Abstract
Background
Abnormal dynamicity of repolarization is considered to be a marker of myocardial vulnerability contributing to increased risk of arrhythmic events and sudden death. However, little is known about QT dynamics in hypertrophic cardiomyopathy (HCM).
In this study, we aimed to evaluate ventricular repolarization by QT dynamicity in patients with HCM, focusing on its value to define if it is able to differentiate among low‐ and high‐risk HCM patients.
Methods
The linear regression slopes of the QT interval, measured to the apex and to the end of the T wave plotted against RR intervals (QTapex/RR and QTend/RR slopes, respectively) were calculated from 24‐hour Holter recordings using a standard algorithm in 36 HCM patients and 64 control subjects.
Results
QTapex/RR and QTend/RR slopes were significantly steeper in the HCM patients in contrary to healthy control subjects (QTapex/RR = 0.22 + 0.08 vs 0.20 + 0.05, P = 0.0367; QTend/RR = 0.25 + 0.10 vs 0.20 + 0.06, P = 0.023). Moreover, the slopes of QTend/RR and QTapex/RR of high‐risk patients were significantly steeper than those of control subjects while no significant differences were found among low‐risk HCM patients and control subjects and only QTe/RR of high‐risk patients was significantly different between low‐ and high‐risk HCM patients.
Conclusions
Our study results suggest that QT dynamicity is impaired in patients with HCM and may help to differentiate among low‐ and high‐risk patients. Further studies are needed to elucidate the prognostic significance and clinical implications of impaired ventricular repolarization in patients with HCM.
Keywords: noninvasive techniques—Holter/event recorders, noninvasive techniques—QT dispersion
Hypertrophic cardiomyopathy (HCM) accounts for more than 50% of cases of sudden death among persons younger than 25 years of age in the general population and is a frequent cause of sudden cardiac death (SCD) in trained athletes.1, 2
Although the arrhythmogenic substrate in patients with HCM is not completely understood, numerous observations suggest that the increased vulnerability to ventricular arrhythmia appears to be the result of repolarization‐related arrhythmogenesis.3
Abnormal dynamicity of repolarization is considered to be a marker of myocardial vulnerability contributing to increased risk of arrhythmic events and sudden death.4
Increased QT/RR slopes were observed in patients at risk of cardiac death, including those with myocardial infarction, long‐QT syndrome, dilated cardiomyopathy, and/or heart failure.5, 6, 7, 8 However, few data exist in patients with HCM concerning QT dynamicity and even somehow, with results that are contradictory. Thus, the aim of this study was to analyze the repolarization abnormalities in patients with HCM focusing on the value QT dynamics in order to define if this parameter is able to differentiate among low‐ and high‐risk HCM patients.
METHODS
Study Population
For this study we selected 36 HCM patients (24 males) that are currently under clinical follow‐up by the Cardiology Division at the Medical Center of the Favaloro University in whom a three‐channel 24‐hour Holter recording was obtained. In order to have a control group 64 Holter recordings pertaining to 37 male and 27 female subjects matched for age and sex with HCM patients, were selected from the healthy database E‐HOL‐03–0202–003 from the Telemetric Holter ECG Warehouse.9
Holter recordings were obtained at a sampling frequency of 200 Hz and a resolution of 10 μV.
In both HCM and control groups more than 70,000 beats were analyzed. Recordings were included if they lasted >20 hours, if they were of good quality, if atrial fibrillation or a paced rhythm was not present and if T‐wave amplitude was >0.15 mV. ECG data were always analyzed on the lead exhibiting the T wave of greatest amplitude.
QT and RR intervals were determined from each 24‐hour Holter ECG digital data of both HCM and control groups using an automatic measurement system (DC‐Pro Holter System Analyzer, Datacardio, Buenos Aires, Argentina). Briefly, consecutive sinus beats during each 15‐second period for 24 hours were averaged. For each 15‐second period a mean RR, QTe, and QTa intervals were calculated. The QTe interval was taken to be from the onset of the QRS complex to the end of the T wave. The QTa interval was taken to be from the onset of the QRS complex to the peak of the T wave. The end of the T wave was determined by the intersection between the maximum descending slope and the isoelectric line and the apex of the T wave was determined by the maximum amplitude of the T wave.
