Abstract
Brugada syndrome (BrS) is an important cause of sudden cardiac death (SCD) with well‐defined ST‐segment elevation patterns on V1–V3. Observation of BrS‐Type‐electrocardiogram (ECG) patterns in medical conditions without true BrS is called “Brugada Phenocopy” (BrP). We present a case of 61‐year‐old male patient with hyperkalemia, hyponatremia, and BrS‐Type‐1 ECG pattern in the setting of acute postrenal failure. He was denying any syncope or family history of SCD. With normalization of electrolyte levels, BrS‐Type‐1‐ECG resolved. Electrolyte disturbances are one of the most common reasons of BrP. Being aware of BrPs and differentiating from an unmasked BrS‐ECG pattern could prevent patients from lethal consequences and unnecessary treatments.
Keywords: acute renal failure, Brugada phenocopy, Brugada syndrome, hyperkalemia, hyponatremia
CASE PRESENTATION
A 61‐year‐old Caucasian male admitted to our emergency department with complaints of lethargy and altered level of consciousness. His Glasgow Coma Scale (GCS) was 13. In physical examination, there were no remarkable findings apart from an obvious distended bladder. The patient denied any history of previous heart/cerebrovascular disease, seizures, syncope, chest pain, or family history of sudden cardiac death (SCD). He had a history of prostate surgery for benign prostate hyperplasia 1 week ago. On admittance electrocardiogram (ECG) (Fig. 1), rhythm was sinus with left posterior hemiblock. In V1, coved‐type‐ST‐elevation of “Brugada‐Type‐I pattern” was observed. In V2, there was typical saddleback ST‐segmemt elevation of “Brugada‐Type‐2 pattern.” Initial blood tests were as follows: blood urea nitrogen: 246.5 mmol/L; creatinine: 680.7 μmol/L; K: 7.1 mmol/L; Na: 124 mmol/L; glucose: 240 mg/dL; pH: 7.4; high sensitivity troponins negative on admission and 3‐hour check. Cranial magnetic resonance imaging and transthoracic echocardiography were normal. Acute postrenal failure was diagnosed and appropriate therapy with urinary catheterization and IV fluids promptly started. After electrolytes were normalized in 10 hours, previously observed Brugada‐Type‐1 pattern completely resolved and GCS developed to 15 (Figs. 2 and 3). No ventricular/atrial dysrhythmias were observed in the 24‐hour cardiac monitoring of the patient in the follow‐up.
Figure 1.
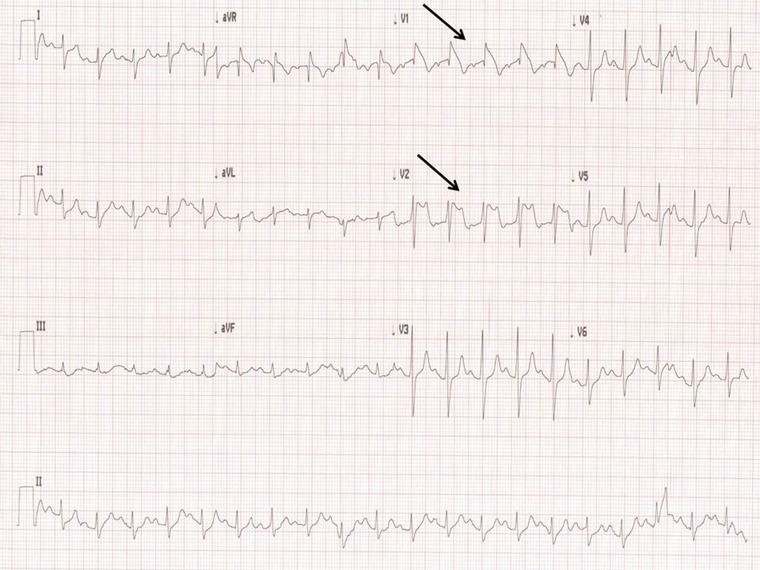
Initial ECG with Brugada‐Type‐1 pattern on V1, Brugada‐Type‐2 on V2, PR interval: 190 ms, and a frontal right axis deviation observed. Black arrows denote Brugada patterns.
Figure 2.
