Abstract
Background
Early repolarization (ER) and acute ST segment elevation myocardial infarction (STEMI) are sharing the pathophysiology of J wave syndromes. It is speculated that early ventricular arrhythmias (VAs) during STEMI may be predisposed by ER. Our aim was to study the association between ER pattern and risk of VAs during acute STEMI.
Methods
The study included 102 male patients with acute STEMI who were divided into two groups: cases and controls. Cases included 52 patients with sustained VAs during the first 48 hours from the onset of STEMI, while controls included 50 patients with no VAs. On 12‐lead surface electrocardiogram, ER was defined as ≥ 1 mm elevation of J point in at least two inferior or lateral leads with or without ST segment elevation.
Results
Mean age was 48.44 ± 10.08 years and mean left ventricular ejection fraction (LVEF) was 42.25 ± 11.1%. ER pattern was more frequent in cases than controls (29 vs 14 patients, P = 0.008). Notched J wave (P = 0.0007) and horizontal ST segment (P = 0.033) were more frequent in cases than controls. On adjusted regression model, LVEF (OR: 0.95, 95% CI: 0.91–0.99, P = 0.015) and ER (OR: 3.39, 95% CI: 1.41–8.12, P = 0.006) could predict VAs, while QTc interval (P = 0.24) and QTd (P = 0.86) did not have predictive effect. Inferior/inferolateral and global ER pattern (P = 0.044 and 0.031 respectively), notched J wave (P = 0.001), increasing J wave amplitude (P = 0.042), and ST segment elevation (P = 0.001) were associated with a higher risk of VAs.
Conclusions
ER is associated with increased risk of VAs in the setting of acute STEMI.
Keywords: early repolarization, ST segment elevation, myocardial infarction, STEMI, ventricular arrhythmias, J wave syndromes
Early repolarization (ER) is a common electrocardiographic pattern manifested as ≥ 1 mm J point elevation with or without ST segment elevation.1 Although it has been widely considered as a benign electrocardiographic finding commonly expressed in young healthy individuals with structurally normal hearts, cumulative data over the last decade suggest its association with idiopathic ventricular fibrillation (VF) and sudden cardiac death.2, 3, 4, 5 Various gene mutations affecting outward potassium current and inward calcium current are implicated in the pathophysiology of early repolarization syndrome (ERS).6, 7
In normal individuals, action potential (AP) notch is slightly deeper in the epicardium than the endocardium, especially in males. The small transmural gradient at the time of AP notch may result into small elevation (< 1 mm) of the J point that may appear as small slurring or notching of the terminal part of QRS complex which usually passes unnoticed. If the AP notch is accentuated in the epicardium, larger transmural gradient will manifest as ≥ 1 mm J point elevation with or without ST segment elevation. This occurs in Brugada syndrome, ERS, ST segment elevation myocardial infarction (STEMI), and hypothermia which are collectively known as J wave syndromes.6 In both ER and STEMI, the J point elevation is due to transmural gradient generated by deep AP notch in the epicardium. When the accentuation of the epicardial AP notch exceeds a certain limit, there is failure of activation of L‐type calcium channels and hence, AP dome. The regional loss of AP dome precipitates phase 2 reentry in which the dome is propagated from the endocardium to the epicardium, then back to the endocardium in the form of a new AP.6, 7 This “ping pong” AP propagation needs a precipitating factor that regionally inhibits AP dome formation, otherwise the pathophysiology of ER will stop at being just an ECG pattern. Such precipitating factors include vagal stimulation during sleep and drugs that increase outflow K+ current, decrease inward Na+ current, or decrease inward Ca++ current.6 In ER pattern, subjects may be asymptomatic for life. However, this may not be the case when they develop acute STEMI. Theoretically, either ER or acute STEMI may further accentuate the epicardial AP notch of one another and precipitate phase 2 reentry. Little is known whether this association increases the risk of ventricular arrhythmias.
METHODS
Study Population
The study prospectively included 102 male patients with acute STEMI that were divided into two groups:
‐ Cases: Included 52 patients with VAs in the form of sustained (≥ 30 seconds) ventricular tachycardia (monomorphic VT, or polymorphic VT) or VF during the first 48 hours of myocardial insult.