For analysis of both QTe/RR and QTa/RR relationships we plotted QTe and QTa against the corresponding RR intervals for long term each ECG recording. A regression line was used according to the equation y = ax + b with their slope a and intercept b, automatically calculated. Also, corrected QTe and QTa intervals were obtained from the linear regression equation for selected RR intervals of 0.5 second, 0.8 second and 1.1 seconds.
Finally, in order to analyze the circadian influences we analyzed Holter recordings of both control and HCM patients in two periods, day (08:00–22:00 hours) and night (22:00–07:00 hours) periods and also for both male and female subjects. For gender influences, in both control and HCM, Holter recordings were analyzed for males against females.
Statistical Analysis
Unless otherwise indicated all data are expressed as mean ± SD. Categorical data were analyzed with the Fisher's chi‐square exact test. The mean differences between the study groups were evaluated by one‐way unpaired Student's t‐test analysis. A P value ≤0.05 was considered to be statistically significant.
RESULTS
Clinical Characteristics
There were 36 patients included in the HCM group (17 patients who were treated with an implanted cardiac defibrillator (ICD) and were considered high‐risk patients and 19 patients who were not treated with an implantable ICD and considered low‐risk patients). The indication of ICD implant was for primary prevention of ventricular fibrillation in 15/17 and for secondary prevention in only 2/17 patients. The indication of ICD for primary prevention was supported by the presence of two or more major criteria of risk in 14/17 patients while in 1/17 the indication of the ICD was supported by the presence of one major (extreme left ventricular hypertrophy) and one minor (23 years of age) criteria of risk. No significant differences were found in diastolic diameter of the left ventricle, systolic diameter of the left ventricle, posterior wall thickness (PW), shortening fraction and ejection fraction. Only the width of the interventricular septum was significantly greater in the high‐risk patients. The phenotype of the hypertrophy was mainly asymmetric since both low‐ and high‐risk patients exhibited an IVS/PW ratio greater than 1.3. Moreover, the IVS/PW ratio of the high‐risk patients was significantly greater than that of the low‐risk patients. Most patients (15/19 of low‐risk patients and 16/17 high‐risk patients) were receiving β‐blockers chronically). Mean ± SD values of clinical characteristics are shown in Table 1.
Table 1.
Clinical Characteristics of HCM Patients
| Low Risk | High Risk | P Value | |
|---|---|---|---|
| Age (years) | 53 ± 14 | 40 ± 17 | 0.039 |
| Syncope | 2/19 | 3/17 | NS |
| Family history of SCD | 0/19 | 7/17 | 0.0023 |
| NSVT | 0/19 | 9/17 | 0.0003 |
| Extreme LVH (mm) | 0/19 | 11/17 | 0.0001 |
| SCD | 0/19 | 2/17 | NS |
| SVT | 0/19 | 1/17 | NS |
| LVDD (mm) | 45 ± 6 | 43 ± 4 | NS |
| LVSD (mm) | 25 ± 5 | 23 ± 8 | NS |
| IVS (mm) | 20 ± 4 | 25 ± 5 | 0.0013 |
| PW (mm) | 12 ± 4 | 12 ± 4 | NS |
| Fractional shortening | 0.43 ± 0.07 | 0.47 ± 0.16 | NS |
| Ejection fraction | 67 ± 6 | 65 ± 8 | NS |
NSVT = non sustained ventricular tachycardia; LVH = left ventricular hypertrophy; SVT = sustained ventricular tachycardia; LVDD = lelft ventricular diastolic diameter; LVSD = left ventricular systolic diameter; SCD = sudden cardiac death; IVS = interventricular septum; PW = posterior wall.
Comparison between Control Subjects and HCM Patients
There was no significant difference in mean age (40 ± 13 years vs 44 ± 18 years for control and HCM groups, respectively; P = NS) or gender (37 males and 27 females vs 24 males and 12 females for control and HCM groups, respectively; P = NS). HCM patients exhibited a lower heart rate than control subjects as expressed by significant differences between both groups in mean RR intervals (919 ± 80 milliseconds vs 808 ± 82 milliseconds for HCM and control groups, respectively; P < 0.000). This lower heart rate could be a consequence that most of HCM patients were receiving β‐blockers (15/19 and 16/17 of low‐ and high‐risk patients respectively; P = 0.3420 with Fischer's exact test).
