Abstract
Introduction
A prolonged P‐wave duration (PWD) in sinus rhythm pre‐ablation has been hypothesized to be a non‐invasive ECG marker associated with increased atrial fibrillation (AF) recurrence after pulmonary vein isolation (PVI). This systematic review and meta‐analysis will assess the latest evidence on the association of prolonged PWD pre‐ablation with AF recurrence after PVI.
Hypothesis
Prolonged PWD pre‐ablation is associated with AF recurrence after PVI.
Methods
The inclusion criteria for this study are all cohort studies that assess prolonged PWD on ECG during sinus rhythm pre‐ablation and its association with AF recurrence in post‐PVI patients.
Results
There were 1,482 patients with AF post‐PVI from twelve cohort studies. The cut‐off points for prolonged PWD ranges from >120 ms to >150 ms. Meta‐analysis on six studies showed a pooled mean difference of PWD in subjects with recurrent AF and non‐recurring AF was 12.54 ms [8.76–16.31], p < 0.001; I 2 78%. Pooled odds ratio was 4.17 [2.10–8.31], p < 0.001; I 2 72% and pooled hazard ratio was 1.93 [1.10–3.39], p = 0.02; I 2 80%. Upon subgroup analysis, the association between prolonged PWD and AF recurrence was significant in signal‐averaged ECG, 12‐lead ECG, paroxysmal AF, >120–130 ms, and >140–150 ms PWD cut‐off point subgroups.
Conclusion
These findings suggest that prolonged PWD with a cutoff of >120 ms to >150 ms in sinus rhythm before ablation may be associated with AF recurrence after PVI regardless of age, gender, left atrial size, and the presence of structural heart disease. We also encouraged further studies that investigate predicting models to include prolonged PWD as one of their parameters.
Keywords: atrial fibrillation, atrial fibrillation recurrence, catheter ablation, prolonged P‐wave duration, pulmonary vein isolation
1. INTRODUCTION
Pulmonary vein isolation (PVI) has become the forefront treatment of atrial fibrillation (AF) with an impressive yet imperfect success rate (60%–80%) (Calkins et al., 2007, 2012). Unfortunately, the AF recurs in 30%–40% of the patients (Williams‐Andrews et al., 2009; Sato et al., 2010; Calkins et al., 2017). Recurrences occurred even in those with full isolation of the pulmonary vein (PV). Hence, re‐conduction is not the only possible cause (Callans et al., 2004; Gerstenfeld, Callans, Dixit, Zado, & Marchlinski, 2003). These causes include extra PV foci, atrial substrate remodeling leading to conduction delays (Van Beeumen, Houben, Tavernier, Ketels, & Duytschaever, 2010; Callans et al., 2004; Gerstenfeld et al., 2003; Ogawa et al., 2007; Redfearn et al., 2007).
There is no established tool to predict the AF recurrence after PVI. The atrial substrate remodeling resulted in conduction delay and an enlarged atrium which might be reflected by prolonged P‐wave duration (PWD) on the electrocardiogram (ECG) (Caldwell et al., 2013; Weber et al., 2018; Mugnai et al., 2016; Salah, Zhou, Liu, & Yan, 2013). A PWD in sinus rhythm pre‐ablation has been hypothesized to be a non‐invasive ECG marker associated with increased AF recurrence after PVI (Caldwell et al., 2013; Weber et al., 2018; Mugnai et al., 2016; Salah et al., 2013; Wu et al., 2016).
If proven to be useful, PWD can be a simple and efficient tool which will nevertheless be used to evaluate these patients. P‐wave duration measurement performed before ablation may give prognostic value and enables a more tailored approach. This systematic review and meta‐analysis will assess the latest evidence on the association of prolonged PWD pre‐ablation with AF recurrence after PVI from studies published until November 2018.
2. METHODS
2.1. Search strategy
We performed a comprehensive search on cohort studies that assess the association between prolonged PWD with the recurrence of AF in post‐PVI patients from inception up until November 2018. We searched [Prolonged p sinus wave atrial fibrillation pulmonary vein isolation recurrence] and its synonyms using PubMed, EuropePMC, EBSCOhost, Cochrane Central Database, ClinicalTrials.gov, and snowballing from potential articles cited by other studies. The records were then systematically evaluated using inclusion and exclusion criteria. Two researchers (E. Y and R. V) independently performed an initial search, discrepancies were resolved by discussion. A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow chart of the literature search strategy of studies was presented in Figure 1.
Figure 1.
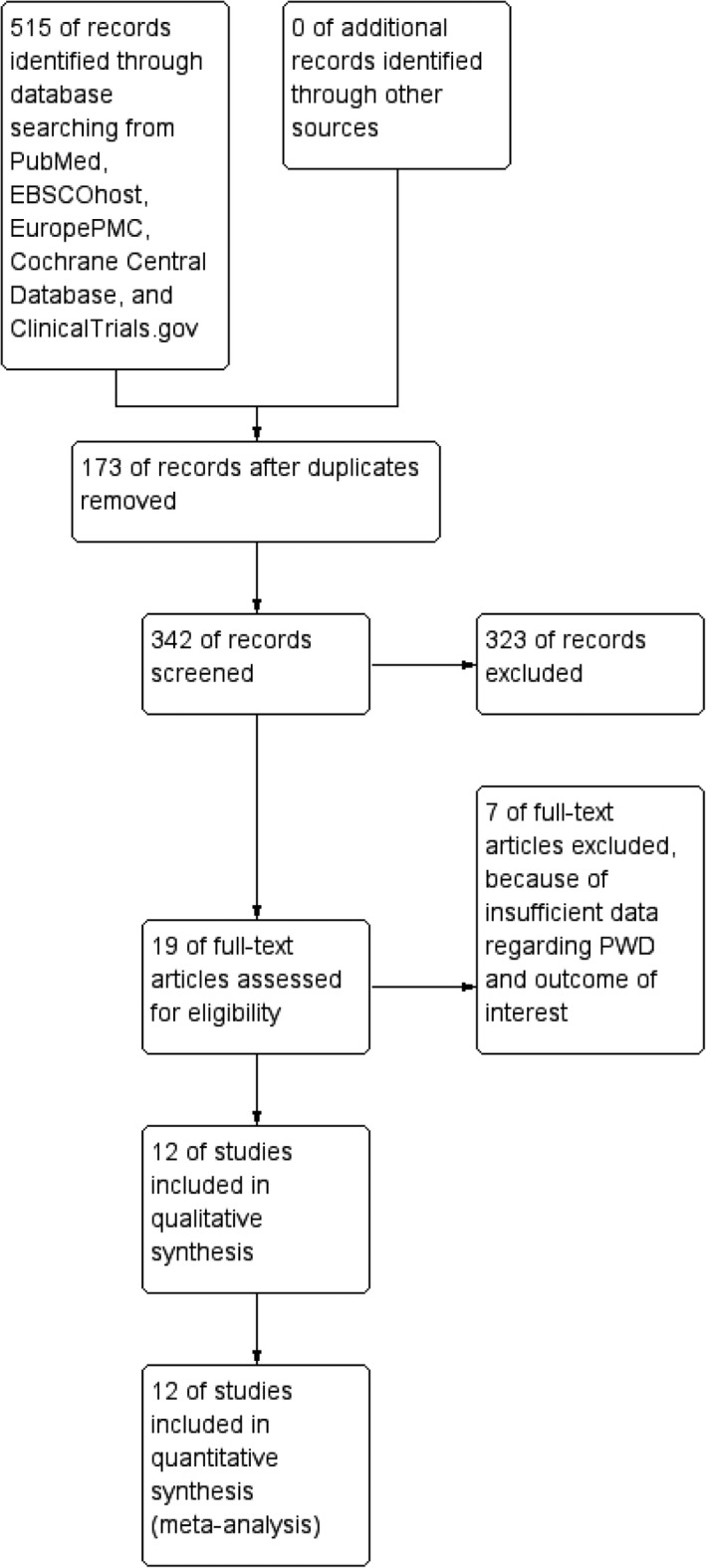
Study flow diagram
2.2. Selection criteria
The inclusion criteria for this study are all cohort studies that assess prolonged PWD on ECG during sinus rhythm pre‐ablation and its association with AF recurrence in post‐PVI patients. Cross‐sectional and case–control studies were excluded, as of those studies with insufficient data to assess the outcome of interest. The outcome measured was AF recurrence after PVI. We include all clinical researches/original articles and exclude case reports, review articles, and non‐English language articles.
