Abstract
Background
New‐onset atrial fibrillation (NOAF) is a common complication in the setting of ST segment elevation myocardial infarction (STEMI), and worsened short/long‐term prognosis. Several clinical parameters have already been associated with NOAF development. However, relationship between NOAF and coronary artery disease (CAD) severity in STEMI patients is unclear. This study evaluates the relationship between NOAF and CAD severity using Syntax score (SS) and Syntax score II (SSII) in STEMI patients who were treated with primary percutaneous coronary intervention (pPCI).
Method
We enrolled 1,565 consecutive STEMI patients who were treated with pPCI. Patients with NOAF were compared to patients without NOAF in the entire study population and in a matched population defined by propensity score matching.
Results
Patients with NOAF had significantly higher SS and SSII than those without, both in the matched population (18.6 ± 4 vs 16.75 ± 3.6; p < .001 and 42 ± 13.4 vs 35.1 ± 13.1; p < .001, respectively), and in all study population (18.6 ± 4 vs 16.5 ± 4.6; p < .001 and 42 ± 13.3 vs 31.5 ± 11.9; p < .001 respectively). SSII, compared to its components, was the only independent predictor of NOAF (OR: 1,041 95% CI: 1.015–1.068; p = .002). In the long‐term follow‐up, all‐cause long‐term mortality was significantly higher in patients with NOAF than those without NOAF (23.3% vs. 11%; p = .032).
Conclusion
This is the first study to comprehensively examine the relationship between NOAF development and CAD severity using SS and SSII. We demonstrated that, in STEMI patients, high SSII was significantly related to NOAF and was an independent predictor of NOAF. Furthermore, patients with NOAF were associated with poor prognosis.
Keywords: atrial fibrillation, ST elevation myocardial infarction, SYNTAX score, SYNTAX score II
1. INTRODUCTION
New‐onset atrial fibrillation (NOAF) is a common complication of acute ST segment elevation myocardial infarction (STEMI). Its incidence varies between 2.3% and 21% depending on the type of the study group, the diagnostic method, and treatment modality used (Schmitt, Duray, Gersh, & Hohnloser, 2009). Previous studies revealed that NOAF development in patients with STEMI was associated with worsened short/long‐term prognosis (Crenshaw et al., 1997; Kinjo et al., 2003; Wong et al., 2003). Several clinical parameters were found to be associated with NOAF development including older age, female gender, history of diabetes mellitus (DM), extensive myocardial damage, higher Killip class, increased heart rate (HR), decreased systolic blood pressure (BP), post PCI <3 thrombolysis in myocardial infarction (TIMI) flow, number of diseased vessels, impaired left ventricular ejection fraction (LVEF) (Crenshaw et al., 1997; Kinjo et al., 2003; Mrdovic et al., 2012; Wong et al., 2003). However, there is limited data regarding the relationship between coronary artery disease (CAD) severity and NOAF development in the setting of STEMI.
Syntax score (SS) is a worldwide used anatomical scoring system which grades CAD severity according to the number of lesions along with their functional impact, location, and complexity (Sianos et al., 2005). The idea of adding clinical variables, determined by applying a Cox proportional hazards model to the results of the Syntax trial, has generated Syntax score II (SSII) (Farooq et al., 2013). SS and SSII have been studied in STEMI patients and both were found to be associated with long term mortality and major adverse cardiac events (Magro et al., 2011; Wang et al., 2016). The purpose of the present study is to investigate the relationship between NOAF development and CAD severity by using SS and SSII in STEMI patients who were treated with primary percutaneous coronary intervention (pPCI).
2. METHOD
2.1. Study population
We conducted this study by retrospectively enrolling 1,847 patients with STEMI who underwent pPCI between January 2010 and June 2016, at Kafkas, and Erzurum Ataturk Universities in Turkey. STEMI was defined based on the following criteria: typical increase or decrease in cardiac biomarkers; ongoing ischemic symptoms (within 12 hr of presentation); newly developed left bundle‐branch block pattern; or a new ST elevation in two or more contiguous leads, with readings of at least 0.2 mV in leads V1, V2, and V3, or at least 0.1 mV in the remaining leads (Steg et al., 2012). Of the enrolled patients 262 were excluded for various reasons. First, 62 were excluded due to already having a documented AF or being diagnosed with AF prior to admission. The remaining 1,785 patients’ ECGs showed sinus rhythm on admission. However, of those, 154 were excluded for having been treated with emergent coronary artery bypass graft (CABG) surgery, or having a previous history of CABG; and finally 66 were excluded due to missing clinical and/or long‐term follow‐up data from their hospital files and computer records; thereby, leaving us with 1,565 patients subject to the study. Long‐term follow‐up data were obtained from hospital records and phone interviews. For patients we were not able to reach, we gathered information from the National Institute of Statistics, and the Registrar of Birth Records to determine whether they were deceased. The study protocol was reviewed and approved by the Local Ethics Committee of the Kafkas University in accordance with the Declaration of Helsinki.
