Abstract
Background
Arrhythmogenic right ventricular dysplasia (ARVD) is characterized by progressive replacement of ventricular myocytes with variable amounts of fibrous and adipose tissue. Several studies have suggested that the interval from the peak to the end of the electrocardiographic T wave (Tp‐e) may correspond to the transmural dispersion of repolarization and that increased Tp‐e interval and Tp‐e/QT ratio are associated with malignant ventricular arrhythmias. The aim of this study was to evaluate repolarization dispersion measured from the 12‐lead surface electrocardiogram (including Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio) in asymptomatic ARVD patients
Methods
We selected 27 patients with asymptomatic ARVD and 27 age‐ and gender‐match young, healthy volunteers.
Results
Tp‐e interval, Tp‐e/QT and Tp‐e/QTc ratio were also significantly higher in ARVD group compared to the control group (all P < 0.001). There were negative correlation between S global and Tp‐e, Tp‐e/QT, Tp‐e/QTc ration (r = −0.57, P = 0.02; r = −0.85, P = 0.02; r = −0.63, P < 0.01; respectively). There were also negative correlation between Sm global and Tp‐e, Tp‐e/QT, Tp‐e/QTc ration (r = −0.61, P < 0.01; r = −0.67, P < 0.01; r = −0.68, P < 0.01; respectively). Moreover, Em global were negative correlation between Tp‐e, Tp‐e/QT, and Tp‐e/QTc (r = − 0.64, P < 0.001, r = − 0.75, P < 0.01; r = −0,69, P < 0.01; respectively)
Conclusion
In conclusion, we have presented strong evidence suggesting that Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio were increased in asymptomatic ARVD patients.
Keywords: Tp‐e interval, arrhythmogenic right ventricular dysplasia, electrocardiography, ventricular arrhythmias
Arrhythmogenic right ventricular cardiomyopathy is characterized by progressive replacement of ventricular myocytes with variable amounts of fibrous and adipose tissue. The loss of normal myocardium is leading to right ventricular (RV) failure, ventricular arrhythmias and sudden death in young adults.1, 2
Myocardial repolarization has been evaluated by various methods including QT dispersion (QTd), corrected QT dispersion (QTcd), and transmural dispersion of repolarization (TDR). Tp‐e interval, which is the interval between the peak and the end of T wave on electrocardiogram (ECG), can be used as an index of total (transmural, apico‐basal, and global) dispersion of repolarization.3, 4 Increased Tp‐e interval might be a useful index to predict ventricular tachyarrhythmias and cardiovascular mortality.5, 6 Recently, a new index, the Tp‐e/QT ratio has been suggested to be more accurate measure for the dispersion of ventricular repolarization compared to QTd, QTcd, and Tp‐e intervals which is independent of alterations in heart rate.7, 8 Tp‐e interval has been examined in symptomatic arrhythmogenic right ventricular dysplasia (ARVD) patients.9, 10 However, it is known ARVD patients are usually asymptomatic at the early disease stages, and ventricular arrhythmias/sudden death can occur primarily in young people.11 Yet, there is no research has been conducted to examine Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio in asymptomatic ARVD patients. Therefore, the aim of this study was to evaluate repolarization dispersion measured from the 12‐lead surface electrocardiogram (including Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio) in asymptomatic ARVD patients.
METHOD
Between May 2009 and May 2014, we recruited 27 patients with ARVD diagnosed using the criteria of the Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology.1 Twenty‐seven age‐ and gender‐match young, healthy volunteers with no medical comorbidities and receiving no cardio‐active medications included in the study. All ARVD patients were asymptomatic and no history of ventricular arrhythmias or survived sudden cardiac arrest. Our study population was consisted of the regularly monitored subjects whose ARVC diagnosis was identified during examination of the first‐degree relatives of the ARVC patients. With 24‐hour Holter ECG, no arrhythmic events were observed in ARVD patients. Prior to data collection written informed consent was obtained from each patient, and the study had been approved by the appropriate institutional ethics review committee. The subjects’ body weight, height and the body surface area (BSA) were measured (m2). Subjects with coronary artery disease, chronic renal failure, chronic liver disorders, chronic lung disease, moderate or severe valvular heart disease, diabetes mellitus, congenital heart disease, left ventricular systolic dysfunction on echocardiography (ejection fraction <50%), recent acute coronary syndrome, anemia, obstructive sleep apnea, hypertension, hematological disorders, known malignancy, thyroid dysfunction, hypercholesterolemia, electrolyte imbalance and atrioventricular conduction abnormalities on ECG and ECGs without clearly analyzable QT segment were excluded from the study. In addition, subjects with ST‐segment elevation, ST‐T changes, wide QRS complexes (>120 ms), LBBB and ventricular preexcitation were not included. All subjects were in sinus rhythm.
