Abstract
One of the more frequent dilemmas in ECG interpretation is the differential diagnosis of an rSr’ pattern in leads V1‐V2. We often face this finding in asymptomatic and otherwise healthy individuals and the causes may vary from benign nonpathological variants to severe or life‐threatening heart diseases, such as Brugada syndrome or arrhythmogenic right ventricular dysplasia. In other cases, a normal variant of rSr’ pattern can be misinterpreted as pathological after the occurrence of certain clinical events such as cardiac arrest or syncope of unknown cause. In this review we analyze in detail all the possible conditions, both benign and pathological that may explain the presence of this electrocardiographic pattern. We also propose a simple electrocardiographic algorithm for differential diagnosis.
Keywords: rSr’ pattern, Brugada ECG pattern, ARVD, algorithm
The normal electrocardiographic QRS morphology that is recorded in leads V1‐V2 is the consequence of the normal electrical activation of the ventricles.1, 2, 3 This is explained by the presence of three vectors; the first, generated by septal activation is directed from left to right and explains the small “r” in lead V1 (and the small “q” in leads V5‐V6). In general, it is directed upwards, but in obese people it may point downwards. The second vector is the consequence of the activation of the free walls of both ventricles from endocardial to epicardial layers. Given the thicker left ventricular wall, the resultant vector is directed leftwards and to the apex and explains the S wave in leads V1‐V2 (and the R wave in leads V5‐V6). Finally, the third vector corresponds to the depolarization of the basal regions of both ventricles; it is directed to the right and upwards and explains the small “s” in leads V5‐V6, the final “r” usually found in lead aVR and part of the ascending slope of the S wave in leads V1‐V2. If the electrodes V1‐V2 are placed in a higher position (second intercostal space), they may face the head of the third vector and a terminal “r” may be recorded in these leads (Fig. 1).
Figure 1.
Leads V1 to V3 of a very lean 15‐year‐old man without heart disease. The rSr’ morphology is due to a misplaced V1 electrode in the second right intercostal space (see negative P wave) and disappears when the electrode is properly positioned (fourth right intercostal space).
An rSr’ pattern in the right precordial leads is a relatively common electrocardiographic finding that has been described in up to 7% of patients without apparent heart disease.4 If the QRS is wide, the presence of an R’ in leads V1‐V2 usually is in the context of a complete right bundle branch block (RBBB), but other causes have been described, including some cases of ventricular preexcitation syndrome.5 We will particularly focus in the differential diagnosis of r’ (R’) in leads V1‐V2 with a QRS duration of less than 120 ms. This morphology can be due to incomplete RBBB or can be a normal electrophysiological variant, especially in young people and athletes. However, it also can be the expression of serious pathological conditions that should be recognized. We have not included in this review the electrocardiographic patterns induced by atrial activation during supraventricular tachycardias, such as atrioventicular node reentrant tachycardia and 2:1 atrial flutter (pseudo r’).
In the present review, the conditions associated with an rSr’ pattern (the r’ usually is small but sometimes may be greater than “r”) are discussed and a diagnostic algorithm is proposed.
CAUSES OF R’ OR R’ IN PRECORDIAL LEADS (V1‐V2)
The entities that may present with r’ or R’ in leads V1‐V2 and a QRS < 120 ms may represent a benign or a pathological condition (Table 1).
Table 1.
Differential Diagnosis for R' ECG Pattern in Leads V1‐V2
| A. Benign patterns: |
|---|
| 1. Higher placement of electrodes V1–V2 |
| 2. Normal variant: The r' is usually of peripheral origin |
| 3. Partial RBBB: The r' is usually of proximal origin |
| 4. Athletes |
| 5. Pectus excavatum |
| B. Pathological patterns: |
| 1. Type‐2 Brugada pattern |
| 2. RV Enlargement that encompasses cases of RV hypertrophy and or dilation often accompanied of peripheral RV delay. These include valvular and congenital heart diseases affecting the RV, pulmonary hypertension, Ebstein disease, arrhythmogenic right ventricular dysplasia (ARVD), etc. |
| 3. Some cases of Ventricular preexcitation (Wolf‐Parkinson‐White) |
| 4. Hyperkalemia |
BENIGN PATTERNS
In general, the r’ is narrow and of low voltage. However, it may evolve to become taller than the r wave (r’ > r).