‐ Controls: Included 50 patients (age matched) with no VAs during the first 48 hours of myocardial insult.
All patients were subjected to thorough clinical assessment, 12‐lead surface ECG, transthoracic echocardiogram, cardiac enzyme assessment, serum electrolyte measurement, and continuous ECG monitoring during the first 48 hours. Any rhythm disturbance was documented with either 12‐lead ECG or rhythm strip.
Exclusion Criteria
Patients with prior STEMI, myocardial aneurysm, suspected pericarditis, other structural heart disease, electrolyte disturbances, wide QRS complex (> 120 ms), and patients with Brugada syndrome (Brugada type 1 ECG) were excluded from the study.
Ventricular Arrhythmias
Sustained monomorphic or polymorphic VT (lasting ≥ 30 seconds) and VF were monitored and documented to assign each of the study groups. Frequency of arrhythmia episodes was recorded. Arrhythmias during reperfusion, percutaneous coronary intervention (PCI), or beyond 48 hours of infarction were excluded from analysis.
Revascularization
Primary PCI was done if presentation was within a proper window or beyond in case of ongoing chest pain. Thrombolytic therapy was given in case of very early presentation (within 30 minutes of infarction) or if the patient refused PCI. Conservative treatment with antiischemic measures was done to patients with completed infarction. The latter category of patients was excluded from the study if presented beyond 48 hours of the onset of chest pain. Coronary angiography was done to patients who received thrombolytic or conservative therapy within 24–48 hours of admission.
Assessment of ER
ER was defined as ≥ 1 mm elevation of J point in at least 2 inferior or lateral leads with or without ST segment elevation.1 Because STEMI would obscure ER, diagnosis of ER was based on either a previous ECG (before infarction), ECG after resolution of ST segment elevation of the infarction (predischarge ECG), or leads not related to the infarction.
ER pattern was classified into three types: type 1 (only in lateral precordial leads), type 2 (inferior or inferolateral leads), and type 3 (all leads). J wave amplitude was measured from the isoelectric line at QRS onset to the summit of QRS‐ST junction (Fig. 1). J wave morphology was classified into either slurred type or notched type. Slurring was defined as smooth transition from of the terminal part of QRS complex to the ST segment with upright concavity, while notching was defined as any positive deflection at the terminal portion of QRS complex (Fig. 2). ST segment elevation related to ER pattern was defined as at least 1 mm elevation of the ST segment following an elevated J point, with either upsloping or horizontal morphology (Figs. 3A and B). In the absence of ST segment elevation, the diagnosis of ER is based only on the presence of J wave (Figs. 3C and D).
Figure 1.
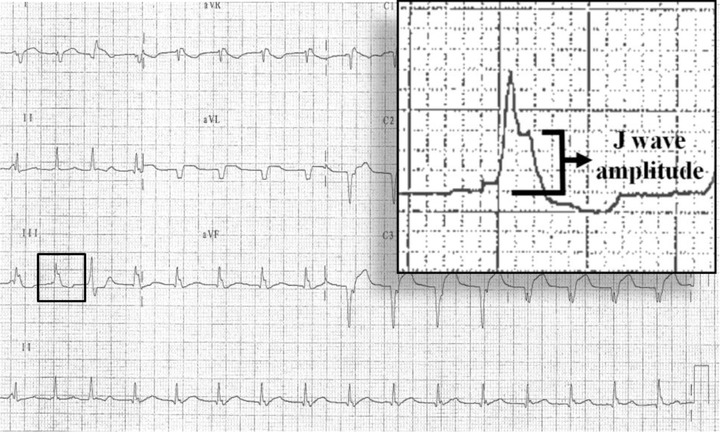
Twelve‐lead surface ECG from a patient with extensive anterior infarction and early repolarization pattern in the form of isolated J waves (without ST segment elevation) in inferior leads (patient number 6 among case group). J wave amplitude was measured from the isoelectric line just before the onset of QRS complex to the summit of J wave (measuring 3 mm).
Figure 2.