Echocardiographic Measurements
Echocardiography was performed in left lateral decubitus position with an ultrasound machine GE‐Vingmed Vivid 7 system (Vivid system 7, GE‐Vingmed Ultrasound AS, Horten, Norway) and 3S‐RS (3.5 MHz) probe. Examinations were performed by a cardiologist who was blinded to the clinical details of each subject. Single‐lead ECG was recorded continuously during the echocardiographic examination. Two‐dimensional, M‐mode, and tissue Doppler images were acquired from the parasternal long and short axis and apical four‐chamber views at end‐expiratory apnea, and were transferred to a customized dedicated software package (EchoPAC, General Electric Vingmed Ultrasound) for offline analysis of stored data. All measurements were averaged from three cardiac cycles. 2D echocardiographic measurements were performed according to standards outlined by the American Society of Echocardiography. LV ejection fraction was calculated according to the Simpson method. 12 Additional off‐plane images of the right ventricle were obtained to maximize the visualization of right ventricular (RV) morphology. RV outflow tract diameter was measured in the parasternal long‐axis (PLAX‐RVOT) and short‐axis (PSAX‐RVOT) views at the aortic valve plane level.13, 14 RV fractional area change (RVFAC) was calculated from the apical four‐chamber view using the percentage change in areas of the end‐diastolic and end‐systolic areas of the RV. 13 The RV outflow diameter was measured from the 2D images and indexed to body surface area. In the apical four‐chamber view, RV long‐axis diameters and short‐axis inflow diameters at the level of the valve leaflet tips were measured at end diastole. Tricuspid annular plane systolic excursion (TAPSE) was recorded at the RV free wall using cross sectional guided M‐mode. Additional off‐plane images of the right ventricle were obtained to maximize the visualization of RV morphology. RV outflow tract diameter was measured in the parasternal long‐axis and short‐axis views at the aortic valve plane level RV fractional area change (RVFAC) was calculated from the apical four‐chamber view using the percentage change in areas of the end‐diastolic and end‐systolic areas of the RV.
The tissue Doppler imaging (TDI) of the RV was performed in the apical four‐chamber view using a 5‐mm pulsed Doppler sample volume with as minimum optimal gain as possible to obtain the best signal‐to‐noise ratio. Care was taken to align the echo image so that the annular motion was parallel to the TDI cursor. Spectral pulsed‐wave Doppler signal filters were adjusted until a Nyquist limit of 15–20 cm/s was reached. The monitor sweep speed was set at 50–100 mm/s to optimize the spectral display of myocardial velocities. For the analysis of regional myocardial velocities and deformation, TDI of the RV lateral free wall was performed. The myocardial peak systolic (Sm), and early diastolic (Em) velocities, and systolic strain (S) were measured using a 5‐mm sample volume positioned in the endocardial half of the basal, middle, and apical segment of the RV free wall. The Sm global, Em global and S global velocities were derived by averaging the velocities from the basal, middle, and apical segment of RV free wall.
Electrocardiographic Measurements
The 12‐lead ECG was recorded at a paper speed of 25 mm/s at rest in the supine position. Resting heart rate was measured from the ECG taken during the patient evaluation. To decrease the percentage of error during measurements, QT and Tp‐e intervals were measured manually with calipers and magnifying glass. Subjects with U waves on their ECGs were excluded from the study. An average value of three readings was calculated for each lead. The QRS‐complex duration was measured from the beginning of the QRS complex to its end in leads V1 through V3. Measurement of S wave upstroke duration from nadir of S to end of QRS in leads V1 through V3. The QT interval was defined as the time from the onset of the QRS complex to the end of the T wave at which the isoelectric line intersected a tangential line drawn at the maximal down slope of a positive T wave, and was corrected for heart rate using the Bazett's formula: cQT = QT/√ (R–R interval. The QTd was defined as the difference between the maximum and minimum QT interval of the 12 leads. 15 The JT interval was derived by subtracting the QRS duration from the QT interval. JT measurement was also corrected for heart rate using the Bazett's formula. The Tp‐e interval was defined as the interval from the peak of T wave to the end of T wave (Fig. 1). Measurements of Tp‐e interval were performed from precordial leads. 5 In the case of more complex T waves, including biphasic and triphasic T waves, the interval from the nadir of the first component of the T wave to the end of the T wave was calculated for Tp‐e interval. Isolated negative T wave in lead V1 was not counted as T‐wave inversion. Tp‐e/QT and Tp‐e/QTc ratio was calculated from these measurements. Mean QT, QTc, Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio in V1–V3/V4–V6 were calculated by averaging each of V1–V2–V3 and V4–V5–V6 leads, respectively. All measurements were performed by 2 experienced investigators unaware of the clinical characteristics of the study participants. Intraobserver and interobserver coefficients of variation (standard deviation [SD] of differences between two observations divided by the mean value and expressed as percent) were found to be 1.3% and 2.2%, respectively.
