Abstract
Ebstein's anomaly is a congenital heart disease where the most important anatomic feature is the inferior displacement of the tricuspid valve leaflets. Vectorcardiographic features are mainly forgotten and electrocardiographic features may be unrecognized by cardiologists handling adult patients.
Keywords: cyanotic congenital heart disease, Ebstein's anomaly, electrocardiogram, electrophysiological abnormalities, vectorcardiogram
1. INTRODUCTION
Ebstein's anomaly is a rare congenital heart defect characterized by inferior displacement of the septal tricuspid leaflets and atrialization of the right ventricle (RV), and accounts for <1% of all newly diagnosed congenital disorders. It encompasses a wide anatomical and physiological spectrum and the disorder can present itself either as cyanosis in the neonatal life and early childhood or exercise intolerance in the older adult.
The heterogeneous spectrum of presentation of its structural anomalies, and associated cardiomyopathy, accounts for a widely varied clinical and hemodynamic manifestation of the pathology and its impact on timing of diagnosis and prognosis. There is a wide spectrum of pathology with an infinite variety of combinations of severity of the involved structures. Neonatal Ebstein's anomaly is characterized by reduced survival, while an average risk of mortality per year of 1%–1.4% has been reported in infancy and adulthood. Accessory pathway‐related arrhythmia, atrial flutter, and atrial fibrillation are frequent complications; it is clear that atrial flutter and atrial fibrillation (AF) are uncommon in the neonatal period. Medical management and a careful clinical and echocardiographic follow‐up are advisable for all asymptomatic patients and those with minimal abnormalities. Radiofrequency catheter ablation (RFCA) is a standard treatment, and depending on the patient's size, first‐line treatment for supraventricular tachycardia supported by pathways and atrioventricular (AV) nodal reentry tachycardia. RFCA is not the treatment of choice for atrial fibrillation unless it is a secondary arrhythmia due to frequent pathway‐induced supraventricular tachycardia. Pulmonary vein trigger and left atrial substrate is probably a relatively rare pathogenesis of AF in patients with Ebstein's anomaly. For the worst spectrum of Ebstein's anomaly surgical palliation and staging to one or one and a half ventricle repair, but biventricular repair is often not applied. Patients often face revision surgery. Surgical correction is also recommended in the presence of progressive dilation of the right atrium (RA) and RV, development of RV dysfunction, or episodes of paradoxical embolization, reduced exercise capacity, or significant desaturation. RFCA or cryoablation is recommended as first‐line treatment for supraventricular and ventricular tachycardia. Prosthetic valve replacement and repair of the tricuspid valve are both common strategies in the correction of tricuspid regurgitation. Mortality is not limited only to the immediate postsurgical period. Only with ample knowledge, this complex and diverse group of patients can be correctly treated in order to improve not only duration, but also quality of life (Geerdink & Kapusta, 2014).
2. ETIOLOGY
Ebstein's anomaly is a rare (≈1–5.2 per 200,000 live births), complex congenital anomaly with a broad pathologic‐anatomical and clinical spectrum accounting for <1% of all congenital heart diseases (Bjornard, Riehle‐Colarusso, Gilboa, & Correa, 2013; Correa‐Villasenor, Ferencz, Neill, Wilson, & Boughman, 1994). Anatomical components of Ebstein's anomaly (Figure 1).
Figure 1.
