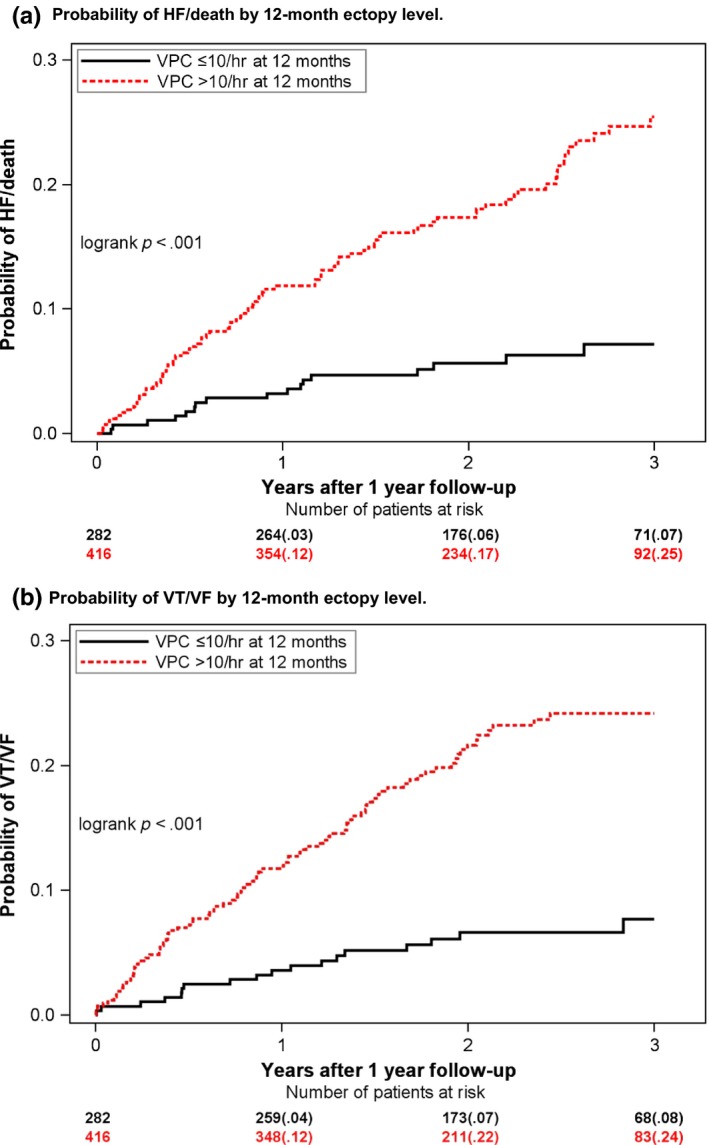
Probability of outcome by 12‐month ectopy level. Kaplan–Meier plots showing the probability of HF/death (a) and ventricular tachyarrhythmias (b) by low (black) or high (red) ectopic burden at 12‐month follow‐up. Ectopic level was evaluated by 24‐hour Holter monitoring at the 12‐month follow‐up visit. Low ectopic burden is defined as ≤ 10 ventricular ectopic beats per hour. High ectopic burden is defined as >10 ventricular ectopic beats per hour
Table 2.
Risk of HF/death and ventricular tachyarrhythmias by ectopic burden
| HF/death | VT/VF | |||||||
|---|---|---|---|---|---|---|---|---|
| Events/patients (105/698) | Hazard ratio | 95% CI | p Value | Events/patients (111/698) | Hazard ratio | 95% CI | p Value | |
| Ectopic burden at 12 months | ||||||||
| Ref: Low ectopic burden | 17/282 | 1.00 | 1.00 | NA | 20/282 | 1.00 | 1.00 | NA |
| High ectopic burden | 88/416 | 2.76 | 1.62–4.70 | <.001 | 91/416 | 2.79 | 1.69–4.58 | <.001 |
| Change in ectopic burden | ||||||||
| Ref: Unchanged low VPC burden | 11/200 | 1.00 | 1.00 | NA | 14/200 | 1.00 | 1.00 | NA |
| Reduction in VPC burden | 6/82 | 1.17 | 0.43–3.18 | .761 | 6/82 | 1.08 | 0.42–2.82 | .869 |
| Increase in VPC burden | 15/80 | 2.97 | 1.35–6.51 | .007 | 13/80 | 2.32 | 1.09–4.97 | .030 |
| Unchanged high VPC burden | 73/336 | 2.90 | 1.51–5.56 | .001 | 78/336 | 2.99 | 1.66–5.37 | <.001 |
CI, confidence intervals; VPC, ventricular premature complexes.