Higher Placement of Electrodes V1 and V2
A spurious rSr´ pattern in the right precordial leads is often induced when electrodes V1 and V2 are placed in higher intercostal spaces.3, 6 The r’ has a fast inscription and the electrode misplacement can be recognized by the presence of a negative P wave in lead V1 (instead of positive or biphasic). This is because the high electrode faces the tail instead of the head of the atrial depolarization vector. The ECG normalizes when is recorded in the appropriate location (Fig. 1). The main differential diagnosis is the ECG of pectus excavatum, which also presents a negative P wave in lead V1 (Figs. 2 and 6).
Figure 2.
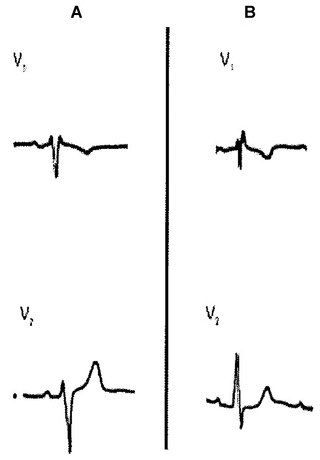
Two cases of rSr’ in lead V1 without associated heart disease. In panel A, note r’ = r and P wave with a ± morphology in lead V1. In panel B, the morphology of the P wave is also ± in lead V1 but r < r’. In an isolated tracing it is not possible to distinguish between the proximal origin in partial RBBB or peripheral RV delay (see text).
Figure 6.
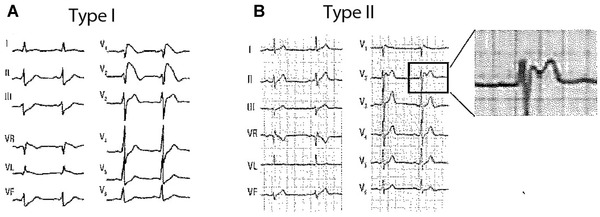
Typical ECG patterns of Type‐1 (coved) Brugada pattern (A) and Type‐2 (saddle‐back) (B).
Normal Variant
The rSr’ pattern can be considered a normal variant due to delay in the activation of the basal part of the right ventricle (RV). It has been reported that an rSr’ pattern is a common finding in the general population. Data from the Copenhagen City Heart Study showed a prevalence of 4.7% in men and 2.3% in women without apparent cardiovascular disease.7 This “normal” rSr’ pattern is more frequent in younger subjects, with male preponderance and by definition is not a precursor of complete (proximal) RBBB. The r’ is of fast ascent/descent inscription and the mechanism is a peripheral conduction delay with late activation of the pulmonary conus and posterobasal portion of the left ventricle.8
Partial or Incomplete RBBB (Figs. 2 and 4)
The mechanism of the typical pattern is a delayed conduction through the right bundle branch. Although it is not a normal variant, it is considered a benign pattern because the presence of isolated partial RBBB is not associated with adverse cardiovascular outcomes.7 Patients with partial RBBB, however, have a higher likelihood of developing complete (advanced) RBBB during the follow‐up.4, 9 Similarly to the normal variant, in partial RBBB the r’ is of fast inscription, the ST‐segment presents a descending slope without ST‐segment ascent and there is not mismatch between the right and left precordial leads duration. Some electrocardiographic features to differentiate partial RBBB from a normal ECG variant have been described.8, 10 These may predict progression to advanced RBBB and include: (1) progressive decrease of the S‐wave voltage; (2) slurring of the ascending slope of the S wave (Fig. 4); (3) inversion of the ratio of the S‐wave depth to SV1 > SV2; and (4) prolongation of the QRS duration to ≥0.10 second.8 However, in an isolated tracing with rSr’ (Fig. 3), it is impossible to differentiate between proximal partial RBBB and mild RV delay of peripheral origin.