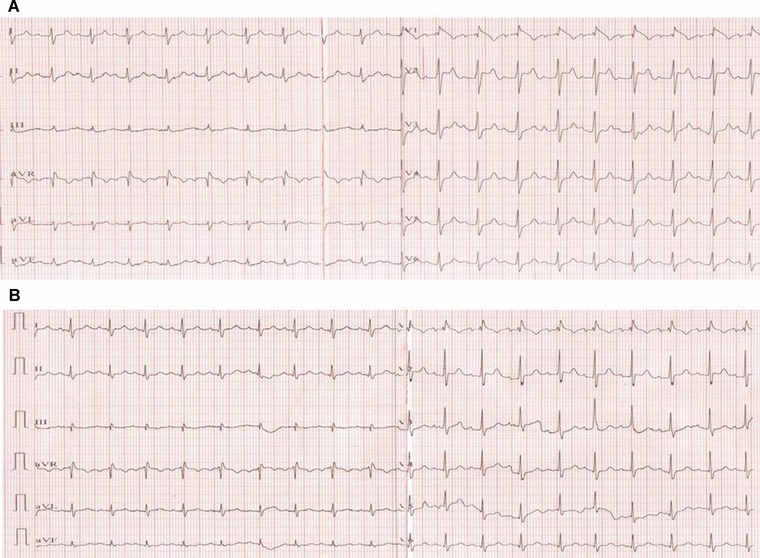
(A) ECG on the second hour of therapy. (B) ECG on the fifth hour of therapy. Changing of the typical patterns together with PR shortening and normalization of frontal QRS axis observed.
Figure 3.
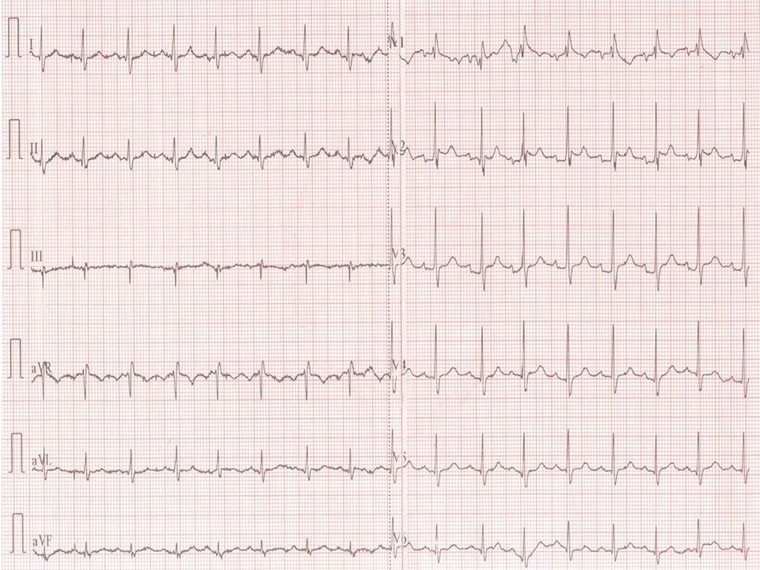
ECG after normalization of serum electrolyte levels on the tenth hour of therapy. Type‐1 pattern diminished on V1 revealing an incomplete right bundle branch block. PR: 170 ms. Brugada‐Type‐2 on V2 still visible with less J point elevation with slight leftward QRS axis.
DISCUSSION
Brugada syndrome (BrS) is a hereditary channelopathy characterized by well‐defined ST‐segment elevation patterns in V1–V3 and a high incidence of SCD in predominantly middle‐aged males with structurally normal hearts.1, 2 Brugada‐Type‐1 pattern, which is defined as a coved‐type slowly descending ST‐segment elevation of at least 2 mm (0.2 mV) followed by a negative T wave in >1 right precordial ECG leads, is the only diagnostic pattern.2 BrS is diagnosed with the observation of Type‐1 with the presence or absence of sodium channel blocking agent (NaCBA) together with at least one of the following clinical entities: documented ventricular fibrillation, polymorphic ventricular tachycardia (VT), family history of SCD less than 45 years old, Type‐1‐ECG in family members, VT inducibility with programmed electrical stimulation, syncope, or nocturnal agonal respiration.2 Suggested mechanisms define a reduced inward positively charged ionic currents taking part in the cardiac action potential (AP) (Na+ and Ca+2) and accentuated/unmatched outward currents (mainly K+) taking part in the repolarization leading to the prominence of the AP notch in the right ventricular (RV) epicardium relative to the endocardium because of the higher abundancy of outwardly directed K+ channels mainly on the RV outflow tract.3, 4 This produces a transmural voltage gradient manifesting the characteristic ECG patterns. BrS demonstrates an autosomal dominant mode of transmission with well‐defined loss‐of‐function mutations in SCN5A, the gene that encode the alpha‐subunit of the cardiac Na channel, but this mutation could be demonstrated only 18–30% of BrS probands.2, 4
Diagnostic pattern in BrS is highly dynamic and NaCBA or fever can unmask this mostly concealed pattern.2, 3, 4 However, a Type‐I pattern might also be observed in some settings without any clinical evidence of BrS. Recently, Riera et al.5 proposed the terminology “Brugada Phenocopy” (BrP) as “an environmental condition that imitates one produced by a gene” defining the conditions with reversible Brugada‐like‐ECG patterns without true BrS. A recently suggested algorithm recommends to sought for the pretest probability of a true congenital BS with the presence of a past medical or family history of syncope or SCD, to observe the clear resolution of the ECG pattern after the treatment of the attributed underlying cause (which shall not be a NaCBA or fever) and trying to perform a NaCBA test in order to differentiate between an unmasked pattern of true congenital BrS and BrP,6, 7 see http://www.brugadaphenocopy.com. BrPs can be observed with many factors such as metabolic conditions (mainly electrolyte disorders), mechanical compressions on