2.3. Data extraction
Data extraction and risk of bias assessment were done by two independent authors (R. P and E. Y) using standardized extraction form which includes authors, year of publication, study design, sample size, type of AF, methods of measurement for P‐wave duration, P‐wave duration pre‐ablation and post‐ablation, cutoff for prolonged PWD, AF recurrence, and follow‐up length.
2.4. Statistical analysis
To perform the meta‐analysis, we used RevMan version 5.3 software (Cochrane Collaboration). We used the odds ratio (OR) and a 95% CI as a pooled measure for dichotomous data. We used mean difference (MD) and its standard deviation (SD) as a pooled measure for the continuous data. Inconsistency index (I 2) test which ranges from 0% to 100% was used to assess heterogeneity across studies. A value above 50% or p < 0.05 indicates statistically significant heterogeneity. We used the Mantel–Haenzsel method (for OR), and the Inverse Variance method (for MD) with a fixed‐effect model for meta‐analysis and a random‐effect model was used in case of heterogeneity. All p values were two‐tailed with a statistical significance set at 0.05 or below.
3. RESULTS
The search for studies that assess the association between prolonged PWD and recurrence of AF in post‐PVI patients yielded a total of potential 515 articles. We removed 173 duplicates. We excluded 323 articles after screening the titles and abstracts. There were 19 potentially relevant articles (Figure 1). We screened the full‐text articles, and after applying the inclusion criteria and exclusion criteria, seven studies had insufficient data regarding PWD and outcome of interest. We included twelve studies for qualitative synthesis, and twelve studies were available for meta‐analysis. There were 1,482 patients with AF post‐PVI from twelve cohort studies (Blanche, Tran, Rigamonti, Burri, & Zimmermann, 2013; Caldwell et al., 2013; Hagiwara et al., 2019; Weber et al., 2018; Knecht et al., 2018; Masuda et al., 2013; Mugnai et al., 2016; Nakatani et al., 2016; Ogawa et al., 2007; Okumura et al., 2007; Salah et al., 2013; Wu et al., 2016; Table 1). The subjects had either persistent or paroxysmal AF, and standard 12‐lead ECG measured the PWD in seven studies and signal‐averaged ECG in four studies. The cutoff for prolonged PWD ranges from >120 ms to >150 ms. The follow‐up ranges from the mean of 4 months to 32 months.
Table 1.
Result of the studies included in the qualitative synthesis
| Author | Year | Study design | Sample size (n) | Patients | Measurement | Prolonged PWD cutoff | Outcome | Follow‐up (mean) |
|---|---|---|---|---|---|---|---|---|
| Caldwell et al | 2014 | Cohort | 100 | Paroxysmal AF undergoing PVI | 12‐lead ECG | >140 ms | OR 2.41 [1.19–4.88]; 0.0214 sensitivity 69%, specificity 53%, PPV 45%, NPV 76% | 32 ± 14 months |
| Jadidi et al | 2018 | Cohort | 143 | Persistent AF undergoing PVI | 12‐lead ECG | >150 ms | OR 3.75 [1.86–7.55]; 0.0002 sensitivity 67%, specificity 65% | 12 months |
| Masuda et al | 2013 | Cohort | 88 | Paroxysmal AF undergoing PVI | Signal‐Averaged ECG | >130 ms | Adjusted OR 4.22 [1.52–11.7]; 0.006 sensitivity 54%, specificity 91%, PPV 73% | 16 ± 4 months |
| Higuchi et al | 2018 | Cohort | 113 | Drug‐resistant symptomatic persistent AF undergoing PVI | 12‐lead ECG | >126 ms | Adjusted HR 2.23 [1.20–4.15]; 0.01, Sensitivity 53%, Specificity 85% | 22.7 months |
| Mugnai et al | 2016 | Cohort | 201 | Drug‐resistant symptomatic Paroxysmal AF undergoing PVI | 12‐lead ECG | >120 ms | OR 5.970 [3.0030–11.8707];<0.0001 sensitivity 77.8%, specificity 62.6%, PPV 49%, NPV 86% | 22 ± 16 months |
| Wu et al | 2016 | Cohort | 204 | Paroxysmal AF undergoing PVI | 12‐lead ECG | >120 ms and biphasic (±) morphology in the inferior leads | HR 2.111 [1.034–4.308]; 0.040 sensitivity 66%, specificity 80% | 13.9 ± 6.2 months |
| Ogawa et al | 2007 | Cohort | 27 | Symptomatic drug‐refractory paroxysmal (92.6%)/persistent AF (6.4%) undergoing PVI | Signal‐Averaged ECG | N/A | PWD in AF recurrence was slightly longer than those without recurrence (168 ± 10 vs. 161 ± 7 msec; p < 0.05) | 16 ± 4 months |
| Blanche et al | 2013 | Cohort | 102 | Paroxysmal (59.8%)/Persistent AF (40.2%) undergoing PVI | Signal‐Averaged ECG | >140 ms | Sensitivity 69.4%, Specificity 53.0%, PPV 44.6%, NPV 76.0% AUC 0.695 [0.5861–0.8040]; 0.0011 | 12 ± 7 months |