Cox proportional hazard regression model assessing the relative risk of the end points by ectopic burden.
Separate models were fitted for each end point. For the HF/death end point, we adjusted for diabetes, LBBB QRS morphology, gender, hospitalizations 1 year prior to enrollment, GFR≥60, and baseline LVESV indexed by BSA. For the end point of VT/VF, we adjusted for prior ventricular arrhythmias, QRS duration, gender, and LVESV indexed by body surface area.
We found no significant differences between patients who experienced increase in VPC burden and those who had an unchanged high VPC burden (HF/death: p = .937, VT/VF: p = .406).
Low VPC burden (≤10 VPCs/hour), high VPC burden (>10 VPCs/hour).
Only 47 patients died during the follow‐up, with significantly more in the high ectopic burden group (38, 9%), as compared with the low ectopic group (9, 3%). In multivariate analyses, high ectopic burden was associated with an increased risk of death although we did not have enough power to detect a significant difference between the groups (HR = 1.86 [0.87–3.95], p = .109).
3.3. Change in ectopic burden
In sensitivity analysis, we investigated the changes in ectopic burden from preimplantation Holter to 12‐month postimplantation Holter.
Out of 698 CRT‐D patients with a preimplantation and 12‐month Holter recording, 10 patients had no ectopy (0 VPCs) in pre‐implantation recording. Of the remaining 688 patients, 242 (35%) patients experienced a reduction ≥50% in the absolute number of VPCs/hour and 267 (40%) patients experienced an increase in ≥50% in the absolute number of VPCs/hour.
As described in the methods, we created four groups of change from preimplantation to 12 months burden. Within these groups, most patients had an unchanged high ectopic burden (>10 VPCs/hour) at 12 months (336 [48%] patients); 200 (29%) had an unchanged low ectopic burden (≤10 VPCs/hour) at 12 months, whereas 82 (12%) patients experienced a reduction (from >10 VPCs/hour to ≤10 VPCs/hour) and 80 (12%) patients experienced an increase in ectopic burden (from ≤10 VPCs/hour to >10 VPCs/hour).
Patient characteristics of all four groups of change are shown in the online appendix (Appendix S1).
Patients with an unchanged low ectopic burden and patients with a reduction in ectopic burden had a similar low risk of HF/death and VT/VF, with 3‐year cumulative incidences of 7%–8% for both end points, whereas patients with an unchanged high ectopic burden, and patients who experienced an increase in ectopic burden had a significantly higher risk of HF/death and VT/VF, with 3‐year cumulative incidences of 24%–25%. (Figure 3).
Figure 3.