Figure 4.
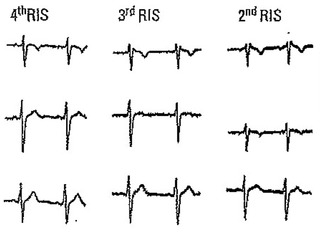
Leads V1 and V2 in four athletes without heart disease.
Figure 3.
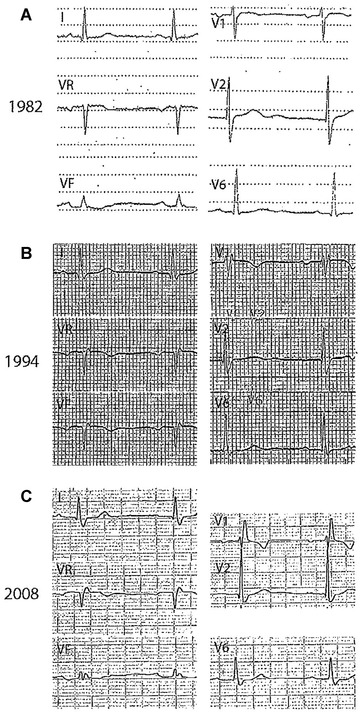
Progressive RBBB. (A) Lead V1 with a notch in the upstroke of the S wave; (B) Lead V1 with r’ and QRS ≥ 120 ms; and (C) Typical rSR’ pattern of RBBB with QRS ≥ 120 ms. In all cases the electrodes are properly placed (note P wave with morphology ± in lead V1).
Athletes (Fig. 4)
An rSr´ pattern can be found in 35–50% of trained athletes, especially in those engaged in endurance sports.11 It is alleged to be consequence of physiological right ventricular enlargement with the resultant delayed peripheral activation. Lead V1 shows an r´ usually peaked and sharp with no ST‐segment elevation or only mildly elevated (< 1 mm). The ST‐segment starts after the end of the QRS and is followed by a negative and sometimes deep T wave in lead V1.3, 12, 13 Corrado et al. described an index based on the slope of the first 80 ms of the ST‐segment in leads V1‐V2 that is of flat or ascendant direction in athletes and of descendant direction in Type‐2 Brugada pattern.11 Athletes exhibit an upsloping ST‐segment with a mean STJ/ST80 ratio (ratio of ST‐segment ascent at high takeoff of QRS/ST‐segment ascent at 80 ms later) ≤1, whereas Brugada patients show a downsloping ST‐segment with a STJ/ST80 ratio >1.11
Pectus Excavatum (Fig. 5)
In these patients, the rSr’ pattern is most likely the consequence of a change on the direction of the third vector, which due to thoracic deformity and change of heart location within the thorax, faces leads V1‐V2.14 This produces a peaked r´ of fast inscription in lead V1 followed by an ST‐segment with descending slope and very small, if exist, ST‐segment ascent. Characteristically, the P wave is negative due to abnormal direction of the P‐wave vector. There is no mismatch between the QRS duration in leads V1‐V2 and leads V5‐V6.
PATHOLOGICAL PATTERNS
Usually the r’ is taller than r, with a less fast ascent–descent morphology than in normal variants.