2.2. Data collection
Baseline clinical and demographic characteristics, and patients’ medical history data were obtained from the hospital records. Records indicated that complete blood count and blood biochemical parameters were measured in all patients on admission to the hospital. Blood samples were retested for troponin T and creatine kinase myocardial band (CK‐MB) every 6 hr, until peak levels were detected. Afterwards, these tests, along with hemograms and creatinine tests, were repeated daily. Estimated glomerular filtration rate (eGFR) was determined using Cockroft‐Gault formula from blood sample obtained on admission. Electrocardiograms (ECG), which were obtained via surface ECG or monitor/defibrillator records (with an ECG readout of at least 30 seconds), were recorded at admission, in the intensive care unit 48–72 hr after pPCI and during hospitalization. New‐onset atrial fibrillation (NOAF) was defined as the arrhythmia developing after hospital admission that included irregular RR intervals on ECG; absence of identifiable P waves, with an unidentifiable isoelectric line; and atrial rhythm >300 beats per minute (Camm et al., 2010). Left ventricular ejection fraction (LVEF) was defined as the postprocedural ejection fraction, and was assessed using a modified Simpson's method (Lang et al., 2005).
2.3. Angiographic analysis
All patients underwent selective coronary angiography, using the Judkins percutaneous trans‐femoral technique. All patients received, on a routine basis, 300 mg acetylsalicylic acid and a 600 mg loading dose of clopidogrel before the intervention, and unfractionated heparin during the intervention. The decision as to whether to use tirofiban was left to the operator's discretion. Culprit lesions were treated with stent implantation and balloon angioplasty if necessary. Coronary angiograms were recorded in digital media for quantitative analysis (Dicom‐viewer; MedCom GmbH, Darmstadt, Germany). Digital angiograms were analyzed by two, independent and experienced, interventional cardiologists, who were blinded to all the data. In case of disagreement, the final decision was made by consensus. Each lesion ≥1.5 mm in diameter and with ≥50% stenosis was scored using the online SS Calculator, version 2.1 (http://www.syntaxscore.com). Because the initial SS algorithm excluded patients with STEMI, we followed an alternate definition which was used in a study of STEMI patients (Magro et al., 2011). In this definition, an occluded infarct related artery is scored as an occluded artery with less than 3‐months duration. SSII was calculated using online calculator (http://www.syntaxscore.com), consisting of two anatomical variables (anatomical SS and unprotected left main coronary artery [LMCA] disease) and six clinical variables (age, creatinine clearance, LVEF, sex, chronic obstructive pulmonary disease (COPD), and peripheral arterial disease (PAD) (Farooq et al., 2013). Coronary blood flow patterns before and after pPCI were subjected to a thorough evaluation on the basis of TIMI flow grade using grades 0, 1, 2, and 3 (Gibson et al., 1996).
2.4. Statistical analysis
SPSS version 22.0 (SPSS Inc. Chicago, Illinois, USA) was used for statistical analysis. Continuous and categorical variables are expressed as mean ± SD and percentages respectively. Differences in patient characteristics between the patients with and without NOAF were analyzed using the t‐test or Mann–Whitney U test for continuous variables, and the chi‐square test for categorical variables. Because the study was nonrandomized, a conditional logistic regression model with propensity scores was created with variables that were shown to be associated with NOAF, excluding clinical variables of SSII in patients with STEMI. This was done to balance patient characteristics and to perform propensity‐matched analysis of the patients with and without NOAF. The variables used in this model were as follows: Diabetes mellitus (DM), hypertension (HT), smoking, Killip class on admission, heart rate, systolic BP, postprocedural TIMI grade, peak CKMB.
In matched population, using multiple logistic regression analysis (stepwise forward likelihood ratio regression [ain = 0.05, aout = 0.10]) two models were constructed for the NOAF prediction. The first model was constructed with the components of SSII (age, EF, SS), but without including SSII itself. The second model was constructed by including all the variables that were significant in univariate analysis (age, EF, SS, SSII), with no exclusion. Multicollinearity between SSII and its components was assessed by Eigen value and condition index. Linearity is tested by interacting with the logarithmic transformation of each parameter itself.