Figure 1.
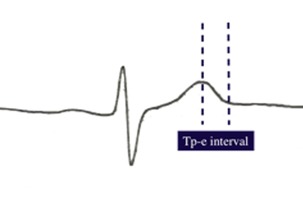
Tp‐e interval was measured from the peak of T wave to the end of T wave.
Statistical Analysis
The SPSS 15.0 statistical program (SPSS Inc., Chicago, IL) was used for the statistical study. All values are given as mean ± standard deviation. Mean values of continuous variables were compared between groups using the Student's t‐test or Mann‐Whitney U test, according to whether normally distributed or not, as tested by the Kolmogorov‐Smirnov test. The chi‐square test was used to assess differences between categorical variables. Pearson's correlation coefficients were used to assess the strength of relationship between continuous variables. A P value of <0.05 was considered significant. The Bland‐Altman analysis (MedCalc, Ostend, Belgium) was used for intra‐ and interobserver reproducibility.
RESULTS
Clinical Characteristics of the Study Population
The characteristics of the subjects are listed in Table 1. No differences between groups emerged in age, sex, BSA, or heart rate.
Table 1.
Clinical Characteristics and Echocardiographic Findings of the Two Groups
| ARVD (n = 27) | Control Group (n = 27) | P Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (year) | 36.1 ± 2.1 | 35.2 ± 3.2 | NS |
| Sex (male) n, % | 17 male (63) | 17 male (63) | NS |
| Heart rate (beats/min) | 64.8 ± 7.3 | 65.8 ± 5.4 | NS |
| BSA (m2) | 1.74 ± 0.12 | 1.81 ± 0.15 | NS |
| 2‐D Echocardiographic parameters | |||
| LV ejection fraction (%) | 60.17 ± 2.4 | 61.86 ± 2.3 | NS |
| PLAX RVOT (mm) | 28.85 ± 1.1 | 25.85 ± 0.98 | <0.01 |
| PSAX RVOT (mm) | 32.66 ± 1.7 | 28.18 ± 1.4 | <0.01 |
| RV‐FAC (%) | 36.7 ± 0.9 | 37.2 ± 1.1 | NS |
| PLAX RVOT/BSA (mm/m2) | 16.6 ± 1.39 | 14.73 ± 1.31 | <0.01 |
| PSAX RVOT/BSA (mm/m2) | 18.8 ± 1.69 | 15.6 ± 1.53 | <0.01 |
| TAPSE (mm) | 2.47 ± 0.27 | 2.59 ± 0.26 | NS |
| Doppler parameters | |||
| Sm global (cm/s) | 7.01 ± 1.94 | 9.17 ± 2.27 | <0.01 |
| Em global (cm/s) | 6.03 ± 2.21 | 9.49 ± 2.28 | <0.01 |
| Strain global (%) | 15,4 ± 4.99 | 25,5 ± 6,1 | <0.01 |
NS = nonsignificant.
Echocardiographic Analysis
Table 1 shows the details of the echocardiographic analysis. PLAX‐RVOT, PSAX‐ RVOT, PLAX‐RVOT/BSA, and PSAX‐RVOT/BSA were significantly greater in ARVD patients than in sedentary controls (P < 0.01). No significant differences were found in RV‐FAC, TAPSE, and LV ejection fraction among the groups.
Analysis of regional myocardial velocities and deformation showed that lateral global Sm, global Em, and global Strain were significantly lower in ARVD patients than in control subjects (6.03 ± 2.21 vs 9.49 ± 2.28, P < 0.01; 7.01 ± 1.97 vs 9.17 ± 2.27, P < 0.01; 15.4 ± 4.99 ± 25.5 ± 6.1; respectively).