| Salah et al | 2013 | Cohort | 198 | Symptomatic drug‐refractory paroxysmal AF undergoing PVI | 12‐lead ECG | >125 ms | Sensitivity 60%, specificity 90%, PPV 72%, NPV 83.7% AUC 0.858 [0.805–0.912];0.001 | 9 ± 3 months |
| Nakatani et al | 2016 | Cohort | 126 | Paroxysmal (62%) and Persistent AF (38%) undergoing PVI | 12‐lead ECG | >130 ms | Sensitivity 68%, specificity 66%, PPV 36%, NPV 88% AUC 0.706;<0.001 | 12 months |
| Okumura et al | 2007 | Cohort | 51 | Paroxysmal (62.7%) and Persistent AF (37.3%) undergoing PVI | Signal‐Averaged ECG | >150 ms | HR 10.3 (1.23–85.3), p = 0.03; sensitivity 93%, specificity 72%, PPV 58%, NPV 96% | 3–4 months |
| Knecht et al | 2018 | Cohort | 129 | Paroxysmal (65%)/Persistent AF (35%) undergoing PVI | 12‐lead ECG | >120 ms | HR: 2.612(1.248–5.466), p = 0.011 | 12 months |
AF: atrial Fibrillation; AUC: area under curve; ECG: electrocardiogram; HR: hazard ratio; NPV: negative predictive value; OR: odds ratio; PPV: positive predictive value; PVI: pulmonary vein isolation; PWD: P‐wave duration.
3.1. AF recurrence and mean difference of P‐wave duration pre‐ablation
Ten studies reported a statistically significant difference between the mean PWD of subjects with recurrent AF and those without (Blanche et al., 2013; Caldwell et al., 2013; Hagiwara et al., 2019; Mugnai et al., 2016; Nakatani et al., 2016; Ogawa et al., 2007; Okumura et al., 2007; Salah et al., 2013; Wu et al., 2016). Pooled mean difference of P‐wave duration in subjects with recurrent AF and non‐recurring AF was 12.54 ms [8.76–16.31]; p < 0.001 and high heterogeneity I 2 78%, p < 0.001 (Figure 2). On sensitivity analysis, no removal of single study resulted in a marked decrease in heterogeneity.
Figure 2.
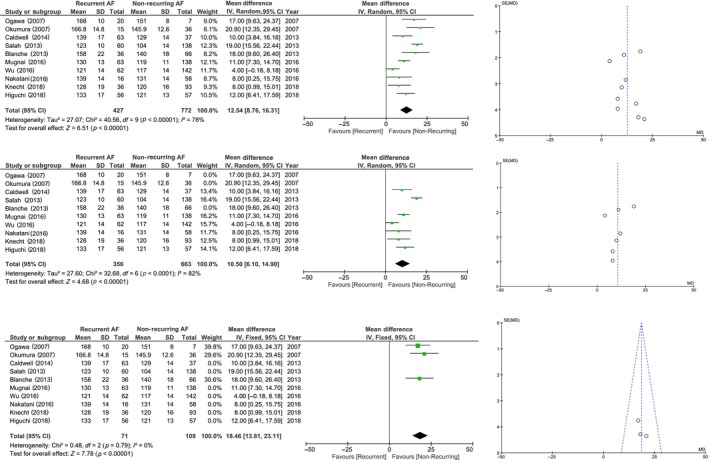
Forest plot and funnel plot pooled analysis of mean difference of P‐wave duration between recurrent and non‐recurring AF. Pooled analysis showed a longer P‐wave duration in recurrent AF compared to non‐recurring AF (a). Subgroup analysis of 12‐lead ECG only group (b) showed a high heterogeneity, meanwhile SAECG only group had low heterogeneity (c); both showed a longer P‐wave duration in recurrent AF group. AF: atrial fibrillation; ECG: electrocardiogram; SAECG: signal averaged electrocardiogram
3.2. AF recurrence and mean difference of P‐wave duration post‐ablation
Three studies reported a significantly longer PWD post‐ablation in those experiencing AF recurrence with a pooled mean difference of 16.54 ms [10.29–22.78], p < 0.001; moderate–high heterogeneity I 2 58%, p = 0.09 (Nakatani et al., 2016; Ogawa et al., 2007; Okumura et al., 2007). Removal of Nakatani et al. study reduces heterogeneity to I 2 0%, mean difference 19.77 ms [14.48–25.06], p < 0.001. Most likely due to Nakatani et al. had a different sample size in the measurement of the pre‐ablation (n = 74) and post‐ablation (n = 126) PWD.
3.3. Prolonged P‐wave duration and AF recurrence
Studies by Caldwell et al., Jadidi et al., Masuda et al., and Mugnai et al. reported that a prolonged PWD has a promising OR ranging from 2.41 to 5.97 in predicting the recurrence of AF in post‐PVI patients (Caldwell et al., 2013; Weber et al., 2018; Mugnai et al., 2016). Four studies reported sensitivity of 60%–93%, specificity of 53%–90%, positive predictive value (PPV) of 36%–72%, negative predictive value (NPV) of 76%–96%, and area under curve (AUC) of 0.695–0.858 (Blanche et al., 2013; Weber et al., 2018; Mugnai et al., 2016; Nakatani et al., 2016; Okumura et al., 2007; Salah et al., 2013; Wu et al., 2016). Quantitative synthesis was done by including five studies with pooled OR of 5.07 [3.43–7.49]; p < 0.001 and low–moderate heterogeneity I 2 45%, p = 0.12 (Figure 3a; Caldwell et al., 2013; Weber et al., 2018; Masuda et al., 2013; Mugnai et al., 2016; Okumura et al., 2007). Upon sensitivity analysis by removing one study at a time, Okumura et al. study were found to be the cause of heterogeneity; after removing their study, OR became 4.51 [3.01–6.75]; p < 0.001 and low heterogeneity I 2 4%, p = 0.37(Figure 3b). When we add a study using different criteria (PWD and biphasic morphology), pooled OR became 4.17 [2.10–8.31]; p < 0.001 and moderate–high heterogeneity I 2 72%, p = 0.004 (Figure 3c; Caldwell et al., 2013; Weber et al., 2018; Masuda et al., 2013; Mugnai et al., 2016; Okumura et al., 2007; Wu et al., 2016). Unfortunately, there is not enough data to obtain OR for the five remaining studies (Blanche et al., 2013; Hagiwara et al., 2019; Nakatani et al., 2016; Ogawa et al., 2007; Salah et al., 2013).
