Abstract
Background
Hemodialysis (HD) patients are at high risk of sudden cardiac death (SCD). HD 6‐times/week (6x/wk) may reduce SCD risk compared to usual 3‐times/week HD (3x/wk) by mechanisms unknown. T‐wave alternans (TWA), heart rate turbulence (HRT), and ventricular ectopy (VE) are elevated in HD patients, but their response to 6x/wk HD has not been assessed.
Methods
Baseline and 1‐year Holter recordings were analyzed from enrollees in the Frequent Hemodialysis Network Daily Trial, a randomized trial comparing 3x/wk to 6x/wk in 245 chronic HD patients. TWA, HRT, and VE were assessed using MARS software.
Results
Sixty‐eight patients (34 with 6x/wk) had complete baseline and 1‐year Holter recordings. Mean age was 50 ± 13 years and 38% were female. Maximum TWA in the 3x/wk and 6x/wk groups were 52.4 μV at baseline and 51.2 μV at 1‐year versus 54.0 and 49.9 μV, respectively (P = 0.28). The proportion of abnormal HRT (scores of 1 or 2) in the 3x/wk group decreased from 65% to 56% at 1‐year versus 53% to 53% in the 6x/wk group (P = 0.58). Mean %VE changed from 1.6% to 2.9% in the 3x/wk group from baseline to 1‐year and from 2.1% to 3.7% in the 6x/wk group (P = 0.85).
Conclusions
There were no significant differences in HRT or VE at 1‐year in chronic HD patients randomized to 6x/wk versus 3x/wk and a trend in TWA reduction. Additional studies are needed to evaluate the impact and mechanisms of SCD in HD.
Keywords: dialysis, heart rate turbulence, sudden cardiac death, T‐wave alternans
Patients on chronic hemodialysis (HD) have a high mortality rate, estimated at 194 deaths per 1000 patient‐years annually. Of these, approximately one‐quarter are estimated to result from sudden cardiac death (SCD) due to arrhythmia, representing the single largest cause of death.1 As SCD typically results from arrhythmogenic triggers in the presence of a vulnerable substrate, many factors can be expected to contribute to this elevated risk in patients on HD. Common structural abnormalities include left ventricular hypertrophy, systolic dysfunction, and coronary artery disease. A number of arrhythmogenic triggers are also frequently found in this population, including fluid and electrolyte shifts related to the episodic nature of conventional HD and alterations in cardiac autonomic function.2, 3
Noninvasive markers of autonomic function and ventricular repolarization including heart rate turbulence (HRT) and T‐wave alternans (TWA) have been used to stratify risk for SCD in various populations and have been shown to be abnormal in a conventional HD cohort.4 Whether these markers of electrophysiologic vulnerability improve with alterations in the HD regimen has not been previously evaluated but has important clinical implications.
The Frequent HD Network Daily Trial examined the effects of frequent HD (six times per week) compared to conventional HD (three times per week). Though not powered to determine differences in rates of mortality or SCD, the study did show that frequent HD improved left ventricular mass as measured by cardiac MR. Furthermore, patients receiving more frequent HD had improved control of hypertension and hyperphosphatemia as well as better physical health composite scores (using the RAND 36‐item health survey). Additionally, a recent substudy showed an increase in the low frequency component of heart rate variability, indicating enhanced sympathetic nerve activity with frequent HD.5
There were, however, more adverse vascular complications associated with frequent HD often necessitating interventional procedures, for example to correct access failures.6
The primary objective of the present analysis is to describe the effects of frequent HD on heart HRT and TWA, and to explore which, if any, baseline patient characteristics appear to modify such an effect.
METHODS
The Frequent Hemodialysis Network (FHN): Daily Trial (NCT00264758) was a multicenter randomized controlled trial comparing conventional three times a week hemodialysis to hemodialysis six times per week.6 The FHN study was approved by the appropriate institutional review boards at the respective sites, and this ancillary study was approved by the institutional review board at Northwestern University. In the FHN study, 245 patients were randomized to either continue three times weekly HD or dialysis six times a week. Details of the inclusion and exclusion criteria as well as the specific details of the dialysis intervention have previously been described.5, 7
The study used the KCI X5 digital Holter recorder (KCI Technology and Services, Branchburg, NJ). Holters were performed on the first HD day after the weekend break and were initiated within one hour prior to the beginning of the HD session.7 Files were transferred to a MARS Holter Analysis Workstation v8.0.2 (GE Medical Systems, Milwaukee, WI) for analysis of HRT and TWA. Measurements were taken by a single observer blinded to the clinical information.