Type‐2 Brugada ECG Pattern (Figs. 6, 11, and 12)
The classic electrocardiographic abnormalities constitute the hallmark of Brugada syndrome. Recently a consensus report endorsed by the ISHNE13 has reduced the Brugada ECG patterns to only two variants: (1) Type 1 (coved pattern) characterized by ST‐segment elevation ≥2 mm in leads V1‐V2 followed by a symmetric negative T wave; and (2) Type 2 (saddle‐back pattern) that includes “old” patterns 2 and 3 from the previous consensus and is characterized by an r´ in leads V1‐V2, followed by convex ST‐elevation ≥ 0.5 mm with positive T wave in lead V2 and of variable morphology in lead V1. Type 1 is very specific and easy to recognize, however, differentiation of Type‐2 Brugada pattern from incomplete RBBB and other benign rSr´ patterns may be challenging. Some features to distinguish these patterns are: (1) the positive terminal r’ wave is peaked in incomplete RBBB, whereas in Type‐2 Brugada pattern is rounded, wide and of relatively low voltage; (2) in incomplete RBBB the QRS duration in leads V1‐V2 is identical to that observed in lead V5‐V6, whereas in Type‐2 Brugada pattern the QRS duration is longer in the right precordial leads than in leads V5‐V6 because the terminal deflection observed in lead V1 (r’ wave) cannot be recorded by the lateral electrodes (Fig. 2). This phenomenon is known as mismatch.15
The typical characteristics of Type‐2 Brugada ECG pattern or saddle‐back pattern in leads V1‐V2 include the following (Figs. 6 and 12):
High takeoff of r’ (that not necessary coincides with J point) ≥ 2 mm.
Descending arm of r’ coincides with the beginning of ST (sometimes is not well seen).
Minimum ST‐segment ascent ≥ 0.5 mm.
T wave usually but not always positive in lead V2 (T peak > ST minimum > 0) and of variable morphology in lead V1 (mildly positive, flat or mildly negative).
Angle between both arms of the r´ wave (ß angle) wider than in other cases with r´ in lead V1.12, 16
Base of triangle of r’ at 5 mm of high takeoff > 4mm12, 13, 15
Mismatch between QRS duration in leads V1 and V6 (longer in lead V1).15
Figure 12.
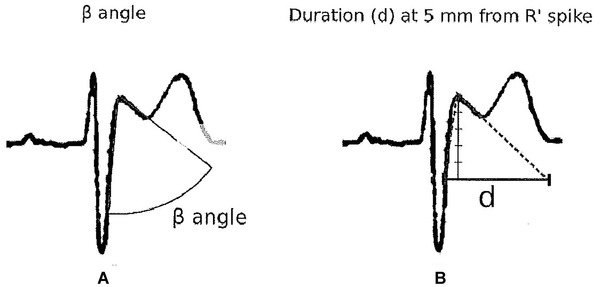
How to measure the ß angle (A) and the base of triangle of the r’ wave (B).
Right Ventricular Enlargement
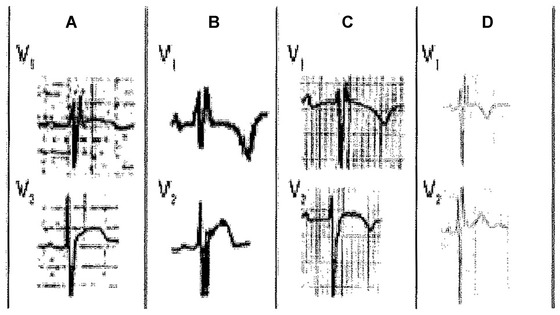
This concept encompasses hypertrophy and/or dilatation of all or part of the RV with the resultant delayed activation of some regions of the RV.3 These cases include different pathologies with RV involvement (enlargement/delayed conduction; Fig. 7A–G), and also some cases with biventricular enlargement (Fig. 7H).
Figure 7.