Figure 3.
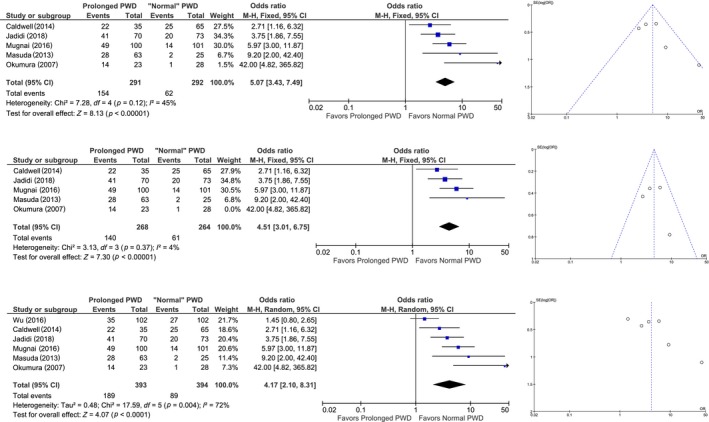
Forest plot and funnel plot of prolonged P‐wave duration and atrial fibrillation recurrence. Pooled analysis of odds ratio showing that prolonged P‐wave duration is associated with AF recurrence (a). Upon sensitivity analysis by removing Okumura et al. study showed a low heterogeneity (b). Upon addition of a study that used slightly different criteria (Wu et al), the heterogeneity was high. (c). AF: atrial fibrillation; PWD: P‐wave duration
Wu et al. reported a hazard ratio (HR) of 2.11, Knecht et al. HR of 2.612, Jadidi et al. HR of 1.91, Higuchi et al. an adjusted HR of 2.23, and Okumura et al. HR of 10.3 (Hagiwara et al., 2019; Weber et al., 2018; Knecht et al., 2018; Mugnai et al., 2016; Okumura et al., 2007). Pooled HR from five studies was 1.93 [1.10–3.39]; p = 0.02 and high heterogeneity I 2 80%, p < 0.001 (Figure 4a; Hagiwara et al., 2019; Weber et al., 2018; Knecht et al., 2018; Mugnai et al., 2016; Okumura et al., 2007). All studies included in the HR analysis had the PWD measured by 12‐lead ECG except Okumura et al. who used SAECG. Upon removal of Okumura et al. study who had a very high confidence interval and the only study in HR analysis using SAECG, the HR became 1.73 [1.01–2.95]; high heterogeneity I 2 81%, p = 0.001. On sensitivity analysis on five pooled studies, upon removal of Mugnai et al. study, the HR became 2.27 [1.59–3.25], p < 0.001; low heterogeneity I 2 0%, p < 0.49 (Figure 4b). Further removal of Okumura et al. study lead to HR of 2.17 [1.51–3.13]; low heterogeneity I 2 0%, p < 0.49.
Figure 4.
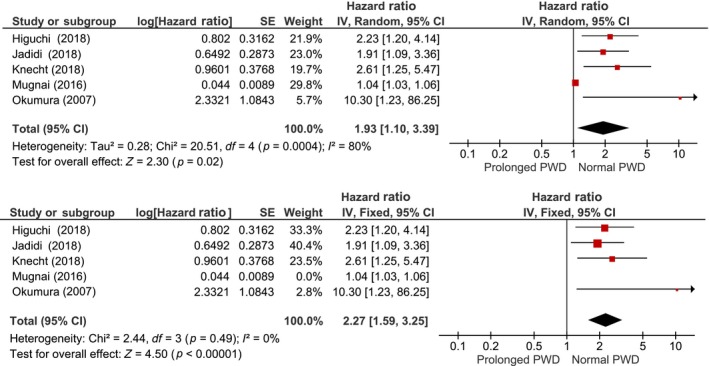
Pooled hazard ratio of prolonged P‐wave duration and atrial fibrillation recurrence. Pooled analysis of hazard ratio showing prolonged P‐wave duration is associated with AF recurrence (a), upon removal of Mugnai et al. study, the heterogeneity become 0% (b). PWD: P‐wave duration
3.4. Subgroup analysis
3.4.1. 12‐lead ECG and signal‐averaged ECG
We performed a subgroup analysis by separating those measured by 12‐lead ECG and SAECG. On a 12‐lead ECG only group (seven studies), the mean difference for the PWD was 10.50 [6.10–14.90]; high heterogeneity I 2 82%, p < 0.001; longer in those with AF recurrence (Caldwell et al., 2013; Hagiwara et al., 2019; Knecht et al., 2018; Mugnai et al., 2016; Nakatani et al., 2016; Salah et al., 2013; Wu et al., 2016; Figure 2b). Upon sensitivity analysis of the subgroup, removal of Salah et al. study resulted in a mean difference of 8.78 [6.01–11.56]; moderate heterogeneity I 2 37%, p = 0.16. Pooled OR from three studies was 3.03 [1.60–5.73], p < 0.001; high heterogeneity I 2 70%, p = 0.02 for those with prolonged PWD to have AF recurrence. Upon removal of Wu et al. study on sensitivity analysis, the OR became 4.16 [2.73–6.34], p < 0.001; low heterogeneity I 2 6%, p = 0.34. Pooled HR from four studies HR was 1.73 [1.01–2.95]; high heterogeneity I 2 81%, p = 0.001.
On subgroup analysis of SAECG only group, the mean difference for the PWD was 18.46 [13.81–23.11]; low heterogeneity I 2 0%, p = 0.79; longer in those with AF recurrence (Blanche et al., 2013; Ogawa et al., 2007; Okumura et al., 2007; Figure 2c). Pooled OR from two studies were 15.16 [4.40, 52.20], p < 0.001; low heterogeneity I 2 21%, p = 0.26 for those with prolonged PWD to have AF recurrence, however, the statistical power is low due to only two studies and pooled OR has a high range of 95% confidence interval.