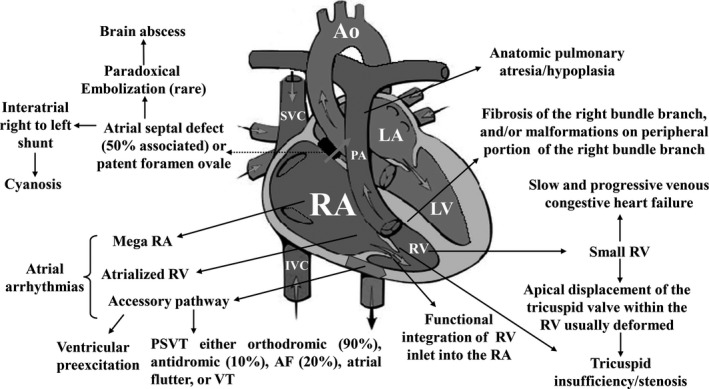
Anatomic aspects and their correlation with clinical manifestations. This figure relates comprehensively the possible modifications found in Ebstein's anomaly, with the potential to cause clinical‐hemodynamic changes. This anomaly is graded as mild (mild cyanosis and symptoms, and mild‐to‐moderate cardiomegaly), moderate (few symptoms despite marked cardiomegaly), and severe (cyanosis and severe symptoms). Ao: aorta; IVC: inferior vena cava; LA: left atrium; PSVT: paroxysmal supraventricular tachycardia; RA: right atrium; RV: right ventricle; SVC: superior vena cava; VT: ventricular tachycardia
In 1988, Carpentier et al. proposed the following classification of Ebstein's anomaly: type A, the volume of the true RV is adequate; type B, a large atrialized component of the RV exists, but the anterior leaflet of the tricuspid valve moves freely; type C, the anterior leaflet is severely restricted in its movement and may cause significant obstruction of the right ventricular outflow tract; and type D, almost complete atrialization of the ventricle except for a small infundibular component.
Celermajer et al. (1992) described an echocardiographic grading score for neonates with Ebstein's anomaly, extended Glasgow Outcome Scale, with grades 1–4. The ratio of the combined area of the RA and atrialized RV is compared with that of the functional RV and left heart (ratio <0.5, grade 1; ratio of 0.5–0.99, grade 2; ratio of 1.0–1.49, grade 3; ratio ≥1.5, grade 4).
3. ECG FEATURES IN EBSTEIN'S ANOMALY
3.1. P wave
Tall P waves (≥2.5 mm) occur in about a third of the patients and are attributable to right atriomegaly. In close to 50% of cases, the P‐wave amplitude is >3 mm. A prolonged P‐wave duration is occasionally registered. This pattern has been ascribed to prolonged aberrant conduction in the enlarged RA (Kastor et al., 1975; Macruz et al., 1968). In some cases, giant tall peaked P waves (called “Himalayan” P waves; Kaushik, Sharma, & Kashyap, 2007), abnormal in height (≥5 mm), duration, and shape may be observed.
An electrocardiographic characteristic very suggestive of Ebstein's anomaly is constituted by the contrast between the giant P wave as opposed to the corresponding atypical right bundle branch block (RBBB) pattern of V1.
3.2. PR interval
The PR interval can be: (a) short when ventricular pre‐excitation is associated with or without delta (δ) wave without a history of paroxysmal tachycardia; (b) prolonged (20% of cases): PR interval duration and the width of the P wave correlate with prolonged conduction in the large RA (Makous & Vander Veer, 1966). First degree AV block occurs in 42% of patients (Ho et al., 2000). Another possibility is intra‐atrial conduction disturbance, AV nodal conduction prolongation, or infranodal conduction disturbance (intra His or infra His with RBBB; 3) normal.
3.3. QRS duration: prolonged
QRS prolongation is the result of prolonged activation of the atrialized RV and is less fully manifest in infants. The conduction disturbance is always distal to the right bundle branch (Kastor et al., 1975) and is sometimes present despite a septal accessory pathway (AP). RBBB is often present after anteroseptal pulmonary artery ablation. One‐third of patients with Ebstein's anomaly and symptomatic tachyarrhythmias have minimal or absent ECG features of ventricular pre‐excitation. In these patients, the absence of RBBB pattern is a strong predictor of an AP (Iturralde et al., 2006). Atypical RBBB is observed in ≈60% of cases and nonspecific intraventricular conduction delay in ≈15% in neonates (Barbara, Edwards, Connolly, & Dearani, 2008).
3.4. QRS axis (SÂQRS)
It is generally inferior and to the right between +90 and +130°, but the range varies widely from −30 to −170°. The QRS axis is inferior, although a splintered polyphasic QRS makes the axis difficult to determine. On the other hand, when associated with right anterior and inferior AP, the QRS axis is located in the left superior quadrant.