HRT was calculated if there was at least one clear ventricular premature depolarization (VPD) in the 24 hour recording. Turbulence onset and turbulence slope were calculated by the MARS software. Turbulence onset was determined as the percent difference between the mean of the first two RR intervals after the VPD and the last two RR intervals before the VPD. Turbulence onset was considered abnormal if it was ≥ 0%.8 Turbulence slope was defined as the slope of the maximum positive regression line assessed over 5 consecutive RR intervals within the 20 RR intervals that followed the VPD and was considered abnormal if it was ≤2.5 msec/RR.8 HRT score was then categorized into three groups. Patients received a score of zero if both turbulence onset and turbulence slope were normal, a score of one if either parameter was abnormal, or a score of two if both parameters were abnormal.
TWA was measured using the time domain modified moving average method, therefore allowing measurement during ambulatory recordings with changing heart rates. This method separates beats into even and odd categories, then averages them and compares those average waveforms.9 In our analysis, the averages were continuously updated by a weighting factor of 1/8th of the difference between the current beat and the average form. TWA were manually over‐read to ensure they were not due to noise or excessive numbers of VPDs with the 15 second window. All TWA were determined in periods of sinus rhythm. Initially, TWA ≥53 μV was set as the cutoff, above which was considered abnormal, as this level has been previously utilized in a dialysis cohort.4
Continuous variables were summarized by mean and standard deviation. Categorical variables were summarized by frequencies and proportions. Descriptive summaries of changes in treatment‐related variables were done for the constant cohort with nonmissing values at baseline and 12 months after randomization. For HRT, estimate of changes was expressed as percent difference in HRT score, both from normal (score of zero) to abnormal (score of one or two), as well as from abnormal to normal. For TWA, estimates of mean changes and treatment effects were expressed as percent differences in geometric means. We specified HRT score and maximum TWA value as the primary outcomes.5
The primary analyses for this manuscript were restricted to those patients with both baseline and follow‐up Holter measurements that could be used for HRT and TWA analyses. Only those patients in sinus rhythm were evaluated. For HRT, at least one VPD had to be present. For TWA, the absence of significant artifact was required to allow for accurate TWA measurements.
RESULTS
Of the 245 subjects enrolled in the FHN study, 207 provided Holter measurements at baseline and 131 of those also had Holter monitors at the 12‐month follow‐up, 68 of which were fully interpretable. Reasons for exclusion included excessive noise (most commonly) and lack of sinus rhythm (primarily due to atrial fibrillation, in one case due to atrial flutter) as is required for TWA analysis. Of these 68 used for analyses, half (34 patients) received dialysis six times per week (6x/wk) while the other half received dialysis three times per week (3x/wk). The vintage of dialysis was similar in both groups. There were no significant differences in baseline characteristics between those patients who had interpretable 12‐month Holters and those who did not. The mean age was 51.2 ± 10.5 in the 6x/wk group and 48.3 ± 11.8 in the 3x/wk group, P > 0.05. Baseline characteristics are listed on Table 1 and were similar with the exception of predialysis systolic blood pressure and interdialytic weight gain. Both of these characteristics were greater in the 3x/wk group than in the 6x/wk group. Additionally, prealbumin was slightly lower in the 6x/wk group. There was no significant difference in percent of patients using beta‐blockers or calcium channel blockers between the two groups.
Table 1.