3.4.2. Prolonged P‐wave duration and cutoff point
We also performed subgroup analysis by separating pooled measure into >120–130 ms and >140–150 ms cutoff point. Two studies were included in the >120–130 ms cutoff point group resulting in an OR of 6.56 [3.51–12.28], p < 0.001; low heterogeneity I 2 0%, p = 0.61 (Masuda et al., 2013; Mugnai et al., 2016). These studies had sensitivity and specificity of 54%–77.8% and 62.6%–91%. If Wu et al. study which used criteria of biphasic (±) morphology in the inferior leads in addition to PWD >120 ms were included, the OR became 3.13 [2.07–4.74]; high heterogeneity I 2 83%, p = 0.003. Three studies were included in the >140–150 ms group with an OR of 4.79 [1.76–13.03]; moderate heterogeneity I 2 63%, p = 0.79 (Caldwell et al., 2013; Weber et al., 2018; Okumura et al., 2007). These studies had sensitivity and specificity of 69%–94.3% and 53%–91.7% upon removal of Okumura et al. study OR became 3.28 [1.91–5.64], p < 0.001; low heterogeneity I 2 0%, p = 0.56.
3.4.3. Paroxysmal atrial fibrillation
We performed a subgroup analysis of studies that only include paroxysmal AF (Caldwell et al., 2013; Mugnai et al., 2016; Salah et al., 2013; Wu et al., 2016). Pooled analysis of four studies showed a mean difference of 11.09 [4.39, 17.79], p = 0.001; high heterogeneity I 2 90%, p < 0.001. No removal of single study resulted in a marked decrease in heterogeneity. A pooled analysis from four studies showed pooled OR of 4.91 [2.99–8.07], p = 0.003; high heterogeneity I 2 74%. Upon removal of Wu et al. study who used slightly different criteria for prolonged PWD, OR become 4.91 [2.99–8.07], p < 0.001; low heterogeneity I 2 30%, p = 0.24.
4. DISCUSSION
All studies demonstrated similar results, supporting the association between prolonged PWD and AF recurrence. These studies had slightly different methods of measuring the PWD, Mugnai et al. and Caldwell et al. assessed the ECG in the sinus rhythm within 6 and 12 months before procedure, respectively. The other studies assessed the ECG recorded before ablation. Blanche et al., Masuda et al., and Ogawa et al. used signal‐averaged ECG while the others used standard 12‐lead ECG. On subgroup analysis for the mean P‐wave duration, the SAECG had low heterogeneity 0%, 12‐lead ECG had high heterogeneity 84% meaning that although most of the studies have a longer P‐wave duration, and 12‐lead ECG was associated with high heterogeneity. Nakatani et al. and Wu et al. also discontinue antiarrhythmic drugs for at least five half‐lives before measuring the ECG, although continued after ablation for 2–3 months, most studies had a proportion of patients continuing antiarrhythmic at the time of ECG recording, and these were reported as the limitation of these studies (Nakatani et al., 2016; Wu et al., 2016). All of these studies, however, reported that the significant association between prolonged PWD and AF recurrence. Varying methods and antiarrhythmic drug discontinuation seemed to have a relatively minimal effect on the outcome of the study, which is further supported by Jadidi et al. and Blanche et al. that antiarrhythmic drug therapy status is not statistically significant on the recurrence of AF (Blanche et al., 2013; Weber et al., 2018). Another limitation of these studies is inter‐observer bias in ECG and echocardiography parameters (which were operator dependent). The studies resolve the possible bias in ECG by two independent cardiologists interpreting the ECG, some of the studies recorded the ECG in the system and enlarged the picture which can also improve the accuracy and reduce inter‐observer bias. Bias in echocardiographic results is more troublesome than ECG results since it is operator dependent. Performing echocardiography twice by different cardiologist also seems exhaustive. The result of Okumura et al. study showed a high HR for the association of prolonged PWD and the AF recurrence, this is probably due to the very small sample size in which the event for AF recurrence was only 15 and the weight in the meta‐analysis was only 5.7%. There is insufficient statistical power to expect a precise risk estimate in this study.
Prolonged PWD and the AF recurrence are independent of gender in all studies and age in all but one study. Salah et al. reported that prolonged PWD is not independent with age (Salah et al., 2013). Five studies reported that either prolonged PWD is independent of the presence of structural heart diseases or there is no significant association between the presence of structural heart disease and the AF recurrence (Blanche et al., 2013; Caldwell et al., 2013; Habib et al., 2015; Hagiwara et al., 2019; Weber et al., 2018; Okumura et al., 2007).
We may assume that prolonged PWD has the potential to become a reliable prognostic tool for both genders and all age groups, with or without structural heart disease.
Mugnai et al. only included patients with normal LA size (indexed diameter <24 mm/m2), a prolonged PWD is still a strong independent predictor of AF recurrence (OR 5.970 [3.0030–11.8707]; <0.0001) (Mugnai et al., 2016). A study by Jadidi et al. also indicated that even though patients with prolonged PWD >150 ms had a significantly larger LA diameter, prolonged PWD is an independent predictor of AF recurrence (OR 3.75 [1.86–7.55]; 0.0002) after adjustment to various covariates including LA diameter >45 mm (Weber et al., 2018). The LA diameter >45 mm by itself is not independently associated with AF recurrence. While Nakatani et al., Okumura et al., Higuchi et al., and Blanche et al. reported no significant difference in LA diameter in those who experienced recurrences versus those who did not (Blanche et al., 2013; Hagiwara et al., 2019; Nakatani et al., 2016; Okumura et al., 2007). A study by Caldwell et al. showed that there is a significant difference in LA dimension between patients that experienced recurrences compared to those that do not. However, there is no multivariate analysis in this study that can ensure its significance after adjustment (Caldwell et al., 2013). Meanwhile, Wu et al. also reported the significant association between LA size and AF recurrence, but since the p‐value was above 0.25, it was not included in the multivariate analysis by the authors and hence not an independent predictor (Wu et al., 2016). Only a study by Salah et al. that indicates that the P‐wave indices including prolonged P‐wave duration are not independent of LA size (Salah et al., 2013). Higuchi et al. concluded that LA volume lost its significance in multivariate analysis; in which only prolonged P‐wave duration ≥126 ms came out as significant (Hagiwara et al., 2019). We can assume that the prolonged PWD rather than LA size is more critical in predicting AF recurrence. The prolonged PWD may be as a result of other pathologies such as conduction delay, inflammation, fibrosis, excessive reactive oxygen species, downregulation of ion channels, and gap junctions (which may also trigger arrhythmias) that might not be accurately reflected by LA size (Pérez‐Riera et al., 2016; Sovari, 2016).