RV, ischemia, myo/pericardial diseases, pulmonary embolism, ECG recording modulations, drugs and substances.7 Specifically, hyperkalemia has been shown to reproduce BrP by decreasing the resting membrane potential causing an indirect inactivation of the Na+ channels.8 Littmann et al.9 demonstrated that hyperkalemia‐induced BrP shows wider QRS, abnormal frontal QRS axis, and diminished P waves, which are generally not the case, in true BrS‐ECG patterns. Hyponatremia might contribute to the reduced Na+ current into the cell due to diminished Na‐gradient leaving the outward K+ channels unopposed also contributing to the typical ECG pattern.8, 10
The pretest probability of our patient for a true BrS was very low.2, 7, 8 The underlying reason of Type‐1‐ECG was not a BrS‐specific one and completely resolved with treatment. Unfortunately; patient did not give consent to an Ajmaline provocation test. Our case seems to fulfill the Type‐1‐BrP class B criteria according to the classification proposed by Gottschalk et al.11 Patient is still doing well without any dysrhythmic events after 1 year.
BrPs should always be kept in mind in the management of patients with Brugada‐like‐ECG patterns in order to prevent patients from further inappropriate tests and treatments. However, care must also be taken not to miss an unmasked true BrS‐ECG pattern with careful history taking and evaluating the precipitating factors for a possible NaCBA or fever episode.
REFERENCES
- 1. Bayes de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol 2012;45(5):433–442. [DOI] [PubMed] [Google Scholar]
- 2. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111:659–670. [DOI] [PubMed] [Google Scholar]
- 3. Antzelevitch C. The Brugada syndrome. Diagnostic criteria and cellular mechanisms. Eur Heart J 2001;22:356–363. [DOI] [PubMed] [Google Scholar]
- 4. Wilde AA, Postema PG, DiDiego JM, et al. The pathophysiological mechanism underlying Brugada syndrome depolarization versus repolarization. J Mol Cell Cardiol 2010;49:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riera ARP, Uchida AH, Schapachnik E, et al. Propofol infusion syndrome and Brugada syndrome electrocardiographic phenocopy. Cardiol J 2010;17:130–135. [PubMed] [Google Scholar]
- 6. Baranchuk A, Nguyen T, Ryu MH, et al. Brugada phenocopy: New terminology and proposed classification. Ann Noninvasive Electrocardiol 2012;17(4):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anselm DD, Evans JM, Baranchuk A. Brugada phenocopy: A new electrocardiogram phenomenon. World J Cardiol 2014;6(3):81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST‐Segment elevation. Circulation 1999;100:1660–1666. [DOI] [PubMed] [Google Scholar]
- 9. Littmann L, Monroe MH, III Taylor L, et al. The hyperkalemic Brugada sign. J Electrocardiol 2007;40:53–59. [DOI] [PubMed] [Google Scholar]
- 10. Mok N, Tong C, Yuen H. Concomitant‐acquired long QT and Brugada syndromes associated with indapamide induced hypokalemia and hyponatremia. Pacing Clin Electrophysiol 2008;31(6):772–775. [DOI] [PubMed] [Google Scholar]
- 11. Gottschalk B, Anselm DD, Baranchuk A. Brugada phenocopy: Morphological classification and importance of provocative testing. Ann Noninvasive Electrocardiol 2014;19(6):604–605. [DOI] [PMC free article] [PubMed] [Google Scholar]