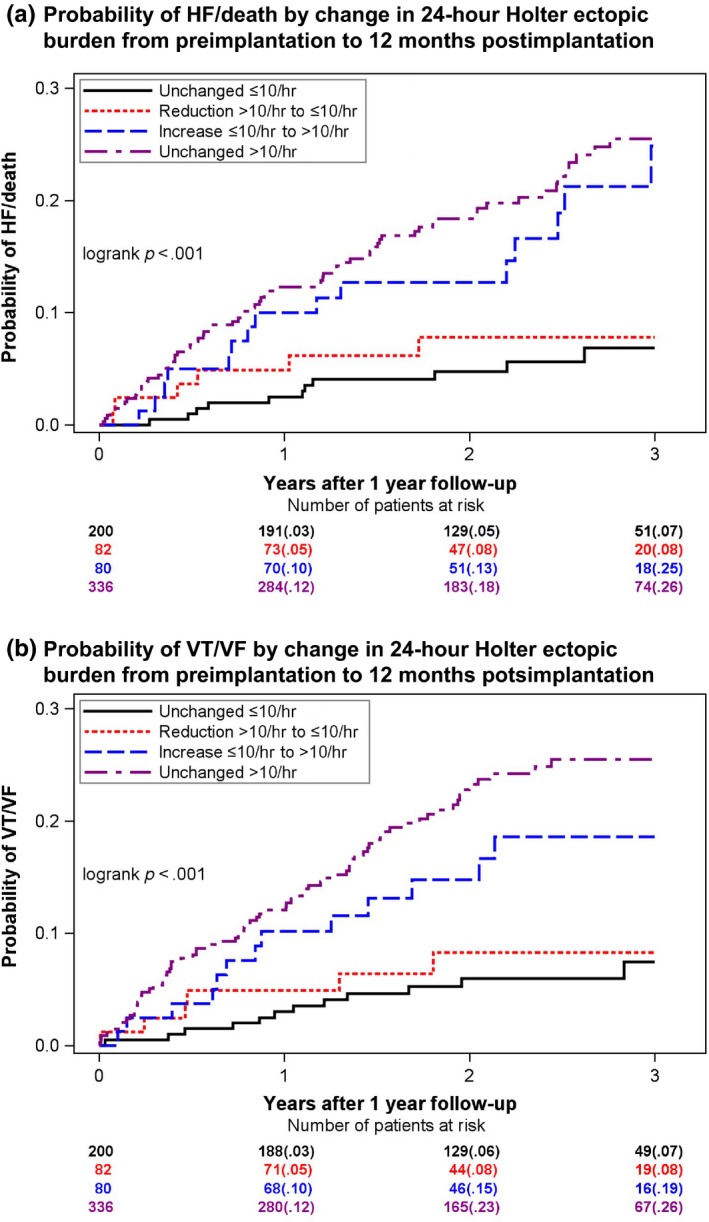
Probability of outcome by change in ectopic burden from preimplantation to 12‐month postimplantation 24‐hour Holter. Kaplan–Meier plots showing the probability of HF/death (a) and ventricular tachyarrhythmias (b) by four groups of changes in ventricular ectopic burden from preimplantation Holter to 12‐month follow‐up Holter. Ectopic burden was evaluated by 24‐hour Holter monitoring prior to device implantation and at the 12‐month follow‐up visit. Low ectopic burden is defined as ≤ 10 ventricular ectopic beats per hour. High ectopic burden is defined as >10 ventricular ectopic beats per hour. Groups of change: Unchanged low burden (black): Patients who presented with both a low preimplantation and low 12‐month ectopic burden. Reduced burden (red): Patients who presented with a high preimplantation ectopic burden and a low 12‐month ectopic burden. Increased burden (blue): Patients who presented with a low ectopic burden preimplantation and a high ectopic burden at 12‐month follow‐up. Unchanged high burden (purple): Patients who presented with both a high preimplantation and high 12‐month ectopic burden
Multivariate Cox proportional hazard regression analyses confirmed that unchanged high ectopic burden and increased ectopic burden were both associated with a similar and significant twofold to threefold increased risk of HF/death and VT/VF, as compared to patients with an unchanged low ectopic burden (Table 2). No difference in the risk of HF/death or VT/VF was evident between patients who experienced a reduction in ectopic burden and patients with an unchanged low ectopic burden (p = .761 and p = .869). The same was true for patients with an unchanged high ectopic burden and patients who experienced an increase in ectopic burden (p = .397 and p = .406), indicating that the 12‐month ectopic level was an equally good predictor of HF/death and VT/VF, as compared to groups of ectopic change. This was further tested statistically, where we found the two models (12‐month ectopic burden vs. groups of ectopic change) to be almost identical when comparing the −2log likelihood values (HF/death: 1,214.250 vs. 1,214.250 and VT/VF: 1,315.782 vs. 1,316.538).