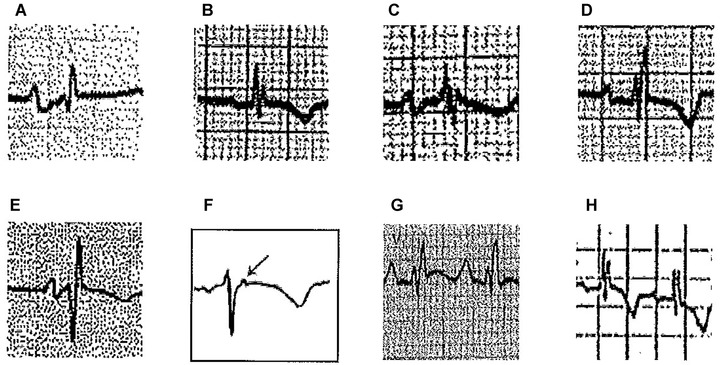
(A) Mitral stenosis with moderate pulmonary hypertension and functional tricuspid regurgitation, (B) A 9‐year‐old girl with mild pulmonary stenosis, (C) Chronic cor pulmonale secondary to chronic obstructive pulmonary disease (COPD) in elderly, (D) Ostium secundum‐type atrial septal defect, (E) ECG pattern after regression of RVE in postsurgery of tetralogy of Fallot, (F) epsilon wave (arrow) in lead V1 in a patient with ARVD, (G) Ebstein disease (note massive atrial enlargement), and (H) Biventricular enlargement in a 8‐year‐old patient with ventricular septal defect and hyperkinetic pulmonary hypertension (Katz‐Watchell pattern)
(1) Mitral valve disease with pulmonary hypertension: Usually the P wave presents the typical pattern of left atrial enlargement (LAE); (2) Congenital pulmonary stenosis 16: The r’ may be lower than r and the P wave does not show LAE criteria; (3) Chronic obstructive pulmonary disease: Usually the QRS in lead V1 is of low voltage, with rSr’ pattern and the P wave does not show a wide negative component; (4) Atrial septal defect (ASD): The typical ECG in the adult with an ASD shows an rSr’ or rsR’ configuration over the right precordial leads.17 This likely reflects right ventricular overload and concomitant RV peripheral delay rather than a true conduction delay in the right bundle branch.18 In ASD, the r´ is often broad and somewhat slurred. A notch near the apex of the R wave in the inferior leads is also frequent in patients with ASD, with a prevalence of 73.1%.19 Criteria for right atrial enlargement are found in one third of patients and right‐axis deviation is frequent in patients with associated pulmonary hypertension20; (5) Arrhythmogenic right ventricular dysplasia (ARVD): The typical ECG in ARVD is characterized by localized prolongation of the QRS complex (≥110 ms) in the right precordial leads (V1‐V3), often associated with an epsilon wave (terminal notch in the QRS complex) and T‐wave inversion. It may emulate an atypical rSr´ pattern because the epsilon wave sometimes is confused with an r´ wave. However usually the epsilon wave is a little separated of the QRS complex21; (6) Ebstein disease: May show a pattern of RBBB with R’ > r and often with very abnormal P wave.
Ventricular Preexcitation (Wolf‐Parkinson‐White Syndrome)
The diagnosis is straightforward when a delta wave is clearly visible and the PR interval is short, but differentiation may be more challenging whit more subtle degrees of preexcitation (Fig. 8). In posteroseptal accessory pathways, the QRS is predominantly negative in the inferior leads, especially in leads III and aVF, with R or RS morphology in lead V1 or V2 and QRS axis between −30° and −90°. Patients with left lateral accessory pathways exhibit a predominantly negative morphology in lateral leads (I, aVL) and usually a high R wave (RSr’) in leads V1 or V2, with right‐axis deviation (QRS axis beyond +90° up to +150°).
Figure 8.
Wolf‐Parkinson‐White with an RSr’ in lead V1 and delta wave suggesting a left lateral accessory pathway.
Hyperkalemia
In hyperkalemia, the T wave in some leads becomes very tall, wide, and peaked, sometimes with a striking elevation of the ST‐segment. With increased severity, the QRS complex becomes wider and the P wave may disappear (Fig. 9).
Figure 9.