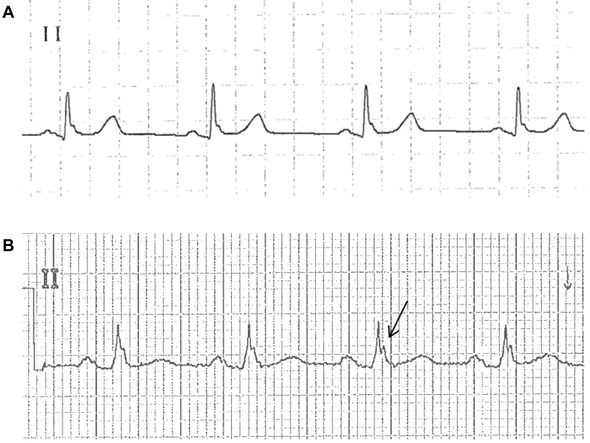
Surface ECG from lead II. (A) Slurred J waves. There is smooth transition from the terminal part of QRS complex to the ST segment despite slight oscillations (patient number 1 among the control group). (B) Notched J waves: Arrow points to the positive deflection at the QRS‐ST junction (patient number 14 among the case group).
Figure 3.
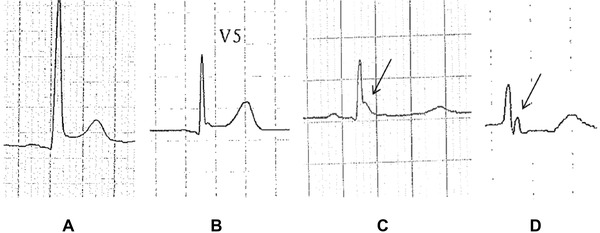
Different early repolarization patterns with different ST segment shifts: upsloping ST segment elevation (A), horizontal ST segment elevation (B), isoelectric ST segment (C), and ischemic ST segment depression (D). In (C) and (D) the diagnosis of early repolarization is based only on the presence of J waves as indicated by arrows (patients 4, 8, 30, and 42, respectively, among the case group).
Other Electrocardiographic Measures
QRS complex duration was measured from the onset to the end of QRS complex. QT interval was measured from the onset of QRS to the end of the T wave defined as the point of return to the isoelectric line. QTc interval was calculated using Bazett's formula8 (QTc = QT/square root of RR interval in seconds). Intervals were measured in leads II, V5, or V6 (selecting the lead with the highest T wave amplitude). QT dispersion (QTd) was measured as the difference between the longest and shortest QT interval in milliseconds among all ECG leads.
Statistical Analysis
Statistical Package for Social Sciences (SPSS, Inc, version 15, Chicago, IL) was used. Categorical data were expressed as frequencies and percentages, while continuous data were expressed as mean ± SD or median according to data distribution. Comparison between categorical data was done using chi‐square or Fisher's exact test as appropriate. Comparison between continuous variables was done using unpaired t‐test or Mann–Whitney test as appropriate. Predictors of VAs were listed in logistic regression model. Unadjusted and adjusted odds ratio (95% confidence intervals) were used to detect significant predictors. P value was considered significant if < 0.05.
RESULTS
Baseline Characteristics
Mean age of study population was 48.44 ± 10.08 years (range 24–74) years. Mean left ventricular ejection fraction (LVEF) was 42.25 ± 11.1% (range 20–65). Apart from LVEF (P = 0.008), there were no differences between cases and controls regarding baseline characteristics (Table 1).
Table 1.