3.4. Factors associated with low ectopic burden at 12 months
Using logistic regression, we identified independent predictors of low ectopic burden at 12 months. Female gender, LBBB QRS morphology, QRS duration>150 ms, blood urea nitrogen <25 mg/dl, younger age, and smaller left atrial volume all independently predicted low ectopic burden at 12 months (Table 3). Furthermore, low ectopy burden at 12 months was the single most significant factor associated with an overall high BIV‐pacing percentage (>97%) (OR = 6.39 [3.85–10.56], p < .001).
Table 3.
Factors associated with low ectopic burden at 12 months
| Odds ratio | 95% confidence interval | p Value | |
|---|---|---|---|
| Female gender | 2.29 | 1.58–3.32 | <.001 |
| BU ≤25 mg/dl | 1.68 | 1.12–2.50 | .012 |
| QRS ≥150 ms | 1.67 | 1.14–2.45 | .008 |
| Left bundle branch block | 1.60 | 1.06–2.40 | .025 |
| Reduction in age by decade (10 years) | 1.24 | 1.06–1.45 | .009 |
| Reduction in LVESV indexed by BSA (10 ml/m2) | 1.16 | 1.07–1.26 | <.001 |
VPC, ventricular premature complexes; LVESV, left ventricular end systolic volume; BSA, body surface area; BUN, blood urea nitrogen.
Low ectopic burden, ≤10 VPCs/hour.
4. DISCUSSION
In the current study, we aimed to evaluate the association between postimplantation 12‐month ectopic burden and clinical outcome in CRT patients. We found that patients with a high ectopic burden at 12 months after implantation had a significant and clinically relevant threefold increase in the risk of HF/death and VT/VF, when compared to patients with a low ectopic burden.
Furthermore, change in ectopic burden from preimplantation to 12 months after implantation was an equally good predictor of the end point, indicating that 12‐month ectopic burden predicts subsequent outcome, irrespective of preimplantation ectopic burden.
The majority of patients implanted with a CRT‐D device, who fulfill guideline criteria, derive a significant benefit from the device in terms of atrial and ventricular remodeling, improvement in NYHA symptoms, and overall better long‐term prognosis (Ge et al., 2014; Goldenberg et al., 2014). However, patients remain, who do not experience the expected clinical benefit from cardiac resynchronization therapy. The efficacy of the device relies on effective BIV‐capture, and thus electromechanical synchronization of the otherwise desynchronized ventricular contraction. Previous studies have shown that BIV‐pacing >90% is needed to derive benefit from a CRT‐D device although there is an additional significant clinical benefit in patients who achieve >97% BIV‐pacing (Hayes et al., 2011; Koplan et al., 2009; Ruwald et al., 2015). There are a substantial number of potentially modifiable factors that may affect the BIV‐capture, and thus the efficacy of the device. In recent studies from our group, we showed that even low ectopic levels measured prior to CRT‐D implantation was associated with a significant increase in HF events and/or death, less reverse remodeling, as well as a higher likelihood of BIV‐pacing <97% (Mittal et al., 2014; Ruwald et al., 2015; Ruwald, Mittal et al., 2014). Furthermore, Cheng et al. reported that frequent VPCs accounted for 17% of loss of BIV‐capture in patients implanted with a CRT device (Cheng et al., 2012). In the current study, a 12‐month low ectopic level was associated with 64% reduced risk of HF/death and VT/VF, as well as a six times higher likelihood of achieving BIV‐pacing >97%. These findings complement the previous reported associations between higher levels of ectopy resulting in lower percentages of BIV‐pacing (Cheng et al., 2012; Ruwald, Mittal et al., 2014). Furthermore, we found that female gender, left bundle branch block, longer QRS duration, lower age, smaller left atrial volumes, and superior renal status all independently predicted a 12‐month low ectopic burden. These factors have all previously been reported to be associated with superior CRT‐D response (van Bommel et al., 2009; Ge et al., 2014; Goldenberg et al., 2011; Ruwald et al., 2014; Van Bommel et al., 2011; Zareba et al., 2011).