Electrocardiographic Parameters
Electrocardiographic parameters of the groups are shown in Table 2. QT, QTc, QTd, and QTcd were significantly increased in ARVD patients compared to the control subjects (all P < 0.05). Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio were also significantly higher in ARVD group compared to the control group (all P < 0.001). QRS V1–V3 and S wave V1–V3 were prolonged in in ARVD group compared to the control group (all P < 0.001). JT and JTc interval were also increased in ARVD patients group compared to the control subjects (all P < 0.05). Mean QT, QTc, Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio of V1–V3 were significantly prolonged rather than in V4–V6 leads in asymptomatic ARVD patients (all P < 0.001) (Table. 3). There were negative correlations between Sm global, Em global, and S global with Tp‐e, Tp‐e/QT, and Tp‐e/QTc ration. There were negative correlation between S global and Tp‐e, Tp‐e/QT, Tp‐e/QTc ration (r = −0.57, P = 0.02; r = −0.85, P = 0.02; r = −0.63, P < 0.01; respectively). There were also negative correlation between Sm global and Tp‐e, Tp‐e/QT, Tp‐e/QTc ration (r = −0.61, P < 0.01; r = −0.67, P < 0.01; r = −0.68, P < 0.01; respectively). Moreover, Em global were negative correlation between Tp‐e, Tp‐e/QT, and Tp‐e/QTc (r = − 0.64, P < 0.001, r = − 0.75, P < 0.01; r = −0,69, P < 0.01, respectively) (Figs. 2, 3, 4)
Table 2.
Electrocardiographic Findings of the Two Groups
| ARVD (n = 27) | Control Group (n = 27) | P Value | |
|---|---|---|---|
| QT (ms) | 408.1 ± 36.5 | 378.1 ± 37.1 | <0.01 |
| QTd (ms) | 47.88 ± 11.8 | 35.67 ± 8.36 | <0.01 |
| QTcd | 49.70 ± 12.6 | 37.34 ± 8.96 | <0.01 |
| QTc | 423.7 ± 44.3 | 396.0 ± 44.5 | 0.026 |
| Tp‐e (ms) | 92.5 ± 10.12 | 75.8 ± 9.02 | <0.01 |
| Tp‐e/QT | 0.22 ± 0.02 | 0.20 ± 0.03 | <0.01 |
| Tp‐e/QTc | 0.21 ± 0.02 | 0.19 ± 0.03 | <0.01 |
| QRS V1–V3 | 110.9 ± 7.64 | 93.7 ± 8.53 | <0.01 |
| S wave V1–V3 | 50.0 ± 6.71 | 32.3 ± 3.64 | <0.01 |
| Epsilon wave n, % | 3 (11) | 0 | – |
| RBBB n, % | 6 (22) | 0 | – |
| Precordial T‐wave inversion, n (%) | 12 (44) | 0 | – |
RBBB = right bundle branch block.
Table 3.
Differences of Electrocardiographic Parameters in ARVD Patients
| V1 –V3 (Mean ± SD) | V4–V6 (Mean ± SD) | P Value | |
|---|---|---|---|
| QT (ms) | 427.1 ± 35.9 | 389.1 ± 37.5 | <0.001 |
| QTc | 443.4 ± 44.2 | 403.8 ± 45,0 | <0.001 |
| Tp‐e (ms) | 105.2 ± 12.9 | 80.0 ± 7.06 | <0.001 |
| Tp‐e/QT | 0.24 ± 0.02 | 0.20 ± 0.02 | <0.001 |
| Tp‐e/QTc | 0.23 ± 0.02 | 0.19 ± 0.02 | <0.001 |
Figure 2.
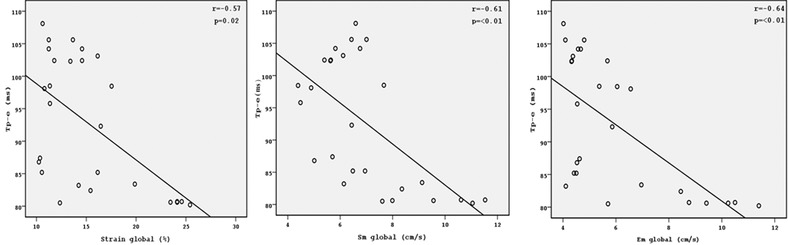
Correlation graphics of Tp‐e with Starin global, Sm global, and Em global.
Figure 3.
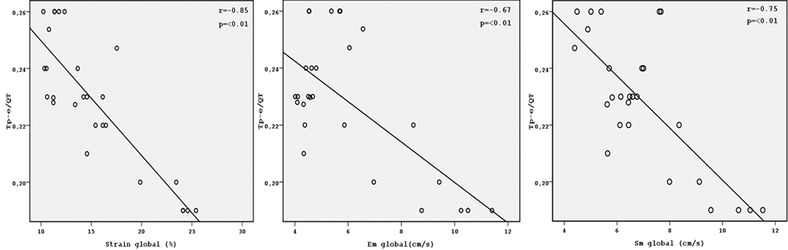
Correlation graphics of Tp‐e/QT with Starin global, Sm global, and Em global.