Baseline Characteristics
| Cases (n = 52) | Controls (n = 50) | P Value | |
|---|---|---|---|
| Age (yrs) | 46.86 ± 10.79 | 50.08 ± 9.1 | 0.1 |
| DM (no., %) | 15 (28.8%) | 24 (48%) | 0.074 |
| HTN (no., %) | 17 (32.7%) | 19 (38%) | 0.72 |
| Smoking (no., %) | 20 (38.5%) | 14 (28%) | 0.36 |
| LVEF (%) | 39.41 ± 11.64 | 45.22 ± 9.77 | 0.008 |
| Infarct location (no., %) | |||
| Anterior | 35 (67.3%) | 27 (54%) | 0.24 |
| Inferior | 16 (30.8%) | 22 (44%) | 0.23 |
| Lateral | 0 (0%) | 1 (2%) | 0.49 |
| Posterior | 1 (1.9%) | 0 (0%) | 1 |
| Coronary lesiona (no., %) | |||
| Left main artery disease | 3 (5.7%) | 0 (0%) | 0.24 |
| Single vessel disease | 23 (44.2%) | 19 (38%) | 0.66 |
| Multivessel disease | 16 (30.8%) | 21 (42%) | 0.33 |
| Revascularization (no., %) | |||
| Thrombolysis | 9 (17.3%) | 9 (18%) | 0.86 |
| PCI | 35 (67.3%) | 37 (74%) | 0.6 |
| CABG | 7 (13.5%) | 4 (8%) | 0.52 |
| Conservative | 1 (1.9%) | 0 (0%) | 1 |
| QRS duration (ms) | 94.19 ± 14 | 90.28 ± 13.48 | 0.15 |
| QTc (ms) | 428.17 ± 40.5 | 419.98 ± 32.46 | 0.26 |
| QTd (ms) | 29.57 ± 22.29 | 27.34 ± 19.73 | 0.72 |
| (median 23.5) | (median 20) | ||
| Deaths (no., %) | 5 (9.6%) | 0 (0%) | 0.056 |
DM = diabetes mellitus; HTN = hypertension; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; QTc = corrected QT interval; QTd = QT dispersion.
Remaining patients refused angiography.
Ventricular arrhythmias among cases included monomorphic VT in 6 patients (17.3%), polymorphic VT in 14 patients (26.9%), and VF in 32 patients (61.5%). Mean frequency of arrhythmia episodes was 2.69 ± 4.32 (median 1, range 1–30 episodes).
ER Pattern
Among the whole study population, 43 patients (42.15%) had ER pattern on surface ECG including 29 and 14 patients among cases and controls respectively (P = 0.008). Inferior/inferolateral ER was the most frequent type (24 patients, 23.52%). Notched J wave and horizontal ST segment morphology were more frequent in cases than controls (P = 0.0007 and 0.033, respectively). Table 2 shows frequency and characteristics of ER in cases and controls.
Table 2.
Frequency and Characteristics of Early Repolarization in Cases and Controls
| Cases (n = 52) | Controls (n = 50) | P Value | |
|---|---|---|---|
| Presence of ER (no., %) | 29 (55.7%) | 14 (28%) | 0.008 |
| Type of ER (no., %) | |||
| Lateral | 7 (13.4%) | 4 (8%) | 0.52 |
| Inferior/inferolateral | 15 (28.8%) | 9 (18%) | 0.29 |
| Global | 7 (13.4%) | 1 (2%) | 0.06 |
| J wave morphology (no., %) | |||
| Slurred | 6 (11.5%) | 8 (16%) | 0.71 |
| Notched | 23 (44.2%) | 6 (12%) | 0.0007 |
| J wave amplitude (mm) | 1.13 ± 1.23 (median 1) | 0.6 ± 1.08 (median 0) | 0.008 |
| ST segment elevationa (no., %) | 28 (53.8%) | 12 (24%) | 0.003 |
| ST segment morphology (no., %) | |||
| Upsloping | 9 (17.3%) | 3 (6%) | 0.12 |
| Horizontal | 19 (36.5%) | 8 (16%) | 0.033 |
ER = early repolarization.
Related to early repolarization.
Predictors of Ventricular Arrhythmias
Each of LVEF, QTc, QTd, and ER pattern were listed as independent variables with VAs as dependent variable in a logistic regression model (Table 3). Adjusting was done for independent variables to each other. Before and after adjusting, QTc and QTd did not have a predictive effect on VAs, whereas LVEF and ER had significant predictive effect.
Table 3.