This study emphasizes the importance of closely monitoring patients with regular follow‐ups after CRT implantation to optimize the benefit of the device. The results suggest that 24‐hour Holter monitoring may prove to be a valuable and cost‐effective method for evaluating the current ectopic status and estimating long‐term prognosis in mild heart failure patients implanted with a CRT‐D device. It has been shown that radiofrequency ablation improves the efficacy of CRT‐D in patients with frequent VPCs (Lakkireddy et al., 2012). Furthermore, some of these patients may benefit from antiarrhythmic drug suppression of ectopy or pharmacological optimization. Therefore, evaluating the burden of ectopy in CRT‐D patients, both before and after implantation, could help guide clinicians to appropriate interventions that may optimize BIV‐capture.
4.1. Clinical implications
Estimating prognosis in patients' post–device implantation is important. Twenty‐four hour Holter monitoring is an inexpensive and simple method for evaluating postimplantation ectopic burden that can be conducted in a wide variety of settings. The results from this study suggest that ectopic burden measured at 12 months after device implantation is valuable for estimating long‐term prognosis in CRT‐D patients with mild HF. Furthermore, the information provided by the Holter recordings may help guide clinicians with further treatment including pharmacological and/or radiofrequency ablation to reduce ectopic burden (Lakkireddy et al., 2012) and/or device programming modifications to potentially improve prognosis. However, the assessment of efficacy of such interventions was not the scope of this study, and thus further studies are needed to evaluate these aspects of patient management.
4.2. Limitations
In the current study, we only included patients with a 12‐month 24‐hour Holter monitoring. We therefore exclude all patients who died prior to the 12‐month follow‐up, which could potentially create a survival bias. However, there were only 20 deaths in CRT‐D patients during the first year, while we do not believe it would have had a significant effect on our results. Although we have tried to adjust for factors that could have affected our results, a risk of unmeasured confounding remains.
Lastly, in the MADIT‐CRT trial, only patients randomized to CRT‐D implantation received a 24‐hour Holter monitoring; therefore, we are unable to evaluate the specific effects of CRT‐D on changes in ectopic burden, since we do not have a comparisons group of ICD patients. Furthermore, it is difficult to establish whether the higher risk of the end points in the group with a high ectopy burden, is due to an ectopy‐driven attenuated efficacy of the CRT‐D device, or if the ectopy burden in itself contribute with independent risk.
5. CONCLUSION
In CRT‐D patients with preexisting ischemic or nonischemic cardiomyopathy and mild heart failure, high ectopic burden (>10 VPCs/hour) at 12‐month follow‐up was associated with a 24% and 25% 3‐year risk of HF/death and VT/VF, respectively, and a threefold increased relative risk of the end points as compared to patients with a low burden (≤ 10 VPCs/hour). Ectopic burden at 12 months may be a valuable approach for evaluating prognosis, and may help identify patients with high ectopic burden that could benefit from appropriate treatment interventions to optimize CRT‐D efficacy. However, further studies are needed to evaluate the potential efficacy of such interventions.
CONFLICT OF INTEREST
This research was performed while Dr. Anne‐Christine Ruwald was a Mirowski‐Moss Awardee. Dr. Anne‐Christine Ruwald has received unrestricted travel grants from The Denmark‐America Foundation, Falck Denmark, the Lundbeck‐Foundation, Bønnelykkefonden, Carl and Ellen Hertz Grant and Torben and Alice Frimodts Foundation. She has further received grant support from Biotronik and travel support from Medtronic.
Dr. Zareba and Dr. Moss reports receiving grant support from Boston Scientific.
Dr. Jons reports receiving fellowship support from Boston Scientific.
Dr. Mittal and Dr. Steinberg reports receiving consulting fees from Boston Scientific.
No other potential conflict of interest relevant to this article was reported.
Supporting information
ACKNOWLEDGMENTS
The authors would like to acknowledge the great work of Bronislava Polonsky. Her skills in statistical programming and data management are an invaluable asset to The University of Rochester, Heart Research Follow‐up Program and without her, we would not be able to have such a productive team of researchers.