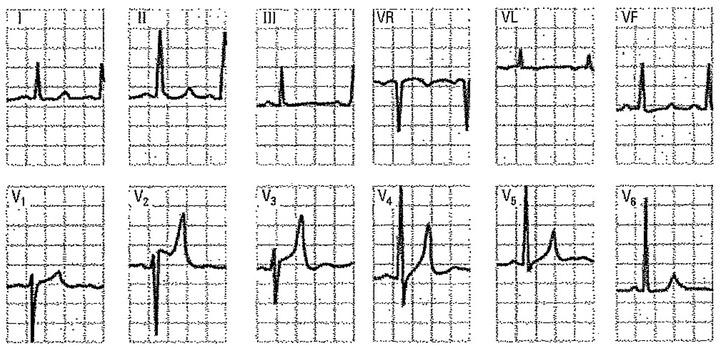
A 20‐year‐old male with chronic renal failure on hemodialysis during the past 2 years. Severe systemic hypertension (210/130 mmHg). Serum potassium levels of 6.4 mEq/1. Note the high and peaked T wave, as well as the ST‐segment elevation in leads V2 and V3. The QT interval duration is relatively long in leads I, II, and III at the expense of the ST‐segment due to the associated hypocalcemia.
CLINICAL APPROACH FOR THE DIFFERENTIAL DIAGNOSIS OF RSR’ (R’) PATTERN IN LEADS V1‐V2
Confirmation of a correct lead placement of the precordial leads during the ECG recording is mandatory. Rerecording is needed when misplacement is suspected (e.g., if P wave is negative in lead V1). Proper anamnesis is of utmost importance to give meaning to an ECG reading: a family history of sudden death or a personal history of recurrent syncope should alert about the possibility of Brugada syndrome or ARVD and eventually prompt further investigations. Physical examination may reveal the typical thoracic deformity in patients with pectus excavatum or a systolic murmur on cardiac auscultation compatible with an ASD mitral stenosis or pulmonary stenosis. Seric potassium should be measured if hyperkalemia is suspected (renal dysfunction, use of ACE inhibitors, etc.) or if other electrocardiographic features or hyperkalemia are evident (e.g., peaked T waves).
Some of the electrocardiographic indexes described above may be also useful in the differential diagnosis, including:
Characteristics of r’
Chevalier et al.16 and Okhubo et al.22 described that the angle of the ascending and descending arm of r’ (ß angle) was wider in patients with Type‐2 Brugada pattern than in those with incomplete RBBB. In the Chevalier study16 a ß angle > 58° yielded a positive predictive value of 73% and a negative predictive value of 87%. However, this angle is not easy to calculate in the clinical practice. For this reason, we have recently published12, 15 an easier method that can be used by any clinical cardiologist. It consists in measuring the base of the triangle formed by the ascending and descending arm of r’ at 5 mm from the apex. If the base is greater than 4 mm, most probably it corresponds to a Type‐2 Brugada pattern. On the contrary, the athletes have a base of triangle <4 mm (Figs. 4, 6, and 12).
There are other conditions to remember that may present with a base of the triangle > 4 mm: (1) ARVD and (2) hyperkalemia (Fig. 11). However in ARVD there is no ST‐segment elevation, the T wave is negative in leads V1 to V3 and usually the r’ corresponds to an epsilon wave that is a little separated of the QRS. Fontaine leads may help in making epsilon waves more obvious.21 In hyperkalemia, the T wave is usually greater than 7 mm in leads V2‐V3, something that is never observed in Type‐2 Brugada pattern.
Figure 11.