Figure 4.
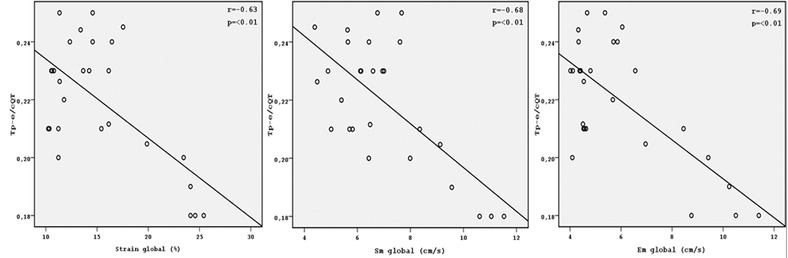
Correlation graphics of Tp‐e/cQT with Starin global, Sm global, and Em global.
DISCUSSION
In our study, we found that mean Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio that are known to be related to various ventricular arrhythmias and sudden death are significantly prolonged in asymptomatic ARVD patients compared with controls.
ARVD is an inherited disease characterized by a progressive replacement of myocytes with fibrofatty tissue, leading to arrhythmias and sudden death in young patients. 1, 2 This loss of myocardial cells also gives rise to areas of diminished contractile function, most often located in the so‐called triangle of dysplasia. 16 As the disease progresses, both global RV systolic and diastolic function become reduced and RV dilatation sets in.17
Unfortunately, on the basis of conventional visual echocardiographic assessment, identification of functional abnormalities is difficult (resulting mainly in false‐positive findings) and RV dilatation is often nonspecific.18, 19, 20 In the diagnostic workup of patients with suspected ARVD, an approach to detect the early loss of function in small areas of the RV could reduce false‐negative results, for instance, when global systolic RV function is still within the normal range because of large parts of still healthy myocardial tissue in the RV. 17 Previous studies demonstrated that, TDI parameters measured in the RV free wall were significantly lower in ARVD patients compared with controls. These data are concordant with a single case report and a study that reported reduced tissue velocities in patients with ARVD. 20, 21, 22, 23 Prakasa et al. showed that peak RV systolic velocity, early diastolic velocity and strain values were significantly lower in patients with ARVD with normal or mildly impaired RV morphology and functions by conventional echocardiography compared with controls. 24 In present study, our ARVD patients were asymptomatic and there were no typical echocardiographic findings. In addition, confirming previous findings, we observed low Sm velocity, low early diastolic peak velocities (Em) and low strain at the lateral RV wall that indicate the early loss of function in small areas of the RV in ARVD patients compared with controls.
It is known that, potentially lethal ventricular arrhythmias are often the first clinical presentation in an early (concealed) phase when typical echocardiographic findings are often still absent or very subtle. 25 Therefore, it is necessary to determine noninvasive markers for unfavorable outcomes such as ventricular arrhythmias and sudden death in asymptomatic ARVD patients. Increased heterogeneity of ventricular myocardial repolarization favors susceptibility to reentrant ventricular tachyarrhythmias. Myocardial repolarization has been evaluated by various methods including QTc, QTd, QTcd, and TDR. 26 Turrini et al. and Fagundes et al. demonstrated that QTd and QTcd were prolonged in ARVD patients who had a history of ventricular arrthythmias or sudden death. 27, 28 It is known that QTc has been used as predictors of increased risk of ventricular arrhythmias and sudden death in several diseases. Previous study demonstrated that QTc was prolonged in symptomatic and asymptomatic ARVD patients when compared with control group. In our study, we showed that QTc, QTd, and QTcd were also significantly greater even in asymptomatic ARVD patients. We also demonstrated that mean QT and QTc duration in V1–V3 leads were significantly longer than V4–V6 in asymptomatic ARVD patients.