Ruwald AC, Aktas MK, Ruwald MH, et al. Postimplantation ventricular ectopic burden and clinical outcomes in cardiac resynchronization therapy‐defibrillator patients: a MADIT‐CRT substudy. Ann Noninvasive Electrocardiol. 2018;23:e12491 10.1111/anec.12491
Funding
The MADIT‐CRT study was supported by a research grant from Boston Scientific to the University of Rochester, with funds distributed to the coordination and data center, enrolling centers, core laboratories, committees, and boards under subcontracts from the University of Rochester.
Clinical trials.gov identifier: NCT00180271..
REFERENCES
- Abraham, W. T. , Fisher, W. G. , Smith, A. L. , Delurgio, D. B. , Leon, A. R. , Loh, E. , & Messenger, J. (2002). Cardiac resynchronization in chronic heart failure. The New England Journal of Medicine, 346(24), 1845–1853. 10.1056/nejmoa013168 [DOI] [PubMed] [Google Scholar]
- Bogun, F. , Crawford, T. , Reich, S. , Koelling, T. M. , Armstrong, W. , Good, E. , & Morady, F. (2007). Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart rhythm: the official journal of the Heart Rhythm Society, 4(7), 863–867. 10.1016/j.hrthm.2007.03.003 [DOI] [PubMed] [Google Scholar]
- van Bommel, R. J. , Bax, J. J. , Abraham, W. T. , Chung, E. S. , Pires, L. A. , Tavazzi, L. , & Ghio, S. (2009). Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: A PROSPECT (Predictors of Response to CRT) sub‐analysis. European Heart Journal, 30(20), 2470–2477. 10.1093/eurheartj/ehp368 [DOI] [PubMed] [Google Scholar]
- Brignole, M. , Auricchio, A. , Baron‐Esquivias, G. , Bordachar, P. , Boriani, G. , Breithardt, O. A. , & Wilson, C. M. (2013). 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). European Heart Journal, 34(29), 2281–2329. 10.1093/eurheartj/eht150 [DOI] [PubMed] [Google Scholar]
- Cairns, J. A. , Connolly, S. J. , Roberts, R. , & Gent, M. (1997). Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet, 349(9053), 675–682. [DOI] [PubMed] [Google Scholar]
- Cheng, A. , Landman, S. R. , & Stadler, R. W. (2012). Reasons for loss of cardiac resynchronization therapy pacing: Insights from 32 844 patients. Circulation Arrhythmia and Electrophysiology, 5(5), 884–888. 10.1161/circep.112.973776 [DOI] [PubMed] [Google Scholar]
- Ge, Y. , Ruwald, A. C. , Kutyifa, V. , McNitt, S. , Polonsky, S. , Klein, H. , & Moss, A. J. (2014). A metric for evaluating the cardiac response to resynchronization therapy. American Journal of Cardiology, 113(8), 1371–1377. 10.1016/j.amjcard.2014.01.410 [DOI] [PubMed] [Google Scholar]
- Goldenberg, I. , Kutyifa, V. , Klein, H. U. , Cannom, D. S. , Brown, M. W. , Dan, A. , & Moss, A. J. (2014). Survival with cardiac‐resynchronization therapy in mild heart failure. The New England Journal of Medicine, 370(18), 1694–1701. 10.1056/nejmoa1401426 [DOI] [PubMed] [Google Scholar]
- Goldenberg, I. , Moss, A. J. , Hall, W. J. , Foster, E. , Goldberger, J. J. , Santucci, P. , & Klein, H. (2011). Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT‐CRT). Circulation, 124(14), 1527–1536. 10.1161/circulationaha.110.014324 [DOI] [PubMed] [Google Scholar]
- Hayes, D. L. , Boehmer, J. P. , Day, J. D. , Gilliam, F. R. 3rd , Heidenreich, P. A. , Seth, M. , & Saxon, L. A. (2011). Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart rhythm: the official journal of the Heart Rhythm Society, 8(9), 1469–1475. 10.1016/j.hrthm.2011.04.015 [DOI] [PubMed] [Google Scholar]