Baseline Characteristics
| Characteristic | 3x/wk | 6x/wk | P Value |
|---|---|---|---|
| Age–Mean ± SD | 51.2 ± 10.5 | 48.3 ± 11.8 | 0.282 |
| Diabetes–N (%) | 13 (38.2) | 16 (47.1) | 0.624 |
| Heart failure–N (%) | 8 (23.5) | 6 (17.6) | 0.765 |
| Left ventricular ejection fraction (%) | 59 ± 8.4 | 59.9 ± 10.3 | 0.705 |
| Physical health composite score | 39.8 ± 7.6 | 41.5 ± 11.9 | 0.499 |
| Short physical performance battery | 8.6 ± 2.7 | 8.9 ± 2.7 | 0.626 |
| Urine volume (L) | 0.1 ± 0.4 | 0 ± 0.2 | 0.228 |
| Predialysis albumin (g/dl) | 4.1 ± 0.3 | 3.9 ± 0.4 | 0.020 |
| Hemoglobin (g/dl) | 11.9 ± 1.2 | 12 ± 0.8 | 0.496 |
| Predialysis systolic blood pressure (mmHg) | 147.7 ± 18.9 | 136.7 ± 18.3 | 0.017 |
| Difference in systolic blood pressure (mmHg) | 30.1 ± 11.6 | 27 ± 14 | 0.327 |
| Intradialytic weight gain (L) | −3.1 ± 0.9 | −2.3 ± 1.1 | 0.004 |
| Extracellular water/total body water | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.165 |
| Beta‐blocker N (%) | 21 (63.6) | 15 (44.1) | 0.144 |
| Calcium channel blocker N (%) | 15 (44.1) | 9 (26.5) | 0.204 |
| Average heart rate (bpm) | 78 ± 11.8 | 78.1 ± 10.5 | 0.974 |
| Max heart rate (bpm) | 149.8 ± 30.9 | 156.4 ± 37 | 0.428 |
Average heart rate remained the same at 77 beats per minute between groups both at baseline and follow‐up. Maximum TWA in the 3x/wk group was 52.4 ± 1.5 μV at baseline and 51.2 ± 1.7 μV at follow‐up. In the 6x/wk group, maximum TWA was 54.0 ± 1.5 μV at baseline and 49.9 ± 1.7 μV at follow‐up (treatment effect P = 0.27; Fig. 1). Patients with higher maximum TWA at baseline showed a greater decrease at 1‐year follow‐up (correlation r = –0.48, P < 0.0001; Fig. 2). This correlation was similar in both the 3x/wk and 6x/wk groups.
Figure 1.
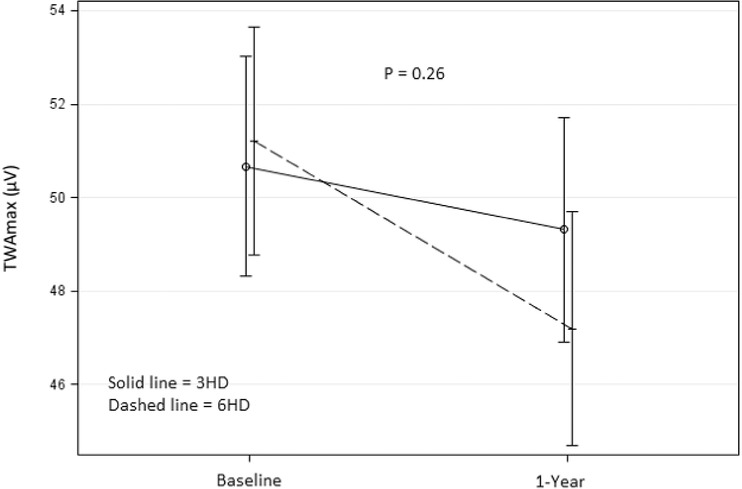
Max TWA change from baseline to follow‐up in 3x/wk (solid) and 6x/wk (dashed). Figure shows the change in maximum TWA from baseline to 1‐year follow‐up visit. Maximum TWA in the 6x/wk group (dashed) shows a greater decrease than the 3x/wk group (solid), however this difference was not significant (P = 0.26).
Figure 2.
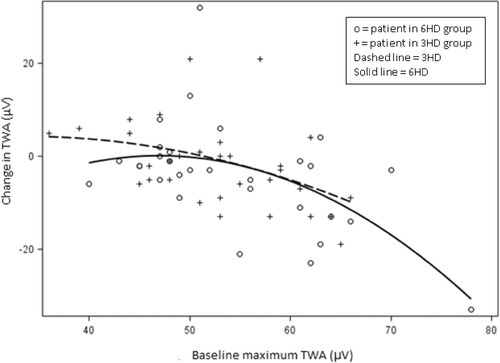
Baseline TWAmax as predictor of TWAmax change in 3x/wk (dashed) and 6x/wk (solid). Figure shows a negative correlation between baseline MaxTWA and MaxTWA change between baseline and follow‐up, r = –0.48, P < 0.0001, indicating that patients with higher MaxTWA at baseline tended to show greater decreases after 1 year. The slopes in the 3x/wk and 6x/wk groups appear similar.