As it is shown in Table 2 the slopes of both QTe/RR and QTa/RR relationships of HCM patients were significantly greater than those of control group. In contrast, no significant differences were found in the intercepts. The relationship between the slopes and intercepts in both control and HCM groups was also analyzed by linear regression between these parameters. Figure 1 shows the scatter plot of the slope and intercept of the QTe/RR relationship of both control subjects and HCM patients. A significant negative correlation was found in both linear relationships and no significant differences were found in the slopes of both regression lines.
Table 2.
QT/RR Relationship and QT Interval Durations at Particular RR Intervals in Both Control Subjects and HCM Patients
| Control | HCM | P Value | |
|---|---|---|---|
| Slope QTe/RR | 0.20 ± 0.06 | 0.25 ± 0.10 | 0.0023 |
| Slope QTa/RR | 0.20 ± 0.05 | 0.22 ± 0.08 | 0.0367 |
| Intercept QTe/RR | 183 ± 43 | 166 ± 75 | 0.1061 |
| Intercept QTa/RR | 133 ± 33 | 123 ± 61 | 0.1838 |
| QTe0.5 second | 0.284 ± 0.018 | 0.293 ± 0.031 | 0.0446 |
| QTe0.8 second | 0.344 ± 0.013 | 0.370 ± 0.023 | 0.0000 |
| QTe1.1 second | 0.404 ± 0.025 | 0.446 ± 0.044 | 0.0000 |
| QTa0.5 second | 0.231 ± 0.014 | 0.234 ± 0.027 | 0.2395 |
| QTa0.8 second | 0.289 ± 0.017 | 0.301 ± 0.023 | 0.0050 |
| QTa1.1 second | 0.348 ± 0.029 | 0.368 ± 0.038 | 0.0044 |
Figure 1.
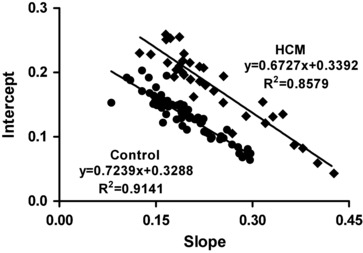
Relationship between the slope and intercept of QTe/RR in control (green dots) and HCM patients (red dots). A significant negative correlation was found in both control and HCM patients. Also, similar slopes of the regression lines of both control and HCM patients were found (F = 0.9886, DFn = 1, DFd = 94, P = 0.3226) while the intercepts were significantly different (F = 35.9784, DFn = 1, DFd = 95, P < 0.0001).
Finally, both corrected QTe and QTa intervals for particular RR intervals of 0.5 second, 0.8 second, and 1.1 seconds were significantly longer in HCM patients as compared with control subjects only at heart rates of 0.8 second and 1.1 seconds.
We also analyzed the circadian and gender influences on QT dynamics and corrected QT intervals at particular heart rates. Table 3 shows the circadian influences on the QT dynamics and the corrected QTe and QTa at similar heart rates in both control subjects and HCM patients. Significant differences were found in the slopes and intercepts of both QTe/RR and QTa/RR relationships of control subjects, with the slopes being greater and the intercepts lower during the day period. In contrast in HCM patients, no significant differences were found between day and night periods. When comparisons were made between control and HCM groups, only the slope of QTe/RR of HCM patients was significantly different during both day and night periods while the intercepts of both QTe/RR and QTa/RR relationships were not significantly different and the corrected QTe and QTa intervals of HCM patients were significantly longer than those of control subjects during both day and night periods.
Table 3.