3.5. QRS patterns
Incomplete or complete RBBB is registered in approximately 44% of cases and is thought to result mainly from a paucity of conduction fibers in the atrialized RV with abnormally low R (>7 mm) and S waves over the right precordium. Incomplete or complete RBBB patterns result from infra‐Hisian conduction disturbance and abnormal activation of the atrialized RV (Hebe, 2000).
It is frequent to record tri‐ or tetraphasic QRS patterns (Figure 2). RBBB is observed in ≈85% of cases (Figure 3).
Figure 2.
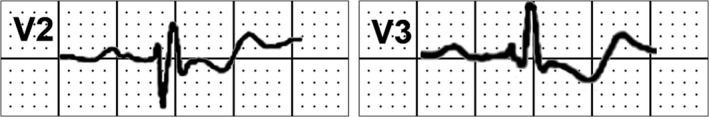
Tetraphasic QRS pattern in leads V2 and V3
Figure 3.
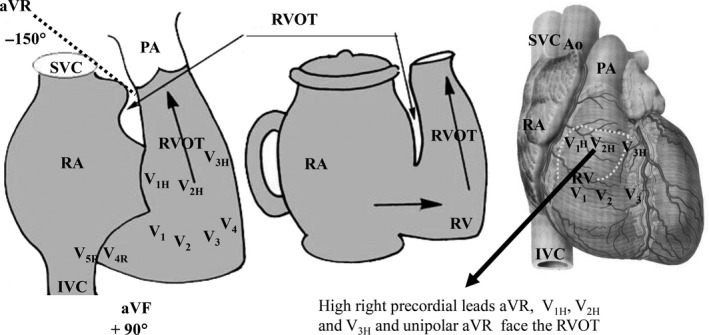
Right ventricular hypertrophy classification by the RV region predominantly hypertrophied and its corresponding lead. RV trabecular region: V2 and V3. When tetraphasic QRS is registered in V2–V3, Ebstein's anomaly is one diagnostic possibility; Inferior right paraseptal region: V3 and V4; RV free wall: from V1 to V4; Basal infundibular region, RVOT or crista supraventricularis: aVR, V1H, V2H, and V3H; RV inflow tract: V4R, V5R, and aVF. Ao: aorta; IVC: inferior vena cava; PA: pulmonary artery; RA: right atrium; RV: right ventricle; RVOT: right ventricular outflow tract; SVC: superior vena cava
Pre‐excitation and WPW syndrome are more frequently seen in Ebstein's anomaly than in any other congenital heart diseases. APs are predominantly right‐sided anterior and inferior, manifest, and localize to the lower half of the anatomic tricuspid annulus (Wei et al., 2014; Figure 4). APs are revealed in 6%–30% of patients with Ebstein's anomaly and can lead to both supraventricular and ventricular tachyarrhythmias. The most common supraventricular tachyarrhythmias are AP‐mediated reciprocating tachycardia, AV nodal reentrant tachycardia, and atrial flutter or AF. Tachyarrhythmias cause palpitation, syncope, or sometimes cardiac arrest and are more poorly tolerated due to the hemodynamic and anatomic abnormalities associated with Ebstein's anomaly. Mahaim nodoventricular fibers are likely to be present when left bundle branch block (LBBB) pattern occurs during sinus rhythm or during an episode of tachycardia (Smith et al., 1982).
Figure 4.