Predictor for Ventricular Arrhythmia within 48 Hours of Myocardial Infarction
| Unadjusted Values | Adjusted Valuesa | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| LVEFb | 0.95 | 0.91–0.98 | 0.01 | 0.95 | 0.91–0.99 | 0.015 |
| QTcb | 1.006 | 0.99–1.017 | 0.26 | 1.007 | 0.99–1.02 | 0.24 |
| QTdb | 1.005 | 0.98–1.024 | 0.58 | 1.002 | 0.98–1.024 | 0.86 |
| ER | 3.24 | 1.42–7.39 | 0.005 | 3.39 | 1.41–8.12 | 0.006 |
Variables were adjusted to each other.
In increments of 1% for LVEF, and 1 ms for QTc and QTd.
Regarding localization and features of ER pattern, inferior/inferolateral ER had significant predictive effect on VAs in adjusted model (Table 4). Global ER, notched J wave, J wave amplitude, ST segment elevation related to ER including both upsloping and horizontal types had significant predictive effect on VAs in both unadjusted and adjusted models.
Table 4.
Early Repolarization Pattern as a Predictor for Ventricular Arrhythmia within 48 Hours of Myocardial Infarction
| Unadjusted Values | Adjusted Valuesa | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Type of ER | ||||||
| Lateral | 2.73 | 0.72–10.41 | 0.13 | 2.63 | 0.66–10.51 | 0.17 |
| Inferior/inferolateral | 2.6 | 0.98–6.93 | 0.055 | 2.9 | 1.03–8.21 | 0.044 |
| Global | 10.95 | 1.26–94.97 | 0.03 | 12.46 | 1.26–122.8 | 0.031 |
| J wave morphology | ||||||
| Slurred | 1.17 | 0.36—3.82 | 0.79 | 1.07 | 0.3–3.73 | 0.91 |
| Notched | 6 | 2.12–16.97 | 0.001 | 6.97 | 2.28–21.29 | 0.001 |
| J wave amplitudeb | 1.5 | 1.04—2.16 | 0.027 | 1.5 | 1.01–2.23 | 0.042 |
| ST segment elevation | 4.13 | 1.74–9.8 | 0.001 | 4.42 | 1.76–11.06 | 0.001 |
| ST segment morphology | ||||||
| Upsloping | 4.87 | 1.2–19.81 | 0.027 | 6.42 | 1.41–29.13 | 0.016 |
| Horizontal | 3.85 | 1.46–10.18 | 0.006 | 3.8 | 1.37–10.51 | 0.01 |
Adjusted for LVEF, QTc, and QTd.
In increment of 0.5 mm.
DISCUSSION
Although cumulative evidence suggested an association between ER and VAs, the vast majority of subjects with ER pattern are asymptomatic.1 Little data are available regarding the risk assessment of asymptomatic ER in the setting of acute STEMI as a related disorder with similar mechanism of arrhythmogenesis.6 In acute STEMI, phase 2 reentry is crucially involved in the genesis of early VAs, while anatomic reentry is the main mechanism of late VAs,6, 9 hence, in the present study our end point was VAs within 48 hours from the onset of STEMI (excluding reperfusion arrhythmias). Because ER is more common in males,2, 3, 4, 5 our study sample was male population, which explains the higher prevalence of ER (42.15%) than that in general population (5 to >10%).6, 10
In the present study, we compared 52 cases with acute STEMI and VAs to 50 controls with acute STEMI and no VAs, and we found that case subjects had a significantly higher prevalence of ER than control subjects (55.7% vs 28%, P = 0.008). Although in our study population cases showed a significantly lower LVEF and therefore might have increased risk of VAs, we included both ER and LVEF in a multiple logistic regression model. There were no differences between the 2 groups in QTc interval and QTd, and despite that, we included them in the regression model due to their clinical relevance. The only significant predictors of VAs were ER and LVEF. This may indicate that phase 2 reentry and anatomic reentry rather than prolonged repolarization and spatial repolarization heterogeneity are implicated in early VAs. Our findings confirm the results of a recent study done by Rudic et al.11 They retrospectively compared the ECGs of 30 patients resuscitated from VF with ECGs of 30 patients without ventricular tachyarrhythmia in the setting of acute MI. They found that case subjects had a significantly higher prevalence of ER than did control subjects (47% vs 13%).