Circadian Influences on QT/RR Relationship and QT Intervals at Particular RR Intervals in Both Control Subjects and HCM Patients
| Control | HCM | |||||
|---|---|---|---|---|---|---|
| Day | Night | P value | Day | Night | P Value | |
| Slope QTe/RR | 0.24 ± 0.09 | 0.21 ± 0.07 | 0.0152 | 0.27 ± 0.09 | 0.25 ± 0.09 | 0.1409 |
| Slope QTa/RR | 0.22 ± 0.07 | 0.20 ± 0.05 | 0.0205 | 0.23 ± 0.08 | 0.22 ± 0.09 | 0.2243 |
| Intercept QTe/RR | 158 ± 60 | 182 ± 50 | 0.0088 | 151 ± 65 | 169 ± 61 | 0.1205 |
| Intercept QTa/RR | 116 ± 47 | 134 ±45 | 0.0113 | 115 ± 54 | 124 ± 63 | 0.2588 |
| QTe0.5 second | 0.301 ± 0.013 | 0.306 ± 0.015 | 0.0140 | 0.313 ± 0.021 | 0.317 ± 0.023 | 0.2183 |
| QTe0.8 second | 0.395 ± 0.029 | 0.389 ± 0.023 | 0.0807 | 0.421 ± 0.035 | 0.416 ± 0.036 | 0.2752 |
| QTe1.1 second | 0.443 ± 0.046 | 0.430 ± 0.035 | 0.0422 | 0.475 ± 0.051 | 0.465 ± 0.051 | 0.2147 |
| QTa0.5 second | 0.247 ± 0.015 | 0.252 ± 0.019 | 0.0436 | 0.254 ± 0.020 | 0.254 ± 0.023 | 0.4986 |
| QTa0.8 second | 0.334 ± 0.028 | 0.330 ± 0.021 | 0.1966 | 0.347 ± 0.036 | 0.341 ± 0.035 | 0.2392 |
| QTa1.1 second | 0.378 ± 0.040 | 0.370 ± 0.028 | 0.0919 | 0.393 ± 0.051 | 0.384 ±0.050 | 0.2246 |
With regard to gender influences, control females exhibit a significantly steeper slope and shorter intercepts of both QTe/RR and QTa/RR than control males. Also, control females exhibited significantly longer corrected QTe and QTa intervals at RR intervals of 0.8 second and 1.1 seconds while at RR intervals of 0.5 second only the corrected QTa interval exhibited significant differences. On the contrary, no significant differences were found in the slopes and intercepts between males and females of the HCM group, while the corrected QTe and QTa intervals were longer in females only at RR intervals of 0.8 second and 1.1 seconds as it is shown in Table 4.
Table 4.
Gender Dependent Influences on QT/RR Relationship and QT Intervals at Particular RR Intervals in Both Control Subjects and HCM Patients
| Control | HCM | |||||
|---|---|---|---|---|---|---|
| Males | Females | P value | Males | Females | P value | |
| Slope QTe/RR | 0.18 ± 0.05 | 0.22 ± 0.07 | 0.0001 | 0.24 ± 0.08 | 0.26 ± 0.14 | 0.1117 |
| Slope QTa/RR | 0.18 ± 0.04 | 0.21 ± 0.07 | 0.0003 | 0.21 ± 0.08 | 0.22 ± 0.11 | 0.1296 |
| Intercept QTe/RR | 198 ± 39 | 163 ± 52 | 0.0005 | 174 ± 63 | 138 ± 96 | 0.2319 |
| Intercept QTa/RR | 142± 29 | 121 ±42 | 0.0083 | 128 ± 57 | 105 ± 69 | 0.2501 |
| QTe0.5 second | 0.305 ± 0.016 | 0.294 ± 0.071 | 0.2507 | 0.317 ± 0.024 | 0.288 ± 0.121 | 0.2261 |
| QTe0.8 second | 0.376 ± 0.018 | 0.382 ± 0.093 | 0.0000 | 0.412 ± 0.034 | 0.388 ± 0.166 | 0.0144 |
| QTe1.1 second | 0.412 ± 0.026 | 0.427 ± 0.105 | 0.0000 | 0.459 ± 0.048 | 0.439 ± 0.189 | 0.0307 |
| QTa0.5 second | 0.248 ± 0.015 | 0.252 ± 0.019 | 0.0420 | 0.255 ± 0.023 | 0.236 ± 0.090 | 0.3218 |
| QTa0.8 second | 0.319 ± 0.024 | 0.330 ± 0.021 | 0.0000 | 0.340 ± 0.033 | 0.318 ± 0.134 | 0.0404 |
| QTa1.1 second | 0.354 ± 0.031 | 0.370 ± 0.028 | 0.0000 | 0.382 ± 0.046 | 0.361 ± 0.153 | 0.0606 |
Comparison between Low‐ and High‐Risk HCM Patients
A significant linear correlation was found between QTe and QTa intervals and the RR interval in low‐risk (r = 0.78 ± 0.13, and r = 0.73 ± 0.17 for QTe/RR and QTa/RR relationships, respectively) and in high‐risk patients (r = 0.84 ± 0.08 and r = 0.82 ± 0.09 for QTe/RR and QTa/RR, respectively). The differences in the correlation coefficients of both groups of patients were not significant. Figure 2 illustrate representative examples of QTe/RR relationships for low‐ and high‐risk HCM patients as compared with a control subject.