The proportion of abnormal HRT (scores of 1 or 2) in the 3x/wk group decreased from 64.7 ± 8.3% to 55.9 ± 8.6% at 1‐year and remained stable from baseline to follow‐up at 52.9 ± 8.7% in the 6x/wk group (treatment effect p = 0.58). The mean percent of ventricular ectopy (VE) increased from 1.6 ± 0.6% to 2.9 ± 1.2% in the 3x/wk group from baseline to 1‐year and from 2.1 ± 0.6% to 3.7 ± 1.2% in the 6x/wk group (treatment effect P = 0.85). There was no significant association between TWA and LV mass in either group overall, however two patients from the 6x/wk group known to have LVH did show a decrease in TWA at 12 months.5
DISCUSSION
In this study of 68 chronic HD patients randomized to 6x/wk versus 3x/wk, there was no significant difference in TWA, HRT, or VE from baseline to 12‐month follow‐up. There was a trend toward improvement in TWA and HRT in the 6x/wk group, though this did not reach statistical significance. This is the largest study to date evaluating TWA and HRT in dialysis patients and the only one to compare TWA and HRT from a randomized trial of dialysis frequency.
HRT is a reflection of the baroreceptor sensitivity following VPDs and increased. HRT has been shown to be associated with an increased risk for SCD, especially in patients with coronary artery disease.10 TWA reflects intracellular calcium handling, resulting in heterogeneity of repolarization, and consequently serves as both a trigger of arrhythmias and as a marker for risk stratification in several populations.11 These measures were previously demonstrated to be reproducible in the dialysis population when evaluated pre‐ and postdialysis. 4
This study confirms that dialysis patients have high rates of abnormal markers of autonomic dysfunction and ventricular repolarization abnormalities, a result that has previously been demonstrated by Secemsky et al. and Green et al.4, 12 In particular the mean TWA of both groups at baseline was close to the previously demonstrated cut‐off for abnormal levels of TWA in dialysis patients. Such a level is above that associated with increased risk of SCD in post‐MI patients and only slightly below the level seen in patients with advanced heart failure.13, 14 This study also demonstrated that patients with the highest maximum TWA value had the greatest decrease from baseline to follow‐up. The implications of this finding are unclear, however, as this correlation was present in both the 3x/wk and 6x/wk groups.
The absence of a statistical difference in change in maximum TWA between the groups has two possible explanations. First, it is likely that the study is underpowered for the observed differences to reach statistical significance, as a sample size of 404 patients would have been needed given the study findings. Second, it is possible that TWA abnormalities in HD patients are not as closely associated with sudden cardiac death as they are in other populations as noted in a study by Green et al. of 19 HD patients, who used mean rather than the recommended peak TWA values 9 and found no association between TWA abnormalities and worse cardiovascular outcomes.12 This approach leaves open the question of whether, had they used peak TWA values, a different result would have been derived. In fact, the levels of TWA observed by Green et al. were four to five times lower than those observed by Secemsky et al.4 and in this study. The population studied here was overall similar to that in the Secemsky study with the exception of percent of patients taking beta‐blockers; 44% of the 3x/wk group and 64% of the 6x/wk group were receiving beta‐blockers as compared to 82% of the patients in the Secemsky study.4
While we did not see a difference in the measured noninvasive electrocardiographic parameters, there did appear to be a clinical benefit of more frequent dialysis with patients in the 6x/week dialysis group showing a decreased LV mass, increased physical health composite scores, lower predialys is phosphorus levels, and lower predialysis systolic blood pressure. 6
Interestingly, Chan et al. analyzed heart rate variability in the FHN Daily Trial and identified several patients with significant reductions in left ventricular mass and noted that these patients had concurrent changes in heart rate variability as well.5 Two of those patients from the 6x/wk group were also included in this study and both cases demonstrated a decrease in TWA, although the change was only 2–3 μV.