Receiver operating characteristics (ROC) curve analysis was performed to calculate the optimal cutoff value of SSII score for predicting NOAF. Survival curves were calculated using the Kaplan–Meier method. Statistical significance was assessed using log‐rank tests. Cox regression analyses were used to identify associations between NOAF and all‐cause long term mortality.
3. RESULT
The study population consisted of 1,565 STEMI patients (mean age: 57 ± 12 years; 79.7% males) who underwent pPCI. New‐onset atrial fibrillation (NOAF) developed in 86 patients (5.8%) of those, during the index hospitalization. Demographic, clinical, laboratory, and coronary angiographic characteristics of patients with NOAF, patients without NOAF, and all patients, are listed in Table 1. Both SS and SSII were significantly higher in patients with NOAF than those without (18.6 ± 4 vs 16.5 ± 4.6; p < .001 and 42 ± 13.3 vs 31.5 ± 11.9; p < .001 respectively). Patients with NOAF were older, had a more frequent history of DM and HT, and had a higher percentage of smoking compared to those without NOAF. Furthermore, compared to patients without NOAF, patients with NOAF had a higher Killip class on admission; longer telemetry monitoring time; lower postprocedural TIMI grade; higher white blood cell (WBC) count; higher level of peak CK‐MB and troponin; and lower hemoglobin, eGFR and LVEF.
Table 1.
Demographic, clinical, laboratory, and coronary angiographic characteristics of all patients, patients with and without NOAF with p value
| New‐onset atrial fibrillation | p Value | |||
|---|---|---|---|---|
| All patients (n = 1,565) | (−;n = 1,479) | (+;n = 86) | ||
| Age (years) | 57 ± 12 | 56 ± 12 | 63 ± 12 | <.001 |
| Male n (%) | 1,247 (79.7) | 1,184 (80.1) | 63 (73.3) | .128 |
| Hypertension n (%) | 629 (40.2) | 575 (38.9) | 54 (62.8) | <.001 |
| DM n (%) | 391 (25) | 357 (24.1) | 34 (39.5) | .001 |
| COPD n (%) | 73 (4.7) | 66 (4.5) | 7 (8.1) | .116 |
| PAD n (%) | 282 (18) | 260 (17.6) | 22 (25.6) | .061 |
| Dyslipidemia n (%) | 622 (39.7) | 593 (40.1) | 29 (33.7) | .240 |
| Smoking n (%) | 842 (53.8) | 811 (54.8) | 31 (36) | .001 |
| Killip class > 1 on admission n (%) | 259 (16.5) | 226 (15.3) | 33 (38.4) | <.001 |
| SBP (mm Hg) | 131 ± 31 | 131 ± 30 | 133 ± 40 | .563 |
| Heart rate (bpm) | 77 ± 16 | 77 ± 15 | 82 ± 19 | .008 |
| eGFR (ml/min) | 87.58 ± 26 | 88.1 ± 25.7 | 77.1 ± 28.8 | <.001 |
| WBC count (103/μl) | 12.3 ± 3.7 | 12.2 ± 3.7 | 13.7 ± 4.4 | <.001 |
| Hemoglobin (g/dl) | 13.7 ± 1.8 | 13.7 ± 1.8 | 13.2 ± 2.1 | .006 |
| Peak CK‐MB (U/L) | 178 (100–306) | 173 (98–298) | 344 (187–430) | <.001 |
| Peak troponin I (ng/ml) | 81 (38–175) | 78 (36–167) | 165 (87–253) | <.001 |
| LVEF (%) | 47 ± 8 | 47 ± 8 | 40 ± 8 | <.001 |
| IRA (%) | ||||
| LAD | 775 (49.5) | 727 (49.2) | 48 (55.8) | .254 |
| Cx | 205 (13.1) | 198 (13.4) | 7 (8.1) | |
| RCA | 557 (35.6) | 526 (35.6) | 31 (36.0) | |
| Other | 28 (1.8) | 28 (1.9) | 0 (0) | |
| LMCA disease (%) | 23 (1.5) | 23 (1.6) | 0 (0) | .244 |
| Post pPCI TIMI grade <3 n (%) | 201 (11.20) | 179.00 (10.50) | 22.00 (25.60) | <.001 |
| Duration of telemetry monitoring (days) | 2.00 (1.50–3.00) | 2.00(1.50–3.00) | 3.00 (2.00–5.00) | <.001 |
| Syntax score | 16.70 ± 4.6 | 16.59 ± 4.6 | 18.63 ± 4 | <.001 |
| Syntax score II | 32.1 ± 12.3 | 31.52 ± 11.9 | 42.09 ± 13.3 | <.001 |
DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; PAD, peripheral artery disease; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; WBC, white blood cell; CK‐MB, Creatine kinase‐myocardial band; LVEF, left ventricular ejection fraction; IRA, infarct related artery; LAD, left anterior descending; Cx, circumflex; RCA, right coronary artery; LMCA, left main coronary artery; SYNTAX, Synergy between PCI with Taxus and Cardiac Surgery.