Figure 2.
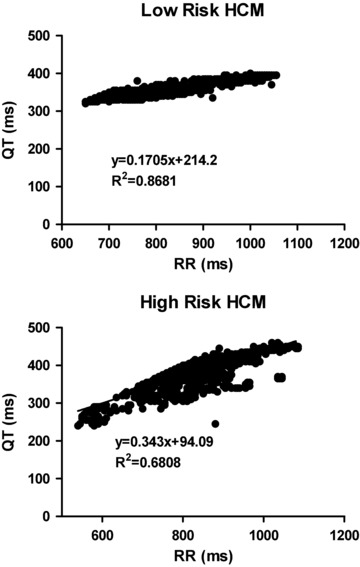
Illustrates the QTe/RR relationship of representative low‐ (upper graph) and high‐risk (lower graph) HCM patients. It can be clearly seen that the slope of the high‐risk patient is steeper than that of the low‐risk patient. A significant correlation between QTe interval and RR interval was found in both patients, though high‐risk patient exhibited a lower correlation than the low‐risk patient.
In Table 5 we show mean ± SD values of QT dynamics parameters of low‐ and high‐risk HCM patients. High‐risk HCM patients showed a significantly steeper slope of QTe/RR but not of QTa/RR as compared with low‐risk HCM patients while no significant differences were found in the corresponding QTe and QTa intercepts between low‐ and high‐risk patients.
Table 5.
Differences in QT/RR Relationship between Control Subjects and HCM Patients
| Low Risk | High Risk | P Value | |
|---|---|---|---|
| Slope QTe/RR | 0.23 ± 0.08 | 0.28 ± 0.13 | 0.0478 |
| Slope QTa/RR | 0.20 ± 0.08 | 0.24 ± 0.10 | 0.0670 |
| Intercept QTe/RR | 179 ± 63 | 152 ± 92 | 0.1516 |
| Intercept QTa/RR | 132 ± 58 | 113 ± 67 | 0.1731 |
When compared to control subjects, low‐risk patients do not show significant differences in the slopes and intercepts of both QTe/RR and QTa/RR relationships (0.23 ± 0.08 vs 0.20 ± 0.06 and 0.20 ± 0.08 vs 0.20 ± 0.05 for QTe/RR and QTa/RR of low risk and control respectively; P = NS). In contrast, high‐risk patients showed a significantly steeper slope of both QTe/RR and QTa/RR (0.28 ± 0.13 vs 0.20 ± 0.06; P = 0.037 and 0.24 ± 0.10 vs 0,20 ± 0.05; P = 0.0132 for QTe/RR and QTa/RR of high risk and control, respectively) and no significant differences were found in the intercepts of QTe/RR and QTa/RR relationships in both high‐ and low‐risk patients as compared with control subjects.
Finally, both QTe and QTa corrected intervals of high‐risk HCM patients were significantly longer than those of low‐risk patients at RR intervals of 0,8s and 1,1s with the percentage of increment of QTe being greater than that of QTa, and the percentage of increment of both QTa and QTe intervals respect to control, were always greater for high‐risk patients. Mean ± SD data of corrected QTe and QTa data of low‐ and high‐risk patients are shown in Table 6.
Table 6.
Differences in the Corrected QTe and QTa Intervals between Low‐ and High‐Risk Patients
| Low‐Risk HCM | High‐Risk HCM | P Value | |
|---|---|---|---|
| QTe0.5 second | 0.293 ± 0.028 | 0.294 ± 0.077 | 0.4373 |
| QTe0.8 second | 0.361 ± 0.025 | 0.380 ± 0.091 | 0.0056 |
| QTe1.1 second | 0.429 ± 0.041 | 0.465 ± 0.116 | 0.0060 |
| QTa0.5 second | 0.234 ± 0.027 | 0.235 ± 0.062 | 0.4750 |
| QTa0.8 second | 0.295 ± 0.025 | 0.308 ± 0.075 | 0.0006 |
| QTa1.1 second | 0.356 ± 0.040 | 0.381 ± 0.095 | 0.0004 |
DISCUSSION
The main findings of this study were: (1) The slope of the linear regression between QTe and QTa intervals and the corresponding RR intervals was significantly higher in patients with HCM than in the control group. (2) Gender and circadian influences on the QTe/RR and QTa/RR relationships that are present in the control group, were lost in patients with HCM. (3) Patients with high‐risk HCM showed a significantly steeper slope of the regression line QT/RR compared to low‐risk patients. (4) Corrected QTe and QTa intervals of high‐risk HCM patients were significantly longer of those of low‐risk patients only at low heart rates (RR intervals of 0.8 second and 1.1 seconds)
These results indicate that patients with HCM can have an impaired repolarization with longer durations of QTa and QTe intervals that increase the steepness of QTe/RR and QTa/RR slopes, mainly at longer RR intervals.