Abnormalities in TWA have been associated with increased left ventricular mass in both the ESRD and non‐ESRD populations. A study by Patel et al. evaluated exercise based TWA and demonstrated an association between abnormal TWA and increased LV mass that was greater in ESRD patients with LVH compared with non‐ESRD patients with LVH from hypertension.15 As Puntmann et al. demonstrated in a study of patients with hypertrophic cardiomyopathy (and normal renal function), increased LV mass, specifically increased inferior LV wall thickness was associated with abnormal TWA.16
This study had several limitations. First, it was based on collected data from a completed trial whose primary endpoint was based on cardiac MR. As a result, many patients lacked complete Holter data that limited the power of the analysis. Second, the nature of ambulatory Holter recordings is that there can be a significant amount of artifact that in several cases made large sections or even the entire recording uninterpretable. Third, the predictive value of abnormal TWA, HRT, and ventricular ectopy in the HD population has not been established, however given the high rates of SCD in this population, evaluation of potential markers appears worthy of investigation.
There were no significant differences in TWA, HRT, or VE with more frequent dialysis in this study of 68 HD patients. Whether more frequent hemodialysis or other changes in dialysate reduce sudden death in this high risk cohort is worthy of further study.
Ann Noninvasive Electrocardiol 2016;21(6):566–571
REFERENCES
- 1. System USRD . USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States In National Institutes of Health NIoDaDaKD (ed.). Bethesda, MD, 2013. [Google Scholar]
- 2. Passman R. Prevention of sudden cardiac death in dialysis patients: Drugs, defibrillators or what else. Blood Purif 2013;35:49–54. [DOI] [PubMed] [Google Scholar]
- 3. Herzog CA, Mangrum JM, Passman R. Sudden cardiac death and dialysis patients. Semin Dial 2008;21:300–307. [DOI] [PubMed] [Google Scholar]
- 4. Secemsky EA, Verrier RL, Cooke G, et al. High prevalence of cardiac autonomic dysfunction and T‐wave alternans in dialysis patients. Heart Rhythm 2011;8:592–598. [DOI] [PubMed] [Google Scholar]
- 5. Chan CT, Chertow GM, Daugirdas JT, et al. Effects of daily hemodialysis on heart rate variability: Results from the Frequent Hemodialysis Network (FHN) Daily Trial. Nephrol Dial Transplant 2014;29:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chertow GM, Levin NW, Beck GJ, et al. In‐center hemodialysis six times per week versus three times per week. N Engl J Med 2010;363:2287–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: Study design. Kidney Int 2007;71:349–359. [DOI] [PubMed] [Google Scholar]
- 8. Bauer A, Malik M, Schmidt G, et al. Heart rate turbulence: Standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol 2008;52:1353–1365. [DOI] [PubMed] [Google Scholar]
- 9. Verrier RL, Klingenheben T, Malik M, et al. Microvolt T‐wave alternans physiological basis, methods of measurement, and clinical utility—consensus guideline by International Society for Holter and Noninvasive Electrocardiology. J Am Coll Cardiol 2011;58:1309–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer A, Zurn CS, Schmidt G. Heart rate turbulence to guide treatment for prevention of sudden death. J Cardiovasc Pharmacol ;55:531–538. [DOI] [PubMed] [Google Scholar]
- 11. Verrier RL, Ikeda T. Ambulatory ECG‐based T‐wave alternans monitoring for risk assessment and guiding medical therapy: Mechanisms and clinical applications. Prog Cardiovasc Dis 2013;56:172–185. [DOI] [PubMed] [Google Scholar]
- 12. Green D, Batchvarov V, Wijesekara C, Kalra PA, Camm AJ. Dialysis‐dependent changes in ventricular repolarization. Pacing Clin Electrophysiol 2012;35:703–710. [DOI] [PubMed] [Google Scholar]
- 13. Sakaki K, Ikeda T, Miwa Y, et al. Time‐domain T‐wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: A prospective study. Heart Rhythm 2009;6:332–337. [DOI] [PubMed] [Google Scholar]
- 14. Verrier RL, Nearing BD, La Rovere MT, et al. Ambulatory electrocardiogram‐based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol 2003;14:705–711. [DOI] [PubMed] [Google Scholar]
- 15. Patel RK, Mark PB, Halliday C, et al. Microvolt T‐wave alternans in end‐stage renal disease patients–associations with uremic cardiomyopathy. Clin J Am Soc Nephrol 2011;6:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puntmann VO, Yap YG, McKenna W, Camm J. T‐wave alternans and left ventricular wall thickness in predicting arrhythmic risk in patients with hypertrophic cardiomyopathy. Circ J 2010;74:1197–1204. [DOI] [PubMed] [Google Scholar]