Our study showed that a longer P‐wave duration [mean difference 16.54 ms [10.29–22.78], p < 0.001] was associated with AF recurrence. Prolonged P‐wave duration has a pooled OR of 4.17 [2.10–8.31]; p < 0.001 of experiencing AF recurrence. Some of the studies reported a cutoff >120 ms to>150 ms. The heterogeneity can be reduced to 4% by excluding Wu et al. and Okumura et al. study. Upon subgroup analysis on cutoff point and exclusion of Okumura et al. study (Wu et al. study is included), we found that by separating cutoff point into >120–130 and >140–150 heterogeneity can decrease to I 2 0% in both groups, respectively. Although the pooled OR was significantly higher in the ≥120–130 ms group, the sensitivity and specificity were found to be greater in >140–150 ms group. We concluded that prolonged P‐wave duration >120 ms to >150 ms in sinus rhythm pre‐ablation is associated with AF recurrence regardless of LA size or presence of structural heart disease. Using the prolonged PWD duration measured before ablation as a variable to predict the recurrence of AF in post‐PVI patients may help the risk stratification, tailor the therapeutic approach, and may serve as a foundation for further research and as inclusion criteria for further studies. Further studies can also create a predictive model using prolonged PWD as one of their variables to create a prognostication tool for AF recurrence after PVI.
It is well known that pulmonary vein is significant in the initiation and maintenance of atrial fibrillation. Therefore, the intervention for AF is aimed to isolate the pulmonary vein from the atria electrically (Ogawa et al., 2007). Human pulmonary veins have muscle sleeves that extend beyond the PV‐left atrium junction and due to the discontinuous nature of these connections, conduction delay exists between LA and PV during both sinus and paced rhythms. Haissaguerre et al. demonstrated that PVs had a strong potential to initiate and perpetuate AF due to shorter refractory periods and abrupt changes in myofibril orientation (Haïssaguerre et al., 1998). This electrical abnormality is generally associated with structural abnormalities such as left atrial enlargement and fibrosis that causes atrial dysfunction (Ariyarajah, Mercado, Apiyasawat, Puri, & Spodick, 2005; Goyal & Spodick, 2001). These changes are called “electro‐anatomic remodeling” and are implicated in the initiation and perpetuation of AF (Daoud et al., 1996; Kumagai et al., 1991). With an additional area in the LA‐PV being conducted electrically, PV muscle sleeves undergo delayed activation compared to the atrial musculature. This conduction delay manifested in the electrocardiogram as a terminal portion of the P‐wave that mainly associated with PV depolarization. The conductance of this additional area of PV also creates an additional terminal segment of the P wave commonly referred to as atrial late potentials (ALP). The presence of ALP in the P wave creates a longer P‐wave duration that can be observed in patients with atrial fibrillations (Ogawa et al., 2007). Several studies have proven that the disappearance of ALP after the disconnection of LA‐PV conduction by ablation is associated with the cessation of atrial fibrillation post‐ablation. In comparison, cases of residual ALP after PV isolation were associated with AF recurrence and reconnection of LA‐PV conduction (Nakatani et al., 2016; Ogawa et al., 2007). P‐Wave morphology represents atrial electrical activation. This activation depends on the distance and velocity of electrical currents on the myocardium. Atrial remodeling caused changes in the conduction pathway on the myocardial tissue of the atrium; these changes are divided into structural and electrical remodeling and most commonly occurs together. Structural remodeling is signified by atrial enlargement visible by imaging modalities and electrical remodeling represented by prolonged electromechanical time which can be assessed using intracardiac electrode positioned in the right atrium. From the surface ECG, this electrical remodeling will be seen as a prolonged P‐wave duration. Specifically, electrical remodeling is a shortening of the refractory period with increased dispersion and reduction of atrial conductivity (Salah et al., 2013). In one particular study, Date et al. studied the association between PV cardiac muscle sleeves to the P wave using standard vectorcardiography and electrocardiography before and after pulmonary vein isolation. The study found that a change in P‐wave morphology occurred after PVI and the myocardial sleeves of the PV played a significant role in the formation of the middle segment of the P wave (DATE et al., 2007). Dabrowska‐Kugacka et al. assessed the association between P‐wave duration and atrial electromechanical delay and concluded that the P‐wave duration of the surface ECG is highly correlated with the atrial electromechanical delay. SALAH 11 This electromechanical delay can be explained by the cellular injury secondary to the atrial fibrillation itself. There is histologic evidence of oxidative stress and injury in the atrial myocardium. This results in peroxynitrite formation, which modifies myofibrillar proteins, contributes to loss of fibrillar protein function and alters myofibrillar energetics (Mihm et al., 2001). Said mechanism regarding cellular injury in AF was further cemented by the findings of Lin et al. in a study that found the extent of left atrial low voltage substrate (LA LVS) increased during the course of AF, with little to no LA LVS in early paroxysmal AF and extensive LA LVS in long‐standing or permanent forms (Lin et al., 2014).
Limitations in this systematic review and meta‐analysis include limitations in searching for literature in which authors tend to publish only positive studies, the literature included in the present study consists of only positive results which means that it is strongly associated or lack of negative study. This bias may lead to overestimating the association. Other limitations include the unavailability of data that needed for OR calculation in several studies. Another limitation is that these studies have different cutoff points varying from ≥120 ms to >150 ms. Fortunately, the difference is acceptably small. The procedural details between these studies also differ slightly in the methods of ECG recording, echocardiographic measurements, to the method of catheter ablation, and skills of the operators performing it may produce heterogeneity in the studies. One of the studies (Okumura et al.) also has a very wide 95% confidence interval, small sample, and event size. These studies also had limited data on post‐ablation P‐wave duration and change in PWD post‐ablation, such information may be valuable for further analysis. Although this study showed that post‐ablation PWD is associated with AF recurrence, it was based only from only three studies that reported post‐ablation PWD. One of them (Nakatani et al.) had a different sample size for pre‐ablation and post‐ablation data, and they also did not report the changes in PWD from pre‐ablation to post‐ablation in their results, and the article contained insufficient information to calculate changes in PWD manually and they also did not present the change in PWD in their results We did not analyze the change in P‐wave duration pre‐ablation and post‐ablation because of only two studies eligible with a small number of events contained sufficient data. Subgroup analysis of cutoff points was also based on a limited number of studies.