Analysis of the QT/RR slope on 24‐hour Holter records is commonly used to assess the adaptation of the QT interval to heart rate and the autonomic influences on repolarization. Moreover, an increase of the slope associated with an increased risk of SCD was observed under different clinical situations including patients with myocardial infarction,7 LQT syndrome,8 dilated cardiomyopathy,10 and congestive heart failure.6, 11 However, little is known about the dynamics of the QT interval in HCM. Moreover, some of the existing data are contradictory.
Piccirillo et al. showed that HCM patients implanted with an ICD had a QT/RR slope steeper than control subjects.12 In contrast, Yi et al. showed that patients with HCM who experienced an episode of sudden death or ventricular fibrillation showed a QT/RR slope similar to those HCM patients who did not showed such events during follow‐up.13 Savelieva et al., also showed that HCM patients exhibited a QT/RR slope steeper than that of control subjects. However they did not analyzed differences in this slope between high‐ and low‐risk HCM patients.14 Similarly, Fei et al. found no significant difference in the slope of the QT/RR relationship between risk groups.15
Our data are consistent with the results of Piccirillo et al. We found that patients with HCM had QTe/RR and QTa/RR slopes significantly steeper than those of the control group. When comparison were made between low‐ and high‐risk HCM patients against control subjects, while high‐risk patients exhibited steeper slopes of QTa/RR and QTe/RR relationships, no significant differences in their slopes were found for the low‐risk group and only the QTe/RR slope of high‐risk patients was significantly more pronounced than that of the corresponding low‐risk group, therefore, a steeper slope of the relationship can help to differentiate between patients at low and high risk.
Both the slope and intercept of the linear regression quantify the relationship between QT interval duration and heart rate. As shown in Figure 1, a significant negative correlation between the slopes and intercepts of the QTe/RR relationships was found in control subjects (y = −0.7239x + 0.3288; r2 = 0.9141) whereas in the group of patients with HCM and upward displacement of the scatter plot with a parallel regression line was observed (y = −0.6727x + 0.3392; r 2 = 0.8579). Our data are consistent with those of Fujiki et al.16 who proposed that this relationship may be another simple and useful technique for quantifying the dynamics abnormality of ventricular repolarization.
Regarding the circadian and gender influences on QT dynamics, Extramiana et al.17 have shown that the slope of the QT/RR relationship of males and females were higher during the day than at night. At the same time, women had a steeper slope than men. Our results in the control group are consistent with these results, as shown in Tables 3 and 4. In HCM patients, however, no changes attributable to gender or circadian influences are observed. Lack of circadian variation in corrected QTe and QTa intervals and in the slope of QT/RR relationships in HCM patients reflects an imbalance in the autonomic nervous system.
Fei et al. showed that patients with HCM showed significant autonomic alteration with decreased sympathetic tone during normal daily activities.18 Moreover, our patients with HCM showed significantly lower heart rate than control subjects. This lower heart rate could be due to the fact that most of them were treated with cardio‐selective β‐blockers as it was shown in Results. Although the reduction in heart rat induced by β‐blockers is considered as a marker of efficacy, its correlation with repolarization changes remains unclear. In this regard, Extramiana et al.19 showed that atenolol treatment significantly depressed the slope of the QT/RR relationship but not during the night. Sundaram et al.20 recently showed that sympathetic blockade with propranolol decreased the QT/RR slope. In contrast, Vaughan Williams et al.21 found that only the cardio‐selective β‐blockers depressed the slope of the QT/RR relationship while no changes were observed with nonselective β‐blockers. Therefore, it could be expected that the slope of QT/RR would be even higher if β‐blockers were suppressed.