To account for the significantly higher number of patients without NOAF than those with, and eliminate the effects of the variables that were earlier found to be associated with NOAF development (excluding clinical variables of SSII), we performed propensity score matching. As a result of this, 84 patients were selected from among those without NOAF, and matched with the 86 patients with NOAF. In the matched population, SS and SSII were significantly higher in patients with NOAF than those without (18.6 ± 4 vs 16.75 ± 3.6; p < .001 and 42 ± 13.4 vs 35.1 ± 13.1; p < .001 respectively). The mean age of patients was higher and LVEF was lower in patients with NOAF than those without NOAF. Demographic, clinical, and coronary angiographic characteristics of the matched population are listed in Table 2. Finally, no statistically significant difference was found in terms of telemetry monitoring between patients with and without NOAF (2.5 [range: 1.5–3.5] vs. 3 [range: 1.5–4.0]; p = .06).
Table 2.
Demographic, clinical, laboratory, and coronary angiographic characteristics of patients with and without NOAF with p value in matched population
| Patients without NOAF (n = 84) | Patients with NOAF (n = 86) | p Value | |
|---|---|---|---|
| Age (years) | 59 ± 12 | 63 ± 12 | .015 |
| Male n (%) | 63 (81.8) | 63 (73.3) | .193 |
| Hypertension n (%) | 49 (63.6) | 54 (62.8) | .911 |
| DM n (%) | 26 (33.8) | 34 (39.5) | .446 |
| COPD n (%) | 5 (6.5) | 7 (8.1) | .688 |
| PAD n (%) | 19 (24.7) | 22 (25.6) | .894 |
| Dyslipidemia n (%) | 34 (44.2) | 31 (36) | .291 |
| Smoking n (%) | 35 (45.5) | 29 (33.7) | .126 |
| Killip class > 1 on admission n (%) | 24 (31.2) | 33 (38.4) | .336 |
| SBP (mm Hg) | 139 ± 41 | 133 ± 40 | .321 |
| Heart rate (bpm) | 78 ± 19 | 81 ± 19 | .365 |
| eGFR (ml/min) | 86 ± 29 | 77 ± 28 | .506 |
| WBC count (103/μl) | 13.3 ± 4.3 | 13.8 ± 4.4 | .265 |
| Hemoglobin (g/dl) | 13.5 ± 1.9 | 13.2 ± 2.1 | .097 |
| Peak CK‐MB (U/L) | 241 (102–398) | 344 (187–430) | .405 |
| Peak troponin I (ng/ml) | 108 (47–216) | 165 (87–253) | .081 |
| LVEF (%) | 44 ± 9 | 40 ± 8 | .005 |
| IRA (%) | |||
| LAD | 46 (59.7) | 48 (55.8) | .629 |
| Cx | 7 (9.1) | 7 (8.1) | |
| RCA | 23 (29.9) | 31 (36) | |
| Other | 1 (1.30) | 0 (0.0) | |
| LMCA disease (%) | 1 (1.3) | 0 (0) | .354 |
| Post‐pPCI TIMI grade <3 n (%) | 9 (11.1) | 22 (25.6) | .015 |
| Duration of telemetry monitoring (days) | 2.50 (1.50–3.50) | 3.00 (1.50–4.00) | .06 |
| SYNTAX score | 16.7 ± 3.6 | 18.6 ± 4 | .002 |
| SYNTAX score II | 35.1 ± 13.1 | 42 ± 13.4 | .001 |
DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; PAD, peripheral artery disease; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; WBC, white blood cell; CK‐MB, creatine kinase‐myocardial band; LVEF, left ventricular ejection fraction; IRA, infarct related artery; LAD, left anterior descending; Cx, circumflex; RCA, right coronary artery; LMCA, left main coronary artery; SYNTAX, Synergy between PCI with Taxus and Cardiac Surgery; NOAF, new‐onset atrial fibrillation.
In the matched population, multivariate regression analysis was used to determine the independent predictors of NOAF development. Age, LVEF, post‐PCI TIMI grade 3, SS, and SSII were significantly associated with NOAF in univariate analysis. In the first multivariate model, age, LVEF, SS were independent predictors of NOAF, whereas post PCI TIMI grade 3 coronary flow, was not. On the other hand, in the second model, SSII, which is calculated from SS, age, LVEF, and other clinical parameters, was found to be the only independent predictor of NOAF (OR: 1,041 95% CI: 1.015–1.068; p = .002). The results of univariate and multivariate analysis are listed in Table 3.