Ventricular myocardium is an electrically heterogeneous structure composed of three distinct cell types with different electrophysiological properties (endocardial layer, M cells, and epicardial layer). 29, 30 The duration of action potential is longer in the midmyocardial M cells compared to other myocardial cells. The earliest completion of repolarization occurs in the epicardial cells. The peak of T wave represents the end of the epicardial action potential, and the end of T wave represents the end of the midmyocardial action potential.10 Therefore, Tp‐e interval is a reflection of TDR. 30 TDR, calculated as the interval between the peak and the end of T wave on electrocardiogram, has been related to ventricular arrhythmias and sudden cardiac death. 5, 6, 26 Recent studies indicated that Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio, can be used as an index of total (transmural, apico‐basal, and global) dispersion of repolarization. 3, 4 Also, these markers may be used as an electrocardiographic index of ventricular arrhythmogenesis and sudden cardiac death. 5, 6 Tp‐e/ QT ratio was reported as a more accurate marker for ventricular arrhythmogenesis compared to Tp‐e interval and QTd, due to its independence from heart rate.31, 32 Previous studies showed that Tp‐e interval and Tp‐e/QT ratio were associated with increased risk for malignant ventricular arrhythmias in a variety of conditions, inculuding long‐QT syndrome (acquired and congenital), Brugada syndrome, acute ST‐segment elevation myocardial infarction, and hypertrophic cardiomyopathy. 5, 33, 34 Moreover, Haapalahti et al. showed that QT and Tp‐e interval were longer in symptomatic ARVD patients.9 In addition, Golcuk et al investigate the possible role of Tp‐e interval to distinguish ARVC and RVOT‐VT. They found that in patients with VT of RV origin, the prolonged Tp‐e interval in sinus rhythm electrocardiogram supports the diagnosis of ARVD. 10 As far as we know, there is no study in the literature has been conducted to examine Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio in asymptomatic ARVD patients. In this study, we showed for the first time that Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio were significantly prolonged in early stage of ARVD patients compared to controls. Furthermore, Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio were prolonged in V1–V3 precordial leads than in V4–V6 precordial leads in asymptomatic ARVD patients.
The possible mechanism for increasing QTd, QTcd, Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/ QTc in early stage of ARVD patients is replacement of myocytes with fibrofatty tissue. With the fibrofatty degeneration characteristically beginning in the subepicardial myocardial layer and often being focal in distribution, myocardial biopsy usually has only a low yield in detecting the ARVC defining histopathological changes, especially in early disease stages. 35 As previously discuss in the text, in patients with ARVD with normal or mildly impaired RV morphology and functions, decreases of Sm, Em and strain values were detect the early replacement of myocytes with fibrofatty tissue in small areas of the RV. It is known that the loss of myocardial cells with fibrofatty replacement and the development of scar is a substrate for potentially lethal ventricular arrhythmias and sudden death in ARVD patients. Zorzi et al. suggests that extent of RV fibrofatty tissue is correlated with amount of repolarization abnormality. 36 In ARVD electrogenesis of repolarization abnormalities and its relation with RV fibrofatty lesions remains to be unexplained. It can be speculated that replacement of RV myocardium by fibrofatty tissue induces a ventricular electrical remodeling with changes of transmural and/or regional activation and repolarization times. It has been demonstrated that in ARVD the RV fibrofatty tissue is much larger in the epicardium than in the endocardium, because the wavefront of RV fibrofatty myocardial replacement progresses from the epicardial to the endocardial layers. 10, 36 This may create transmural repolarization heterogeneity and prolong Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio in patients with early phases of ARVD. In our study, we found that Sm, Em and strain velocities which detect the early replacement of myocytes with fibrofatty tissue have negative correlation with Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio. Therefore, we can also speculated that the loss of normal myocardium and the development of fibrofatty tissue in early phase of ARVD patients may be the reason underlying the prolongation of Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio.
In ARVD patient's exercise‐related ventricular atthytmias were possibly based on delayed repolarization in the diseased RV. The clinical findings suggest that sympathetic activation was responsible for malignant arrtythmias.10, 37 Wichter et al. found a significant reduction of myocardial β‐adrenergic receptor density in patients with ARVC than controls, which was consistent with autonomic dysfunction.37 Also, Shan et al, comparing Doppler tissue imaging and histologic findings in patients affected by coronary artery disease, demonstrated that Sm and Em are strongly dependent on the number of myocytes, myocardial β‐adrenergic receptor density, and the amount of interstitial fibrosis.38 In our study Sm and Em velocities were also reduced in ARVD patients, which may shows density of myocardial β‐adrenergic receptor. Therefore, alterations in cardiac autonomic activity in asymptomatic ARVD may be another possible mechanism for ventricular arrhythmias by increasing the heterogeneity of ventricular repolarization.