We have recently found that the well‐known gender‐dependent differences in repolarization were lost during cardiac hypertrophy probably due to the electrical remodeling that occurs under this condition.22 So, both the electrical remodeling due to hypertrophy and the autonomic imbalance in patients with HCM can contribute to loss of circadian and gender influences on QT dynamics.
Finally, based on the individual regression parameters, we have also shown that corrected QTe and QTa intervals of HCM patients were significantly longer than those of control subjects. Moreover, the percentage of increment in corrected QTe and QTa of high risk respect to those of low‐risk patients were always greater for the corrected QTe interval. It is long been known that, compared with endocardium and mid‐myocardium, epicardial action potentials show a briefer duration. Epicardial‐endocardial differences in the duration of action potentials are crucial for the genesis of the T wave. Taking into account the basis for the genesis of the T wave postulated by Antzelevitch,23 the T wave begins when the plateau of epicardial action potential separates from that of the M cell. As epicardium repolarizes, the voltage gradient between epicardium and the M region continues to grow giving rise to the ascending limb of the T wave. The voltage gradient between the M region and epicardium reaches a peak when the epicardium is fully repolarized. Therefore,
QTa interval reflects the end of repolarization at the epicardium. On the endocardial side of the ventricular wall, the plateau of the endocardial action potential deviates from that of the M cell, generating an opposing voltage gradient and corresponding current that limits the amplitude of the T wave and contributes to the descending limb of the T wave. The voltage gradient between the endocardium and the M region reaches a peak when the endocardium is fully repolarized and all gradients are extinguished when the longest M cells are fully repolarized. Hence, the QTe reflects the end of repolarization at the endocardium/mid myocardium. The hallmark of the M cell is the ability of its action potential to prolong more than that of epicardial or endocardial cells in response to a slowing of rate and/or in response to drugs with QT‐prolonging actions. The ionic basis for these features includes the presence of a smaller, slowly activating, delayed rectifier current (IKs). These epicardial‐endocardial/midmyocardial differences in the duration of action potentials can be even greater due to the downregulation of the delayed rectifier during the electrical remodeling of the hypertrophic process, and therefore, can be postulated a greater increase of action potential duration in endocardial and midmyocardial cells leading to an increase in the QTe interval with respect to QTa interval and an enhancement of the transmural dispersion of repolarization with it consequent arrhythmogenic effect.
CONCLUSION
In conclusion, our study findings suggest that QT dynamicity is altered in patients with HCM. These results may highlight the value of QT dynamics in differentiating between patients at low and high risk. Further studies to elucidate the prognostic significance and clinical implications of abnormal ventricular repolarization in patients with HCM are needed
LIMITATIONS OF THE STUDY
Our study should be evaluated with some limitations. This is a case‐control study in a small number of patients. Therefore, larger sample sizes are required to provide more accurate information. Also, the study does not provide genotype‐phenotype correlations.
REFERENCES
- 1. Ly HQ, Greiss I, Talajic M, et al. Sudden death and hypertrophic cardiomyopathy: A review. Can J Cardiol 2005;21(5):441–448. [PubMed] [Google Scholar]
- 2. Maron BJ. Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation 2010;121:445–456. [DOI] [PubMed] [Google Scholar]
- 3. Barletta G, Lazzeri C, Franchi F, et al. Hypertrophic cardiomyopathy: Electrical abnormalities detected by the extended‐length ECG and their relation to syncope. Int J Cardiol 2004;97(1):43–48. [DOI] [PubMed] [Google Scholar]