Table 3.
Multivariate logistic regression analysis of the matched population. Independent predictors of new‐onset atrial fibrillation (NOAF) with univariate and multivariate p value, OR with 95% CI
| Variable | Univariate analysis of NOAF | Multivariate analysis of NOAF (first model) | Multivariate analysis of NOAF (second model) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p Value | Odds ratio | 95% CI | p Value | Odds ratio | 95% CI | p Value | |
| Age (years) | 1.040 | 1.012–1.068 | .004 | 1.043 | 1.015–1.072 | .003 | – | – | |
| LVEF (%) | 0.949 | 0.915–0.984 | .005 | 0.957 | 0.920–0.995 | .026 | – | – | |
| Post‐PCI TIMI grade 3 flow | 0.359 | 0.154–0.835 | .017 | – | – | – | – | – | |
| Syntax score/per unit | 1.097 | 1.014–1.187 | .021 | 1.103 | 1.010–1.203 | .029 | – | – | |
| Syntax score II/per unit | 1.043 | 1.016–1.072 | .002 | 1.041 | 1.015–1.068 | .002 | |||
LVEF, left ventricular ejection fraction; Post‐PCI TIMI flow grade, postpercutaneous coronary intervention, thrombolysis in myocardial infarction flow grade; Syntax, Synergy between PCI with Taxus and Cardiac Surgery.
Receiver operating characteristics (ROC) curve analysis was performed to determine SSII cutoff value to predict NOAF in the matched population. The cutoff value of SSII for NOAF prediction was found to be 40.6 with sensitivity of 55.3% and specificity of 71.1% (AUC: 0.667 95% CI: 0.589–0.740; p = .003) (Figure 1).
Figure 1.
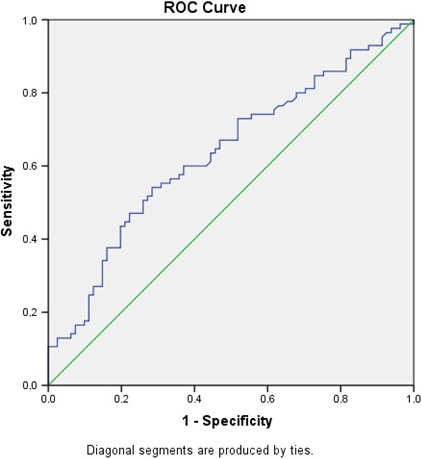
Receiver operating characteristics (ROC) graphics to detect best cutoff value of Syntax score II (SSII) for new‐onset atrial fibrillation (NOAF) prediction
In the study, NOAF patients were older, had higher SS and lower LVEF. Cox regression analysis was performed to determine whether NOAF was a risk factor independent of these parameters in long‐term mortality. In multivariate analysis, NOAF was found to be a risk factor for long‐term mortality independent of age and LVEF (HR: 2.246 95% CI: 1.022–4.936; p = .044). The results of Cox regression analysis are listed in Table 4.
Table 4.
Adjusted and unadjusted hazards ratios for long‐term mortality
| Variable | Univariate analysis of long term mortality | Multivariate analysis of long term mortality | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p Value | Hazard ratio | 95% CI | p Value | |
| Age (years) | 1.041 | 1.009–1.073 | .011 | 1.037 | 1.006–1.070 | .019 |
| LVEF (%) | 0.881 | 0.841–0.923 | <.001 | 0.882 | 0.841–0.924 | <.001 |
| Syntax score/per unit | 1.06 | 0.982–1.144 | .138 | – | – | – |
| NOAF | 2.278 | 1.802–5.005 | .023 | 2.246 | 1.022–4.936 | .044 |
LVEF, left ventricular ejection fraction; NOAF, new‐onset atrial fibrillation.
The mean follow‐up was 30.8 ± 16.3 months. Twenty‐nine patients (17.3%) died during long‐term follow‐up. The incidence of all cause long‐term mortality was significantly higher in patients with NOAF than those in patients without NOAF (23.3% vs 11%; p = .032) (Figure 2). The matched group was stratified into 3 groups according to their SSII values. Low (SSII < 31.6 n: 56), moderate (SSII: 31.6–45.3 n: 57) and high SSII (SSII > 45.3 n: 57). There was no long‐term mortality in the low SSII tertile, while mortality was 15.78% in the moderate and 35.08% in the high SSII tertiles (p < .001) (Figure 3).