- Koplan, B. A. , Kaplan, A. J. , Weiner, S. , Jones, P. W. , Seth, M. , & Christman, S. A. (2009). Heart failure decompensation and all‐cause mortality in relation to percent biventricular pacing in patients with heart failure: Is a goal of 100% biventricular pacing necessary? Journal of the American College of Cardiology, 53(4), 355–360. 10.1016/j.jacc.2008.09.043 [DOI] [PubMed] [Google Scholar]
- Lakkireddy, D. , Di Biase, L. , Ryschon, K. , Biria, M. , Swarup, V. , Reddy, Y. M. , & Natale, A. (2012). Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. Journal of the American College of Cardiology, 60(16), 1531–1539. 10.1016/j.jacc.2012.06.035 [DOI] [PubMed] [Google Scholar]
- Mittal, S. , Aktas, M. K. , Moss, A. J. , McNitt, S. , Kutyifa, V. , Steinberg, J. S. , & Zareba, W. (2014). The impact of nonsustained ventricular tachycardia on reverse remodeling, heart failure, and treated ventricular tachyarrhythmias in MADIT‐CRT. Journal of Cardiovascular Electrophysiology, 25(10), 1082–1087. 10.1111/jce.12456 [DOI] [PubMed] [Google Scholar]
- Moss, A. J. , Brown, M. W. , Cannom, D. S. , Daubert, J. P. , Estes, M. , Foster, E. ,… Zareba, W. . (2005). Multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy (MADIT‐CRT): Design and clinical protocol. Annals of Noninvasive Electrocardiology: The Official Journal of the sInternational Society for Holter and Noninvasive Electrocardiology, Inc, 10(Suppl 4), 34–43. 10.1111/j.1542-474x.2005.00073.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padeletti, L. , Paoletti Perini, A. , & Gronda, E. (2012). Cardiac resynchronization therapy: The issue of non‐response. Heart Failure Reviews, 17(1), 97–105. 10.1007/s10741-011-9250-6 [DOI] [PubMed] [Google Scholar]
- Ruwald, A. C. , Kutyifa, V. , Ruwald, M. H. , Solomon, S. , Daubert, J. P. , Jons, C. , & Zareba, W. (2015). The association between biventricular pacing and cardiac resynchronization therapy‐defibrillator efficacy when compared with implantable cardioverter defibrillator on outcomes and reverse remodelling. European Heart Journal, 36(7), 440–448. 10.1093/eurheartj/ehu294 [DOI] [PubMed] [Google Scholar]
- Ruwald, M. H. , Mittal, S. , Ruwald, A. C. , Aktas, M. K. , Daubert, J. P. , McNitt, S. , & Zareba, W. (2014). Association between frequency of atrial and ventricular ectopic beats and biventricular pacing percentage and outcomes in patients with cardiac resynchronization therapy. Journal of the American College of Cardiology, 64(10), 971–981. 10.1016/j.jacc.2014.06.1177 [DOI] [PubMed] [Google Scholar]
- Ruwald, M. H. , Solomon, S. D. , Foster, E. , Kutyifa, V. , Ruwald, A. C. , Sherazi, S. , & Zareba, W. (2014). Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: Results from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT‐CRT) trial. Circulation, 130(25), 2278–2286. 10.1161/circulationaha.114.011283 [DOI] [PubMed] [Google Scholar]
- Van Bommel, R. J. , Mollema, S. A. , Borleffs, C. J. , Bertini, M. , Ypenburg, C. , Marsan, N. A. , & Bax, J. J. (2011). Impaired renal function is associated with echocardiographic nonresponse and poor prognosis after cardiac resynchronization therapy. Journal of the American College of Cardiology, 57(5), 549–555. 10.1016/j.jacc.2010.06.060 [DOI] [PubMed] [Google Scholar]
- Zareba, W. , Klein, H. , Cygankiewicz, I. , Hall, W. J. , McNitt, S. , Brown, M. , & Moss, A. J. (2011). Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial‐Cardiac Resynchronization Therapy (MADIT‐CRT). Circulation, 123(10), 1061–1072. 10.1161/circulationaha.110.960898 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