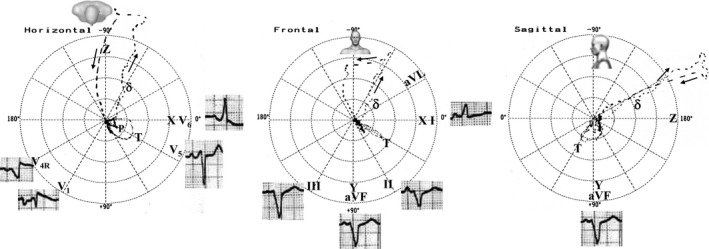
ECG/VCG with right‐sided anterior and inferior AP pre‐excitation. VCG of a 13‐year‐old boy with Ebstein's anomaly and right‐sided anterior and inferior AP. Note the “comets” of the initial part of the QRS are closer and erratic, indicating slow conduction on initial activation via AP (δ wave). In the horizontal plane, the QRS loop is directed backwards (right‐sided anterior and inferior AP). The electric axis shows upper displacement in the frontal plane. In the absence of WPW, the QRS axis is lower and the rotation is clockwise or in eight, except in some cases, which are counterclockwise (Ellison & Restieaux, 1972). S > R in V1 indicates that the AP is on the right side and lower because the QRS complexes in II, III, and aVF are predominantly negative
3.6. Fragmented QRS
Abnormal conduction through the atrialized RV leads to fragmented QRS (fQRS) on the ECG. Its presence suggests a more severe abnormality and a higher risk of arrhythmia (Park et al., 2013). fQRS disappears after corrective surgery with resection of the atrialized RV (Acharya, Ang, & Gitler, 2017).
Deep Q waves in the right and middle precordial leads (from V1 to V4) are recorded in 50% of the cases. These distinctive Q waves occur because the precordial surface leads record RV intracavitary potentials and usually far leftward as a result of the large size of the RA (Bialostozky, Medrano, Munoz, & Contreras, 1972; Sodi‐Pallares, Bisteni, & Herrmann, 1952). This explains the indirect sign of atrialized RV enlargement: qR in V1 (Sodi‐Pallares sign). The volumetric increase of the RA gets it closer to the exploring electrode V1, recording initial QRS negativity in this lead, because this electrode records the epicardial morphology of the RA (Sodi‐Pallares et al., 1952).
3.7. Arrhythmias
In ~30% of the cases, paroxysmal supraventricular tachycardia (orthodromic or antidromic), atrial flutter, AF, and ventricular tachycardia are registered. Permanent atrial standstill has been reported in familial Ebstein's anomaly. (Pierard, Henrard, & Demoulin, 1985). The persistent atrial standstill with slow escape rhythm was most probably a consequence of the abnormalities in both the atrial wall and the His bundle which, together with the abnormal attachment of the tricuspid valve, may be features of the same congenital cardiac anomaly.
4. MAHAIM ACCESSORY PATHWAYS
4.1. Mahaim fibers
The term Mahaim conduction is used to describe decremental conduction connections between the RA or between the AV node and the RV or near the right branch. Although these conductions are rare, their unique electrophysiological properties make their diagnosis and treatment complicated. The true nature of these still obscure pathways makes them particularly interesting from the electrophysiological point of view (Katritsis et al., 2017). In 1941, Mahaim and Winston described the histology of abnormal connections originating from the AV node with RV distal insertion. This was the first description of the Mahaim Node‐Ventricular Pathways or “Mahaim Accessory Pathways” (MAPs). Ueshima et al. reported a case of a 7‐year‐old girl with atriofascicular Mahaim (AFM) pathway concomitant with Ebstein's anomaly. The ECG showed LBBB. Holter showed PR interval prolongation and QRS morphological change during sinus tachycardia. An electrophysiological study demonstrated that the distal His potential appeared earlier than the proximal His potential, which suggested retrograde His conduction through the AV node. Conduction from the Mahaim fiber to the His bundle was faster than that from the AV‐node toward the His bundle. The findings allowed a differential diagnosis between AFM and WPW syndrome (Ueshima, Nakamura, Takeno, Miyake, & Takemura, 2017). Ebstein's anomaly is often accompanied by either WPW syndrome or AFM. Therefore, it is important to differentiate between them with regard to treatment and associated risks. AFM pathway indicates AP that demonstrate decremental conduction property without ventriculoatrial conduction (Tchou, Lehmann, Jazayeri, & Akhtar, 1988). Differential diagnosis between AFM and WPW syndrome is very important because both of them cause antidromic AV reciprocating tachycardia; sometimes it can only be diagnosed in the electrophysiology laboratory. One of the points to consider differentiating AFM from WPW syndrome is the change of PR interval. In WPW syndrome, PR interval during sinus rhythm is stable because APs do not exhibit decremental conduction property. Meanwhile in AFM, both PR interval and QRS waveform often change due to decremental conduction property in the Mahaim fiber. In this case, the Holter monitoring demonstrated a change in the PR interval and narrowing of the QRS complex, which supported the diagnosis of AFM rather than WPW syndrome.