- 4. Zareba W, Bayes de Luna A. QT dynamics and variability. Ann Noninvasive Electrocardiol 2005;10(2):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faber TS, Grom A, Schopflin M, et al. Beat‐to‐beat assessment of QT/RR ratio in severe heart failure and overt myocardial ischemia: A measure of electrical integrity in diseased hearts. Pacing Clin Electrophysiol 2003;26:836–842. [DOI] [PubMed] [Google Scholar]
- 6. Pathak A, Curnier D, Fourcade J, et al. QT dynamicity: A prognostic factor for sudden death in chronic heart failure. Eur J Heart Fail 2005;7:269–275. [DOI] [PubMed] [Google Scholar]
- 7. Järvenpää J, Oikarinen L, Korhonen P, et al. Dynamic QT/RR relationship in post‐myocardial infarction patients with and without cardiac arrest. Scand Cardiovasc J 2010;44(6):352–358. [DOI] [PubMed] [Google Scholar]
- 8. Halamek J, Couderc JP, Jurak P, et al. Measure of the QT–RR dynamic coupling in patients with the long QT syndrome. Ann Noninvasive Electrocardiol 2012;17(4):323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Couderc JP. The Telemetric and Holter ECG Warehouse Initiative (THEW): A Data Repository for the Design, Implementation and Validation of ECG related Technologies 32nd Annual International Conference of the IEEE EMBS Buenos Aires, Argentina, August 31–September 4, 2010: 6252–6255. [DOI] [PMC free article] [PubMed]
- 10. Iacoviello M, Forleo C, Guida P, et al. Ventricular repolarization dynamicity provides independent prognostic information toward major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2007;50:225–2312. [DOI] [PubMed] [Google Scholar]
- 11. Cygankiewicz I, Zareba W, Vazquez R, et al. MUSIC Investigators. Prognostic value of QT/RR slope in predicting mortality in patients with congestive heart failure. J Cardiovasc Electrophysiol 2008;19:1066–1072. [DOI] [PubMed] [Google Scholar]
- 12. Piccirillo G, Germanoa G, Quaglione R, et al. QT‐interval variability and autonomic control in hypertensive subjects with left ventricular hypertrophy. Clin Sci (Lond) 2002;102(3):363–371. [PubMed] [Google Scholar]
- 13. Yi G, Poloneecki J, Dickie S, et al. Can the assessment of dynamic qt dispersion on exercise electrocardiogram predict sudden cardiac death in hypertrophic cardiomyopathy? Pacing Clin Electrophysiol 2000;23[(Pt.II]:1953–1956. [DOI] [PubMed] [Google Scholar]
- 14. Savelieva I, Yap YG, Yi G, et al. Relation of ventricular repolarization to cardiac cycle lenght in normal subjects, hypertrophic cardiomyopathy and patients with myocardial infarction. Clin Cardiol 1999;22:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fei L, Slade AK, Grace AA, et al. Ambulatory assessment of the QT interval in patients with hypertrophic cardiomyopathy: Risk stratification and effect of low dose amiodarone. Pacing Clin Electrophysiol 1994;17(11 Pt2):2222–2227. [DOI] [PubMed] [Google Scholar]
- 16. Fujiki A, Yoshioka R, Sakabe M. Evaluation of repolarization dynamics using the QT–RR regression line slope and intercept relationship during 24‐h Holter ECG. Heart Vessels 2014 Jan 25 [Epub ahead of print]. [DOI] [PubMed]
- 17. Extramiana F, Maison‐Blanche P, Badilini F, et al. Circadian modulation of QT rate dependence in healthy volunteers: Gender and age differences. J Electrocardiol 1999;32(1):33–43. [DOI] [PubMed] [Google Scholar]
- 18. Fei LJ, Slade AK, Prasad K, et al. Is there increased sympathetic activity in patients with hypertrophic cardiomyopathy? J Am Coll Cardiol 1995;26:472–480. [DOI] [PubMed] [Google Scholar]
- 19. Extramiana F, Maison‐Blanche P, Tavernier R, et al. Cardiac effects of chronic oral beta‐blockade. Ann Noninvasive Electrocardiol 2002;7(4):379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sundaram S, Carnethon M, Polito K, et al. Autonomic effects on QT‐RR interval dynamics after exercise. Am J Physiol Heart Circ Physiol 2008;294(1):H490‐H497. [DOI] [PubMed] [Google Scholar]
- 21. Vaughan Williams EM, Hassan MO, Floras JS, et al. Adaptation of hypertensives to treatment with cadioselective and non‐selective beta blockers. Br Heart J 1980;44:473–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biagetti MO, Quinteiro RA. Gender differences in electrical remodeling and susceptibility to ventricular arrhythmias in rabbits with left ventricular hypertrophy. Heart Rhythm 2006;3:832–839 [DOI] [PubMed] [Google Scholar]
- 23. Antzelevitch C. Cellular basis for the repolarization waves of the ECG. Ann NY Acad Sci 2006;1080:268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]