Figure 2.
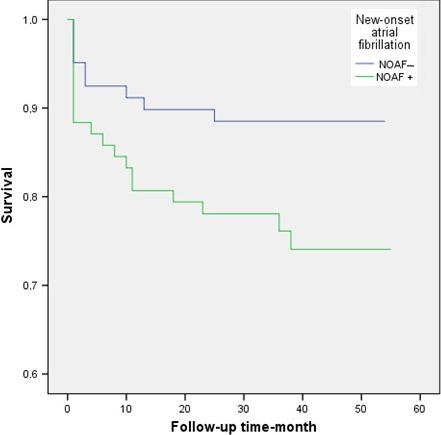
Kaplan–Meier survival curve of patients with and without new‐onset atrial fibrillation (NOAF)
Figure 3.
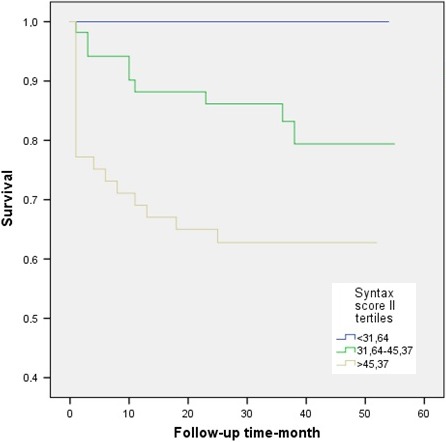
Long‐term survival curve comparison between Syntax score II tertiles
4. DISCUSSION
In the present study, we focused on the potential relationship between SS, SSII and the development of NOAF in patients undergoing pPCI for STEMI. Our study demonstrated that STEMI patients with NOAF had a higher SS and SSII. SSII was shown to be an independent predictor of NOAF development during hospitalization and patients that had NOAF, had a worse prognosis in the long‐term follow‐up.
Previous studies demonstrated that NOAF development in patients with acute myocardial infarction during the hospitalization is associated with a worse short‐ and long‐term prognosis in patients undergoing thrombolysis or pPCI (Crenshaw et al., 1997; Kinjo et al., 2003; Wong et al., 2003). Also, effect of NOAF on worse prognosis was shown to be free of other independent predictors including re‐infarction, heart failure, cardiogenic shock, and life threatening arrhythmias (Wong et al., 2003). To date, several clinical parameters related to the development of NOAF have been established in STEMI patients. Consistent with the results of previous studies (Crenshaw et al., 1997; Iguchi et al., 2008; Kinjo et al., 2003; Mrdovic et al., 2012; Schmitt et al., 2009; Wong et al., 2003); we found that older age; history of HT, DM or smoking; higher Killip class, heart rate, and extensive myocardial damage (determined by peak CK‐MB level); reduced eGFR and LVEF; imperfect reperfusion (determined by post pPCI TIMI flow grade) were associated with increased likelihood of NOAF development in all study populations. In the present study we also observed that patients with NOAF had a reduced hemoglobin level. Although there is no definitive relationship between anemia alone and atrial fibrillation, anemia could probably facilitate AF in STEMI patients through increase in sympathetic activity and heart rate; reduction in oxygen presentation and emergence of tissue hypoxia; and through ventricular remodeling associated with increased stroke volume and cardiac output secondary to anemia (Metivier, Marchais, Pannier, & London, 2000).
In the matched population SS was higher in NOAF patients and this observation is the first to be reported in the literature. There are limited data in literature regarding the association between CAD severity and NOAF in STEMI patients. And in those studies, CAD severity was determined only by the number of vessels involved (Crenshaw et al., 1997; Kinjo et al., 2003). In the present study, CAD severity was assessed by SS, which is able to provide more important prognostic information in stable CAD (Sianos et al., 2005) and STEMI (Magro et al., 2011) patients, due to being based not only on anatomical/morphological properties, but also on functional properties of coronary lesions. The reason STEMI patients with more extensive CAD have an increased risk of NOAF could be explained by potentially being more likely to have atrial ischemia (Sinno et al., 2003), high atrial pressure (Satoh & Zipes, 1996), and autonomic nervous system alterations (Coumel, 1996) that caused structural and functional remodeling in atrium.
One of the most important findings of the present study is that SSII was significantly higher in NOAF patients. This is true for both the matched population as well as the entire study population. Although the first model of multivariate analysis showed that the SSII parameters (Age, SS and LVEF) were separately independent predictors of NOAF, the second model of multivariate analysis showed SSII was superior to its own parameters, and only SSII was found to be an independent predictor by itself. It is not surprising that the present study has found LVEF and older age to be predictors for NOAF, and this finding is compatible with other studies (Ciçek et al., 2003; Schmitt et al., 2009). However, the most important finding of this study is that SSII's predictive power is superior to its own parameters. This could be because SSII reflects the cumulative effect of its parameters. Also, the coexistence of the parameters is SSII could be increasing the likelihood of AF with additive effect.