Main electrophysiological features in Ebstein's anomaly:
Intra‐atrial conduction disturbance: (RA) P‐wave abnormalities, PR interval prolongation
AV nodal conduction: PR prolongation
Infranodal conduction: intra‐His or infra‐His conduction abnormalities
RBBB distal to the right bundle branch
Right‐sided anterior and inferior AP
Supraventricular tachycardias
Atrial fibrillation
Atrial flutter
Arrhythmias originating from the atrialized portion of the RV
Mahaim fibers‐mediated arrhythmias
Deep Q waves from V1 to V4 (it is an indirect signal of RA enlargement)
5. VECTORCARDIOGRAM
The Frank VCG in Ebstein's anomaly shows prolongation of the QRS loop/complex owing to RBBB. In patients between 1 day and 31 years old, with the mean age of 8.6 years, the QRS duration was increased in all patients, averaging 103 ms (Ellison & Restieaux, 1972). The delay is secondary to RBBB because delayed conduction being primarily in the terminal of the QRS loop/complex. In patients with right‐sided anterior and inferior AP, QRS prolongation is a consequence of delayed initial forces. In infants from 1 to 3 days of age there is prolongation of QRS (between 80–100 ms). In normal conditions, the newborns have a QRS duration average of 50 ms.
RV voltages are reduced as shown by decrease in the right maximum spatial voltage (RMSV; 0.6 ± 0.4 mV) and 10–20 ms vectors, and by the predominantly leftward orientation of the HP and FP QRS loops. The RMSV is usually of greater magnitude in patients with little or without tricuspid regurgitation than in those with severe tricuspid insufficiency. The QRS axis in the majority of patients shows a leftward inferior frontal axis. In presence of right‐sided anterior and inferior AP, the QRS axis has extreme left axis deviation.
HP: QRS loop rotation is counterclockwise (CCW) or eight‐shaped, and the loop is oriented to the left and predominantly posteriorly. This reflects the reduction in the RV potentials.
In the presence of right‐sided anterior and inferior AP, initial conduction delay is observed (δ loop) and the early forces directed to the left.
FP: QRS axis is located in the left inferior quadrant, and the QRS loop rotation is clockwise or eight‐shaped. In the presence of right‐sided anterior and inferior AP, the QRS axis has extreme left axis deviation.
LSP: Rotation is predominantly CCW and the reduction in anterior forces could be seen. P loop is oriented in the normal direction, to the left, inferiorly and anteriorly. Clear right atrial enlargement loop is the rule. The T loops are directed predominantly to the left and posteriorly; 50% directed inferiorly and 50% directed superiorly.
6. CONCLUSIONS
Ebstein's anomaly encompasses a wide anatomical and physiological spectrum. Accessory pathway‐related arrhythmia, atrial flutter, and atrial fibrillation are frequent complications, although that atrial flutter and atrial fibrillation are uncommon in the neonatal period. The ECG is of great value to raise the suspicion of Ebstein's anomaly, and it is very useful to guide future diagnostic imaging methods, both noninvasive and invasive. The association of giant P waves with prolonged PR interval and atypical, low voltage RBBB in the right precordial leads constitute the hallmark of this entity. Pre‐excitation and WPW syndrome are more frequently seen in Ebstein's anomaly than in any other congenital heart diseases, and APs can lead to both supraventricular and ventricular tachyarrhythmias. Ebstein's anomaly is also often accompanied by AFM, and certain ECG features help to distinguish between the WPW syndrome and AFM. The VCG is a relevant additional diagnostic tool, because it allows for the diagnosis of ventricular pre‐excitation (right‐sided anterior and inferior AP) with the presence of comets very close to one another in the initial part of the QRS loop.
CONFLICTS OF INTEREST
None.