In the present study, the location and morphology of ER played a role in the prediction of VAs. In logistic regression model (after adjusting for LVEF, QTc and QTd), type 2 and 3 ER, notched J wave morphology, increasing J wave amplitude, and the presence of ST segment elevation were associated with higher risk of VAs. Similarly, Rudic et al.11 found that notching of the terminal portion of QRS complex was more common in cases than in control subjects after unadjusted analysis (40% vs 7%, P = 0.002) and after adjustment for LVEF and QTc (P = 0.007). They also found significant association between horizontal ST segment elevation and VF, and trend toward more J point elevation in patients with VF. Tikkanen et al.12 showed that the magnitude of J point elevation further increases the risk of sudden death, with a relative risk of 2.92 (P = 0.01) in J point elevations of 0.2 mV. Antzelevitch and Yan6 demonstrated that the risk of VF is related to the location of ER on 12‐lead ECG. ER in lateral leads (type 1 ER) was rarely associated with VF; ER in inferior/inferolateral leads (type 2 ER) was associated with higher risk of VF, while ER in all leads (type 3 ER) was associated with the highest level of risk and is often associated with electric storms.6
Up to our knowledge, Rudic et al.11 were the only who addressed the relation between ER and risk of VAs in MI patients. However, there were some differences between their study and the present study in methodology, which included the following: (a) our study had a prospective design, (b) we included more number of patients, (c) we included only male population in order to include more frequent ER and, hence, more informative statistics (20% of their study were females), (d) we analyzed pre‐infarction ECGs, and we excluded suspected pericarditis or myocardial aneurysm to avoid bias caused by ST segment elevation not related to ER, (e) we included QTd in the analysis, (f) we did not include patients with wide QRS complex which might hinder the diagnosis of ER, (g) we included revascularization in analysis which might influence the risk of VAs. However, our main finding was similar to that of Rudic et al.11 Further studies are warranted.
LIMITATIONS
The results of our study might be more informative if we performed signal average ECG. The onset of STEMI could not be accurately determined in case of completed infarction. We did not restrict ER to the infarction territory. However, since type 2 and 3 rather than type 1 ER were associated with higher risk of VAs, it is likely that infarction territory was overlapped with ER location in patients with VAs.
Conflict of interest: The authors have no conflict of interests to disclose.
REFERENCES
- 1. Klatsky AL, Oehm R, Cooper RA, et al. The early repolarization normal variant electrocardiogram: Correlates and consequences. Am J Med 2003;115:171–177. [DOI] [PubMed] [Google Scholar]
- 2. Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358:2016–2023. [DOI] [PubMed] [Google Scholar]
- 3. Tikkanen JT, Anttonen O, Junttila MJ, et al. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med 2009;361:2529–2537. [DOI] [PubMed] [Google Scholar]
- 4. Haruta D, Matsuo K, Tsuneto A, et al. Incidence and prognostic value of early repolarization pattern in the 12‐lead electrocardiogram. Circulation 2011;123:2931–2937. [DOI] [PubMed] [Google Scholar]
- 5. Rosso R, Kogan E, Belhassen B, et al. J‐point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J Am Coll Cardiol 2008;52:1231–1238. [DOI] [PubMed] [Google Scholar]
- 6. Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm 2010;7:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haissaguerre M, Chatel S, Sacher F, et al. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol 2009;20:93–98. [DOI] [PubMed] [Google Scholar]
- 8. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920;7:353–357. [Google Scholar]
- 9. Kleber A, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 2004;84:431. [DOI] [PubMed] [Google Scholar]
- 10. Stern S. Clinical aspects of the early repolarization syndrome: A 2011 update. Ann Noninvasive Electrocardiol 2011;16:192–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rudic B, Veltmann C, Kuntz E, et al. Early repolarization pattern is associated with ventricular fibrillation in patients with acute myocardial infarction. Heart Rhythm 2012;1295–1300. [DOI] [PubMed] [Google Scholar]
- 12. Tikkanen JT, Anttonen O, Junttila MJ, et al. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med 2009;361:2529–2537. [DOI] [PubMed] [Google Scholar]