5. CONCLUSION
These findings suggest that prolonged PWD with a cutoff of >120 ms to >150 ms in sinus rhythm before ablation may be associated with AF recurrence after PVI regardless of age, gender, LA size, and the presence of structural heart disease. With current evidence, a cutoff of ≥120–130 ms may be used albeit with a lower sensitivity and specificity of >140–150 ms. We encourage further investigations to include a recording using SAECG for a more objective and accurate results, more focus on determining a single cutoff point for a prolonged PWD to by performing multiple analysis for different cutoff points to narrow down the >120 to >150 ms range in order to become more clinically applicable, and a longer follow‐up above 5 years to determine the long‐term significance. We also suggest future studies to include post‐ablation PWD and change in PWD after ablation. We also encouraged further studies that investigate the predictive model for post‐PVI prognostication to include prolonged PWD as one of their variables.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
AUTHORS' CONTRIBUTION
Raymond Pranata conceived and designed the study, interpreted the data, and drafted the manuscript. Emir Yonas and Rachel Vania performed a systematic search for the literature. Raymond Pranata and Emir Yonas extracted the data and assessed the risk of bias. Raymond Pranata performed the meta‐analysis in this study. All authors contributed to the writing of the manuscript.
Pranata R, Yonas E, Vania R. Prolonged P‐wave duration in sinus rhythm pre‐ablation is associated with atrial fibrillation recurrence after pulmonary vein isolation—A systematic review and meta‐analysis. Ann Noninvasive Electrocardiol. 2019;24:e12653 10.1111/anec.12653
REFERENCES
- Ariyarajah, V. , Mercado, K. , Apiyasawat, S. , Puri, P. , & Spodick, D. H. (2005). Correlation of left atrial size with P‐wave duration in interatrial block. Chest, 128(4), 2615–2618. 10.1378/chest.128.4.2615 [DOI] [PubMed] [Google Scholar]
- Bhargava, M. , Di Biase, L. , Mohanty, P. , Prasad, S. , Martin, D. O. , Williams‐Andrews, M. , … Natale, A. (2009). Impact of type of atrial fibrillation and repeat catheter ablation on long‐term freedom from atrial fibrillation: Results from a multicenter study. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 6(10), 1403–1412. 10.1016/j.hrthm.2009.06.014 [DOI] [PubMed] [Google Scholar]
- Blanche, C. , Tran, N. , Rigamonti, F. , Burri, H. , & Zimmermann, M. (2013). Value of P‐wave signal averaging to predict atrial fibrillation recurrences after pulmonary vein isolation. Europace, 15(2), 198–204. 10.1093/europace/eus251 [DOI] [PubMed] [Google Scholar]
- Caldwell, J. , Koppikar, S. , Barake, W. , Redfearn, D. , Michael, K. , Simpson, C. , … Baranchuk, A. (2013). Prolonged P‐wave duration is associated with atrial fibrillation recurrence after successful pulmonary vein isolation for paroxysmal atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 39(2), 131–138. 10.1007/s10840-013-9851-1 [DOI] [PubMed] [Google Scholar]
- Calkins, H. , Brugada, J. , Packer, D. L. , Cappato, R. , Chen, S.‐A. , Crijns, H. J. G. , … Shemin, R. J. (2007). HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow‐up. A report of the heart rhythm society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. developed in partnership with the european heart rhythm association (EHRA). Heart Rhythm: the Official Journal of the Heart Rhythm Society, 4(6), 816–861. 10.1016/j.hrthm.2007.04.005 [DOI] [PubMed] [Google Scholar]
- Calkins, H. , Kuck, K. H. , Cappato, R. , Brugada, J. , Camm, A. J. , Chen, S.‐A. , … Wilber, D. (2012). 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Journal of Interventional Cardiac Electrophysiology, 33(2), 171–257. 10.1007/s10840-012-9672-7 [DOI] [PubMed] [Google Scholar]
- Callans, D. J. , Gerstenfeld, E. P. , Dixit, S. , Zado, E. , Vanderhoff, M. , Ren, J. F. , & Marchlinski, F. E. (2004). Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. Journal of Cardiovascular Electrophysiology, 15(9), 1050–1055. 10.1046/j.1540-8167.2004.04052.x [DOI] [PubMed] [Google Scholar]
- Daoud, E. G. , Bogun, F. , Goyal, R. , Harvey, M. , Ching Man, K. , Adam Strickberger, S. , & Morady, F. (1996). Effect of atrial fibrillation on atrial refractoriness in humans. Circulation, 94(7), 1600–1606. 10.1161/01.CIR.94.7.1600 [DOI] [PubMed] [Google Scholar]
- Date, T. , Yamane, T. , Inada, K. , Matsuo, S. , Kanzaki, Y. , Miyanaga, S. , … Mochizuki, S. (2007). The effects of pulmonary vein isolation on the morphology of P waves: The contribution of pulmonary vein muscle excitation to the formation of p waves. Pacing and Clinical Electrophysiology, 30(1), 10.1111/j.1540-8159.2007.00570.x [DOI] [PubMed] [Google Scholar]
- Gerstenfeld, E. P. , Callans, D. J. , Dixit, S. , Zado, E. , & Marchlinski, F. E. (2003). Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: Implications for ablation strategies. Journal of Cardiovascular Electrophysiology, 14(7), 685–690. 10.1046/j.1540-8167.2003.03013.x [DOI] [PubMed] [Google Scholar]
- Goyal, S. B. , & Spodick, D. H. (2001). Electromechanical dysfunction of the left atrium associated with interatrial block. American Heart Journal, 142(5), 823–827. 10.1067/mhj.2001.118110 [DOI] [PubMed] [Google Scholar]
- Habib, G. , Lancellotti, P. , Antunes, M. J. , Bongiorni, M. G. , Casalta, J.‐P. , Del Zotti, F. , … Zamorano, J. L. (2015). 2015 ESC Guidelines for the management of infective endocarditis. European Heart Journal, 36(44), 3075–3123. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- Haïssaguerre, M. , Jaïs, P. , Shah, D. C. , Takahashi, A. , Hocini, M. , Quiniou, G. , … Clémenty, J. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England Journal of Medicine, 339(10), 659–666. 10.1056/NEJM199809033391003 [DOI] [PubMed] [Google Scholar]