STUDY LIMITATIONS
Our study has several limitations. The most important limitations of our study are the small sample size and cross‐sectional design of the study, in which we could not follow up the patients prospectively for future arrhythmic events. We did not observe any arrhythmias in the study population. Therefore, we do not know whether prolongation of Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio predicts ventricular arrhythmias in asymptomatic ARVD patients. Further studies need to be conducted with a larger number of patients and a longer follow‐up time in order to increase the accuracy of the results. The prevelance of ECG signs of ARVC disease differs among studies; the diagnostic value of Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio should be tested in different series and in larger cohorts.
All patients with ARVD met the task force criteria, and most had mild RV dilatation and asymptomatic; thus, the present results should not be extrapolated to subjects with signs of more than moderate RV dilation.
CONCLUSION
In conclusion, in this cross‐sectional study, we have presented an strong evidence suggesting that Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio were increased in asymptomatic ARVD patients. Furthermore, replacement of RV myocardium by fibrofatty tissue and alterations in cardiac autonomic activity may be the reason underlying the prolongation of Tp‐e interval, Tp‐e/QT ratio, and Tp‐e/QTc ratio in early stage of ARVD patients. In addition we also found that QTc, QTd, and QTcd were also significantly greater even in asymptomatic ARVD patients. These markers may be a better predictor of sudden death and ventricular arrhythmias with occult ARVD patients.
REFERENCES
Ann Noninvasive Electrocardiol 2017;22(1):e12362, DOI: 10.1111/anec.12362
The authors declare that there are no conflicts of interest.
References
- 1. McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 1994;71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D): Proposed modification of the task force criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp‐Te interval and its diagnostic value. J Electrocardiol 2008;41:575–580. [DOI] [PubMed] [Google Scholar]
- 4. Antzelevitch C, Sicouri S, Di Diego JM, et al. Does Tpeak‐Tend provide an index of transmural dispersion of repolarization? Heart Rhythm 2007;4:1114–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, et al. Tpeak‐Tend and Tpeak‐Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol 2006;47:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smetana P, Schmidt A, Zabel M, et al. Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease:peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol 2011;44:301–308. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol 2004;37:191–200. [DOI] [PubMed] [Google Scholar]
- 8. Gupta P, Patel C, Patel H, et al. T(p‐e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 2008;41:567–574. [DOI] [PubMed] [Google Scholar]
- 9. Haapalahti P, Viitasalo M, Kaartinen M, et al. Electrocardiographic ventricular repolarization during cardiovascular autonomic function testing in patients with arrhythmogenic right ventricular cardiomyopathy. Scand Cardiovasc J 2008;42(6):375–382. [DOI] [PubMed] [Google Scholar]
- 10. Golcuk E, Yalin K, Bilge Kaya, et al. Usefulness of T(peak)‐T(end) interval to distinguish arrtythmogenic right ventricular cardiomyopathy from idiopathic right ventricular out flow tract tachycardia. Pacing Clin Electrophysiol 2014;37(12):1665–1670. [DOI] [PubMed] [Google Scholar]
- 11. Vitarelli A, Cortes Morichetti M, Capotosto L, et al. Utility of strain echocardiography at rest and after stress testing in arrhythmogenic right ventricular dysplasia. Am J Cardiol. 2013;111(9):1344–1350. [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography: A branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 13. Lindqvist P, Henein M, Kazzam E. Right ventricular outflow‐tract fractional shortening: An applicable measure of right ventricular systolic function. Eur J Echocardiogr. 2003;4(1):29–35. [DOI] [PubMed] [Google Scholar]
- 14. Yoerger DM, Marcus F, Sherrill D, et al. Echocardiographic findings in patients meeting task force criteria for arrhythmogenic right ventricular dysplasia: new insights from the multidisciplinary study of right ventricular dysplasia. J Am Coll Cardiol 2005;45(6):860‐865. [DOI] [PubMed] [Google Scholar]
- 15. Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J 1990;63:342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation 1982;65:384–398. [DOI] [PubMed] [Google Scholar]
- 17. Teske AJ, Cox MG, De Boeck BW, et al. Echocardiographic tissue deformation imaging quantifies abnormal regional right ventricular function in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Soc Echocardiogr 2009;22(8):920–927. [DOI] [PubMed] [Google Scholar]