Pérez‐Riera AR, Barbosa‐Barros R, Daminello‐Raimundo R, de Abreu LC, Nikus K. Electro‐vectorcardiographic and electrophysiological aspects of Ebstein's anomaly. Ann Noninvasive Electrocardiol. 2019;24:e12590 10.1111/anec.12590
REFERENCES
- Acharya, P. , Ang, J. R. , & Gitler, B. (2017). Ebstein anomaly with QRS fragmentation on electrocardiogram. Journal of Investigative Medicine High Impact Case Reports, 5(1), 2324709616688710 10.1177/2324709616688710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara, D. W. , Edwards, W. D. , Connolly, H. M. , & Dearani, J. A. (2008). Surgical pathology of 104 tricuspid valves (2000–2005) with classic right‐sided Ebstein's malformation. Cardiovascular Pathology, 17(3), 166–171. 10.1016/j.carpath.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Bialostozky, D. , Medrano, G. A. , Munoz, L. , & Contreras, R. (1972). Vectorcardiographic study and anatomic observations in 21 cases of Ebstein's malformation of the tricuspid valve. American Journal of Cardiology, 30(4), 354–361. 10.1016/0002-9149(72)90565-6 [DOI] [PubMed] [Google Scholar]
- Bjornard, K. , Riehle‐Colarusso, T. , Gilboa, S. M. , & Correa, A. (2013). Patterns in the prevalence of congenital heart defects, metropolitan Atlanta, 1978 to 2005. Birth Defects Research Part A: Clinical and Molecular Teratology, 97(2), 87–94. 10.1002/bdra.23111 [DOI] [PubMed] [Google Scholar]
- Carpentier, A. , Chauvaud, S. , Mace, L. , Relland, J. , Mihaileanu, S. , Marino, J. P. , … Guibourt, P. (1988). A new reconstructive operation for Ebstein's anomaly of the tricuspid valve. Journal of Thoracic and Cardiovascular Surgery, 96(1), 92–101. [PubMed] [Google Scholar]
- Celermajer, D. S. , Cullen, S. , Sullivan, I. D. , Spiegelhalter, D. J. , Wyse, R. K. , & Deanfield, J. E. (1992). Outcome in neonates with Ebstein's anomaly. Journal of the American College of Cardiology, 19(5), 1041–1046. 10.1016/0735-1097(92)90291-T [DOI] [PubMed] [Google Scholar]
- Correa‐Villasenor, A. , Ferencz, C. , Neill, C. A. , Wilson, P. D. , & Boughman, J. A. (1994). Ebstein's malformation of the tricuspid valve: Genetic and environmental factors. Teratology, 50(2), 137–147. 10.1002/tera.1420500208 [DOI] [PubMed] [Google Scholar]
- Ellison, R. C. , & Restieaux, N. J. (1972). Vectorcardiography in congenital heart disease. A method for estimating severity (pp. 175–183). Philadelphia, PA; London, UK; Toronto, ON: W.B. Saunders Company. [Google Scholar]
- Geerdink, L. M. , & Kapusta, L. (2014). Dealing with Ebstein's anomaly. Cardiology in the Young, 24(2), 191–200. 10.1017/S1047951113001169 [DOI] [PubMed] [Google Scholar]
- Hebe, J. (2000). Ebstein's anomaly in adults. Arrhythmias: Diagnosis and therapeutic approach. Thoracic and Cardiovascular Surgeon, 48(4), 214–219. 10.1055/s-2000-6897 [DOI] [PubMed] [Google Scholar]
- Ho, S. Y. , Goltz, D. , McCarthy, K. , Cook, A. C. , Connell, M. G. , Smith, A. , & Anderson, R. H. (2000). The atrioventricular junctions in Ebstein malformation. Heart, 83(4), 444–449. 10.1136/heart.83.4.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturralde, P. , Nava, S. , Salica, G. , Medeiros, A. , Marquez, M. F. , Colin, L. , … Gonzalez, M. D. (2006). Electrocardiographic characteristics of patients with Ebstein's anomaly before and after ablation of an accessory atrioventricular pathway. Journal of Cardiovascular Electrophysiology, 17(12), 1332–1336. 10.1111/j.1540-8167.2006.00617.x [DOI] [PubMed] [Google Scholar]