It is possible that, for patients that already have some clinical parameters of SSII, medications being used might be causing development of NOAF. For instance, COPD patients with STEMI were found to need more inotropic agents, and to be able to use fewer b‐blockers (Lazzeri et al., 2013). Similarly, in another study, PAD patients with acute coronary syndrome were able to use less beta‐blockers and had more heart failures and recurrent ischemic episodes (Al‐Thani et al., 2011). While SSII has a low AUC, it has been found to be a predictor for NOAF, which is associated with long‐term mortality, and thereby SSII is still a useful metric. And while it is not being claimed that SSII is a screening method for NOAF, higher SSII may suggest the clinician should be watchful for the likelihood of NOAF, and should avoid medications that will precipitate it.
4.1. Limitations
Coronary artery disease severity was evaluated only by visual assessment which did not include further information about atherosclerosis extensity such as lumen area, plaque size, composition, and distribution. The frequency of NOAF could have been higher than it is observed, as it is possible to miss silent AF attacks. Furthermore, inability to identify the silent/asymptomatic paroxysmal AF episodes in patients prior to admission is also a limitation of this study.
5. CONCLUSION
We presented the first study to comprehensively examine the relationship between NOAF development and CAD severity using SS and SSII. In this study we have shown that high SSII is significantly related to NOAF, and patients that had NOAF had a worse prognosis in the long‐term follow‐up. Due to poor prognosis of NOAF in patients with STEMI, ability to predict NOAF is crucial. In the setting of STEMI, determination of CAD severity by SS, or SSII, which we have shown to be a stronger predictor than its components, can help identify NOAF patients.
ACKNOWLEDGMENTS
Thanks to http://www.metastata.com for their contributions to statistical analysis and trial design.
Rencuzogullari I, Çağdaş M, Karakoyun S, et al. Propensity score matching analysis of the impact of Syntax score and Syntax score II on new onset atrial fibrillation development in patients with ST segment elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2018;23:e12504 10.1111/anec.12504
REFERENCES
- Al‐Thani, H. A. , El‐Menyar, A. , Zubaid, M. , Rashed, W. A. , Ridha, M. , Almahmeed, W. , … Al Suwaidi, J. (2011). Peripheral arterial disease in patients presenting with acute coronary syndrome in six middle eastern countries. International Journal of Vascular Medicine, 2011, 815902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm, A. J. , Kirchhof, P. , Lip, G. Y. , Schotten, U. , Savelieva, I. , Ernst, S. , … Rutten, F. H. (2010). European Heart Rhythm Association, European Association for Cardio‐Thoracic Surgery, Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European Heart Journal, 31, 2369–2429. [DOI] [PubMed] [Google Scholar]
- Ciçek, D. , Camsari, A. , Pekdemir, H. , Kiykim, A. , Akkuş, N. , Sezer, K. , & Diker, E. (2003). Predictive value of P‐wave signal‐averaged electrocardiogram for atrial fibrillation in acute myocardial infarction. Annals of Noninvasive Electrocardiology, 8(3), 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumel, P. (1996). Autonomic influences in atrial tachyarrhythmias. Journal of Cardiovascular Electrophysiology, 7(10), 999–1007. [DOI] [PubMed] [Google Scholar]
- Crenshaw, B. S. , Ward, S. R. , Granger, C. B. , Stebbins, A. L. , Topol, E. J. , & Califf, R. M. (1997). Atrial fibrillation in the setting of acute myocardial infarction: The GUSTO‐I experience. Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries. Journal of the American College of Cardiology, 30, 406–413. [DOI] [PubMed] [Google Scholar]
- Farooq, V. , van Klaveren, D. , Steyerberg, E. W. , Meliga, E. , Vergouwe, Y. , Chieffo, A. , … Serruys, P. W. (2013). Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery andpercutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet, 381(9867), 639–650. [DOI] [PubMed] [Google Scholar]