- 18. Teske AJ, Prakken NH, De Boeck BWL, et al. Echocardiographic tissue deformation imaging of right ventricular systolic function in endurance athletes. Eur Heart J 2009;30:969–977. [DOI] [PubMed] [Google Scholar]
- 19. Sievers B, Addo M, Franken U, et al. Right ventricular wall motion abnormalities found in healthy subjects by cardiovascular magnetic resonance imaging and characterized with a new segmental model. J Cardiovasc Magn Reson 2004;6:601–608. [DOI] [PubMed] [Google Scholar]
- 20. Lindstrom L, Wilkenshoff UM, Larsson H, et al. Echocardiographic assessment of arrhythmogenic right ventricular cardiomyopathy. Heart 2001;86:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herbots L, Kowalski M, Vanhaecke J, et al. Characterizing abnormal regional longitudinal function in arrhythmogenic right ventricular dysplasia. The potential clinical role of ultrasonic myocardial deformation imaging. Eur J Echocardiogr 2003;4:101–107. [DOI] [PubMed] [Google Scholar]
- 22. Donal E, Raud‐Raynier P. Transthoracic tissue Doppler study of right ventricular regional function in a patient with an arrhythmogenic right ventricular cardiomyopathy. Heart 2004;90:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kraiem S, Chehaibi N, Bouladi W, et al. Doppler echocardiography and arrhythmogenic right ventricular dysplasia. Tunis Med 2002;80:801–806. [PubMed] [Google Scholar]
- 24. Prakasa KR, Wang J, Tandri H, et al. Utility of tissue Doppler and strain echocardiography in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol 2007;100(3):507–512. [DOI] [PubMed] [Google Scholar]
- 25. Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation 2005;112:3823–3832. [DOI] [PubMed] [Google Scholar]
- 26. Demir M, Uyan U. Evaluation of Tp‐e interval and Tp‐e/QT ratio in patients with non‐dipper hypertension. Clin Exp Hypertens 2014;36(5):285–288. [DOI] [PubMed] [Google Scholar]
- 27. Turrini P, Corrado D, Basso C, et al. Dispersion of ventricular depolarization:repolarization:a noninvasive marker for risk stratification inarrhythmogenic right ventricular cardiomyopathy. Circulation 2001;103(25):3075–3080. [DOI] [PubMed] [Google Scholar]
- 28. Fagundes ML, Maia IG, Cruz FE, et al. Arrhythmogenic cardiomyopathy ofthe right ventricle. Predictive value of QT interval dispersion to assessarrhythmogenic risk and sudden death. Arq Bras Cardiol 2000;75(2):115–124. [DOI] [PubMed] [Google Scholar]
- 29. Antzelevitch C, Shimizu W, Yan GX. Electrical heterogeneity and thedevelopment of arrhythmias In Olsson SB, Yuan S, Amlie JP. (eds.): Dispersion of Ventricular Repolarization: State of the Art. Armonk, NY: Futura Publishing Company, Inc;2000, p. 3 [Google Scholar]
- 30. Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle: The M cell. Circ Res 1991;68:1729. [DOI] [PubMed] [Google Scholar]
- 31. Gupta P, Patel C, Patel H, et al. T(p‐e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 2008;41:567–574. [DOI] [PubMed] [Google Scholar]
- 32. Zhao X, Xie Z, Chu Y, et al. Association between Tp‐e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. Clin Cardiol 2012;35:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung T, Kelleher S, Liu PY, et al. Effects of testosterone and nandrolone on cardiac function: A randomized, placebo‐controlled study. Clin Endocrinol 2007;66:235–245. [DOI] [PubMed] [Google Scholar]
- 34. Erikssen G, Liestøl K, Gullestad L, et al. The terminal part of the QT interval (T peak to T end): a predictor of mortality after acute myocardial infarction. Ann Noninvasive Electrocardiol 2012;17:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kayser HW, van der Wall EE, Sivananthan MU, et al. Diagnosis of arrhythmogenic right ventricular dysplasia: A review. Radiographics 2002;22:639–648. [DOI] [PubMed] [Google Scholar]
- 36. Zorzi A, Migliore F, Elmaghawri M, et al. Electrocardiographic predictors of electroanatomic scar size in arrhythmogenic right ventricular cardiomyopathy: Implications for arrhythmic risk stratification. J Cardiovasc Electrophysiol 2013;24:1321–1327. [DOI] [PubMed] [Google Scholar]
- 37. Wichter T, Schaffers M, Rhodes CG, et al. Abnormalities of cardiac sympathetic innervation in arrhythmogenic risht ventricular cardiomyopathy: Quantitative assessment of presynaptic norepinephrine reuptake and postsynaptic beta‐adrenergic receptor density with positron emission tomography. Circulation 2000;101:1552–1558. [DOI] [PubMed] [Google Scholar]
- 38. Shan K, Bick RJ, Poindexter BJ, et al. Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta‐adrenergic receptor density in humans. J Am Coll Cardiol 2000;36:891–896. [DOI] [PubMed] [Google Scholar]