- Higuchi, S. , Ejima, K. , Shoda, M. , Yamamoto, E. , Iwanami, Y. , Yagishita, D. , & Hagiwara, N. (2019). Impact of a prolonged interatrial conduction time for predicting the recurrence of atrial fibrillation after circumferential pulmonary vein isolation of persistent atrial fibrillation. Heart and Vessels, 34(4), 616–624. 10.1007/s00380-018-1272-8 [DOI] [PubMed] [Google Scholar]
- Jadidi, A. , Müller‐Edenborn, B. , Chen, J. , Keyl, C. , Weber, R. , Allgeier, J. , … Arentz, T. (2018). The duration of the amplified sinus‐p‐wave identifies presence of left atrial low voltage substrate and predicts outcome after pulmonary vein isolation in patients with persistent atrial fibrillation. JACC: Clinical Electrophysiology, 4(4), 531–543. 10.1016/j.jacep.2017.12.001 [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Pradella, M. , Reichlin, T. , Mühl, A. , Bossard, M. , Stieltjes, B. , … Sticherling, C. (2018). Left atrial anatomy, atrial fibrillation burden, and P‐wave duration‐relationships and predictors for single‐procedure success after pulmonary vein isolation. Europace, 20(2), 271–278. 10.1093/europace/euw376 [DOI] [PubMed] [Google Scholar]
- Kumagai, K. , Akimitsu, S. , Kawahira, K. , Kawanami, F. , Yamanouchi, Y. , Hiroki, T. , & Arakawa, K. (1991). Electrophysiological properties in chronic lone atrial fibrillation. Circulation, 84(4), 1662–1668. 10.1161/01.CIR.84.4.1662 [DOI] [PubMed] [Google Scholar]
- Lin, Y. , Yang, B. , Garcia, F. C. , Ju, W. , Zhang, F. , Chen, H. , … Chen, M. (2014). Comparison of left atrial electrophysiologic abnormalities during sinus rhythm in patients with different type of atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 39(1), 57–67. 10.1007/s10840-013-9838-y [DOI] [PubMed] [Google Scholar]
- Masuda, M. , Inoue, K. , Iwakura, K. , Okamura, A. , Toyoshima, Y. , Doi, A. , … Fujii, K. (2013). Impact of pulmonary vein isolation on atrial late potentials: Association with the recurrence of atrial fibrillation. Europace, 15(4), 501–507. 10.1093/europace/eus326 [DOI] [PubMed] [Google Scholar]
- Mihm, M. J. , Yu, F. , Carnes, C. A. , Reiser, P. J. , McCarthy, P. M. , Van Wagoner, D. R. , & Bauer, J. A. (2001). Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation, 104(2), 174–180. 10.1161/01.CIR.104.2.174 [DOI] [PubMed] [Google Scholar]
- Miyazaki, S. , Kuwahara, T. , Kobori, A. , Takahashi, Y. , Takei, A. , Sato, A. , … Takahashi, A. (2010). Long‐term clinical outcome of extensive pulmonary vein isolation‐based catheter ablation therapy in patients with paroxysmal and persistent atrial fibrillation. Heart, 97(8), 668–673. 10.1136/hrt.2009.186874 [DOI] [PubMed] [Google Scholar]
- Mugnai, G. , Chierchia, G.‐B. , de Asmundis, C. , Juliá, J. , Conte, G. , Sieira‐Moret, J. , … Brugada, P. (2016). P‐wave indices as predictors of atrial fibrillation recurrence after pulmonary vein isolation in normal left atrial size. Journal of Cardiovascular Medicine, 17(3), 194–200. 10.2459/JCM.0000000000000220 [DOI] [PubMed] [Google Scholar]
- Nakatani, Y. , Sakamoto, T. , Mizumaki, K. , Nishida, K. , Kataoka, N. , Tsujino, Y. , … Inoue, H. (2016). Coefficient of variation of p‐wave duration is a novel atrial heterogeneity index to predict recurrence of atrial fibrillation after catheter ablation. Journal of Cardiovascular Electrophysiology, 27(5), 542–548. 10.1111/jce.12920 [DOI] [PubMed] [Google Scholar]
- Ogawa, M. , Kumagai, K. , Vakulenko, M. , Yasuda, T. , Siegerman, C. , Garfinkel, A. , … Saku, K. (2007). Reduction of P‐wave duration and successful pulmonary vein isolation in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology, 18(9), 931–938. 10.1111/j.1540-8167.2007.00890.x [DOI] [PubMed] [Google Scholar]
- Okumura, Y. , Watanabe, I. , Ohkubo, K. , Ashino, S. , Kofune, M. , Hashimoto, K. , … Saito, S. (2007). Prediction of the efficacy of pulmonary vein isolation for the treatment of atrial fibrillation by the signal‐averaged P‐wave duration. PACE ‐ Pacing and Clinical Electrophysiology, 30(3), 304–313. 10.1111/j.1540-8159.2007.00670.x [DOI] [PubMed] [Google Scholar]
- Pérez‐Riera, A. R. , de Abreu, L. C. , Barbosa‐Barros, R. , Grindler, J. , Fernandes‐Cardoso, A. , & Baranchuk, A. (2016). P‐wave dispersion: An update. Indian Pacing and Electrophysiology Journal, 16(4), 126–133. 10.1016/j.ipej.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfearn, D. P. , Skanes, A. C. , Gula, L. J. , Griffith, M. J. , Marshall, H. J. , Stafford, P. J. , … Klein, G. J. (2007). Noninvasive assessment of atrial substrate change after wide area circumferential ablation: A comparison with segmental pulmonary vein isolation. Annals of Noninvasive Electrocardiology, 12(4), 329–337. 10.1111/j.1542-474X.2007.00182.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah, A. , Zhou, S. , Liu, Q. , & Yan, H. (2013). P wave indices to predict atrial fibrillation recurrences post pulmonary vein isolation. Arquivos Brasileiros de Cardiologia, 519–527, 10.5935/abc.20130214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasambu, B. , Balouch, M. A. , Zghaib, T. , Bajwa, R. J. , Chrispin, J. , Berger, R. D. , … Spragg, D. D. (2017). Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. Journal of Cardiovascular Electrophysiology, 29(2), 239–245. 10.1111/jce.13388 [DOI] [PubMed] [Google Scholar]
- Sovari, A. A. (2016). Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiology Research and Practice, 2016, 1–7. 10.1155/2016/9656078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beeumen, K. , Houben, R. , Tavernier, R. , Ketels, S. , & Duytschaever, M. (2010). Changes in P‐wave area and P‐wave duration after circumferential pulmonary vein isolation. Europace, 12(6), 798–804. 10.1093/europace/eup410 [DOI] [PubMed] [Google Scholar]
- Wu, J.‐T. , Long, D.‐Y. , Dong, J.‐Z. , Wang, S.‐L. , Fan, X.‐W. , Yang, H.‐T. , … Yang, C.‐K. (2016). Advanced interatrial block predicts clinical recurrence of atrial fibrillation after catheter ablation. Journal of Cardiology, 68(4), 352–356. 10.1016/j.jjcc.2015.10.015 [DOI] [PubMed] [Google Scholar]