- Gibson, C. M. , Cannon, C. P. , Daley, W. L. , Dodge, J. T. Jr , Alexander, B. Jr , Marble S.J., … Braunwald E.. (1996). TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation, 93, 879–888. [DOI] [PubMed] [Google Scholar]
- Iguchi, Y. , Kimura, K. , Kobayashi, K. , Aoki, J. , Terasawa, Y. , Sakai, K. , … Shibazaki, K. (2008). Relation of atrial fibrillation to glomerular filtration rate. American Journal of Cardiology, 102(8), 1056–1059. [DOI] [PubMed] [Google Scholar]
- Kinjo, K. , Sato, H. , Sato, H. , Ohnishi, Y. , Hishida, E. , Nakatani, D. , … Hori, M. (2003). Prognostic significance of atrial fibrillation/atrial flutter in patients with acute myocardial infarction treated with percutaneous coronary intervention. American Journal of Cardiology, 92, 1150–1154. [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Bierigi, M. , Devereux, R. B. , Flachskampf, F. A. , Foster, E. , Pellikka, P. A. , … Stewart, W. J. (2005). Recommendations for chamber quantification: A report from the American society of echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. Journal of the American Society of Echocardiography, 18(12), 1440–1463. [DOI] [PubMed] [Google Scholar]
- Lazzeri, C. , Valente, S. , Attanà, P. , Chiostri, M. , Picariello, C. , & Gensini, G. F. (2013). The prognostic role of chronic obstructive pulmonary disease in ST‐elevation myocardial infarction after primary angioplasty. European Journal of Preventive Cardiology, 20(3), 392–398. [DOI] [PubMed] [Google Scholar]
- Magro, M. , Nauta, S. , Simsek, C. , Onuma, Y. , Zhang, Y. J. , Garcia‐Garcia, H. M. , … Serruys, P. W. (2011). Value of the SYNTAX score in patients treated by primary percutaneous coronary intervention for acute ST‐elevation myocardial infarction: The MI SYNTAX score study. American Heart Journal, 161, 771–781. [DOI] [PubMed] [Google Scholar]
- Metivier, F. , Marchais, S. J. Guerin , Pannier, B. , & London, G. M. (2000). Pathophysiology of anaemia: Focus on the heart and blood vessels. Nephrology, Dialysis, Transplantation, 15, 14–18. [DOI] [PubMed] [Google Scholar]
- Mrdovic, I. , Savic, L. , Krljanac, G. , Perunicic, J. , Asanin, M. , Lasica, R. , … Ostojic, M. (2012). Incidence, predictors, and 30‐day outcomes of new‐onset atrial fibrillation after primary percutaneous coronary intervention: Insight into the RISK‐PCI trial. Coronary Artery Disease, 23, 1–8. [DOI] [PubMed] [Google Scholar]
- Satoh, T. , & Zipes, D. P. (1996). Unequal atrial stretch in dogs increases dispersion of refractoriness conducive to developing atrial fibrillation. Journal of Cardiovascular Electrophysiology, 7(9), 833–842. [DOI] [PubMed] [Google Scholar]
- Schmitt, J. , Duray, G. , Gersh, B. J. , & Hohnloser, S. H. (2009). Atrial fibrillation in acute myocardial infarction: A systematic review of the incidence, clinical features and prognostic implications. European Heart Journal, 30(9), 1038–1045. [DOI] [PubMed] [Google Scholar]
- Sianos, G. , Morel, M. A. , Kappetein, A. P. , Morice, M. C. , Colombo, A. , Dawkins, K. , … Serruys, P. W. (2005). The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention, 1, 219–227. [PubMed] [Google Scholar]
- Sinno, H. , Derakhchan, K. , Libersan, D. , Merhi, Y. , Leung, T. K. , & Nattel, S. (2003). Atrial ischemia promotes atrial fibrillation in dogs. Circulation, 107, 1930–1936. [DOI] [PubMed] [Google Scholar]
- Steg, P. G. , James, S. K. , Atar, D. , Badano, L. P. , Blömstrom‐Lundqvist, C. , Borger, M. A. , … Zahger, D. (2012). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. European Heart Journal, 33, 2569–2619. [DOI] [PubMed] [Google Scholar]
- Wang, G. , Wang, C. , Zhang, Y. , Onuma, Y. , Garg, S. , van der Heide, E. , & Serruys, P. W. (2016). Usefulness of the SYNTAX score II to predict 1‐year outcome in patients with primary percutaneous coronary intervention. Coronary Artery Disease, 27(6), 483–489. [DOI] [PubMed] [Google Scholar]
- Wong, C. K. , White, H. D. , Wilcox, R. G. , Criger, D. A. , Califf, R. M. , Topol, E. J. , & Ohman, E. M. (2003). Significance of atrial fibrillation during acute myocardial infarction, and its current management: Insights from the GUSTO‐3 trial. Cardiac Electrophysiology Review, 7, 201–207. [DOI] [PubMed] [Google Scholar]