- Kastor, J. A. , Goldreyer, B. N. , Josephson, M. E. , Perloff, J. K. , Scharf, D. L. , Manchester, J. H. , … Hirshfeld, J. W. (1975). Electrophysiologic characteristics of Ebstein's anomaly of the tricuspid valve. Circulation, 52(6), 987–995. 10.1161/01.CIR.52.6.987 [DOI] [PubMed] [Google Scholar]
- Katritsis, D. G. , Boriani, G. , Cosio, F. G. , Hindricks, G. , Jais, P. , Josephson, M. E. , … Rickard, J. (2017). European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE). Europace, 19(3), 465–511. 10.1093/europace/euw301 [DOI] [PubMed] [Google Scholar]
- Kaushik, M. L. , Sharma, M. , & Kashyap, R. (2007). 'Himalayan' p wave. Journal of the Association of Physicians of India, 55, 856. [PubMed] [Google Scholar]
- Macruz, R. , Tranchesi, J. , Ebaid, M. , Pileggi, F. , Romero, A. , & Decourt, L. V. (1968). Ebstein's disease. Electrovectorcardiographic and radiologic correlations. American Journal of Cardiology, 21(5), 653–660. 10.1016/0002-9149(68)90262-2 [DOI] [PubMed] [Google Scholar]
- Makous, N. , & Vander Veer, J. B. (1966). Ebstein's anomaly and life expectancy. Report of a survival to over age 79. American Journal of Cardiology, 18(1), 100–104. 10.1016/0002-9149(66)90201-3 [DOI] [PubMed] [Google Scholar]
- Park, S. J. , Chung, S. , On, Y. K. , Kim, J. S. , Yang, J. H. , Jun, T. G. , … Huh, J. (2013). Fragmented QRS complex in adult patients with Ebstein anomaly and its association with arrhythmic risk and the severity of the anomaly. Circulation: Arrhythmia and Electrophysiology, 6(6), 1148–1155. 10.1161/CIRCEP.113.000636 [DOI] [PubMed] [Google Scholar]
- Pierard, L. A. , Henrard, L. , & Demoulin, J. C. (1985). Persistent atrial standstill in familial Ebstein's anomaly. British Heart Journal, 53(6), 594–597. 10.1136/hrt.53.6.594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W. M. , Gallagher, J. J. , Kerr, C. R. , Sealy, W. C. , Kasell, J. H. , Benson, D. W., Jr., … Grant, A. O. (1982). The electrophysiologic basis and management of symptomatic recurrent tachycardia in patients with Ebstein's anomaly of the tricuspid valve. American Journal of Cardiology, 49(5), 1223–1234. 10.1016/0002-9149(82)90048-0 [DOI] [PubMed] [Google Scholar]
- Sodi‐Pallares, D. , Bisteni, A. , & Herrmann, G. R. (1952). Some views on the significance of qR and QR type complexes in right precordial leads in the absence of myocardial infarction. American Heart Journal, 43(5), 716–734. 10.1016/0002-8703(52)90047-1 [DOI] [PubMed] [Google Scholar]
- Tchou, P. , Lehmann, M. H. , Jazayeri, M. , & Akhtar, M. (1988). Atriofascicular connection or a nodoventricular Mahaim fiber? Electrophysiologic elucidation of the pathway and associated reentrant circuit. Circulation, 77(4), 837–848. 10.1161/01.CIR.77.4.837 [DOI] [PubMed] [Google Scholar]
- Ueshima, K. , Nakamura, Y. , Takeno, S. , Miyake, T. , & Takemura, T. (2017). Atriofascicular Mahaim with Ebstein anomaly: A case report. Journal of Arrhythmia, 33(5), 508–510. 10.1016/j.joa.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, W. , Zhan, X. , Xue, Y. , Fang, X. , Liao, H. , Deng, H. , … Wu, S. (2014). Features of accessory pathways in adult Ebstein's anomaly. Europace, 16(11), 1619–1625. 10.1093/europace/euu028 [DOI] [PubMed] [Google Scholar]