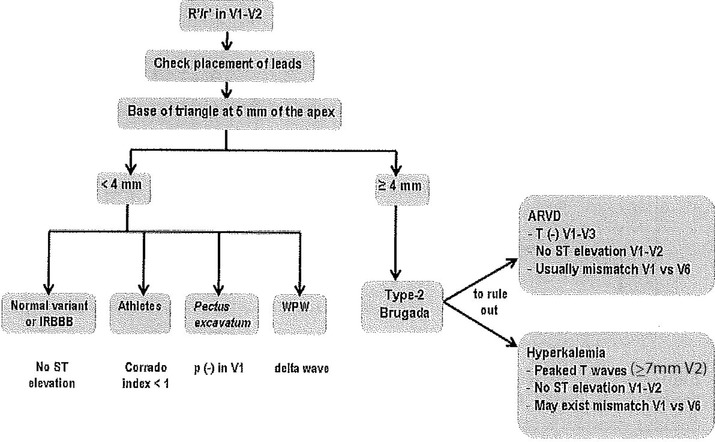
Proposed diagnostic algorithm in patients with r’ in leads V1‐V2.
Index Ratio of “ST Elevation at High Takeoff of QRS/ST Elevation at 80 ms” (Corrado Index)
According to Corrado et al.11 the ratio is >1 in type 1 Brugada pattern and <1 in athletes. Although this ratio usually allows to differentiate between Type‐1 Brugada pattern (see Fig. 10), it is not necessary true that the takeoff of QRS coincides always with the J point.12
Figure 10.
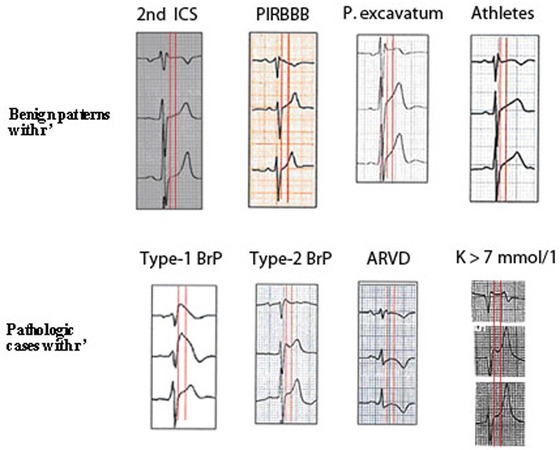
This figure shows (first red vertical line) the difference in the duration of QRS in lead V1 compared to lead V3 in Brugada patterns 1 and 2, ARVD and hyperkalemia (low row). However, in 4 variants of normality (upper row), the duration of QRS in leads V1‐V3 is the same. The second vertical line measured 80 ms later, shows that in the two Brugada patterns, the ST‐segment is downsloping (ratio ST at J point/ST 80 ms later > 1; Corrado index), but it is upsloping (in at least lead V2) in normal variants (upper row) (ratio < 1).
Discrepancy between the QRS Duration in Leads V1‐V2 and V5‐V6 (Mismatch)
This is usually seen in Type‐2 Brugada pattern, ARVD and hyperkalemia. However this is not easy to measure because the exact definition of the location of the J‐point (end of the QRS) in leads V1‐V2, as we previously said, can be very difficult to determine in these conditions.15 The mismatch phenomenon needs to be investigated in a larger cohort of patients with type‐2 Brugada pattern, using blind readers. This requires recording of simultaneous precordial leads.
Proposed Diagnostic Algorithm
In the presence of an rSr’ pattern in leads V1‐V2; we propose an algorithm to facilitate the differential diagnosis (Fig. 11). The algorithm focuses in patients without apparent structural heart disease. We excluded patients with ASD or other congenital heart diseases since the diagnosis usually is evident from physical examination and echocardiography.
The first step is to measure the base of the triangle of r´ (Figs. 11 and 12).
If the base of the triangle is >4 mm, it probably corresponds to a Type‐2 Brugada pattern, but still ARVD and hyperkalemia need to be ruled out. In all 3 cases usually there is mismatch between leads V1‐V2 and V5‐V6. However, in cases of ARVD T‐wave inversion in leads V1‐V3 is frequently seen and there is no ST‐segment elevation. In addition, low voltages and epsilon wave are quite frequent.21 In hyperkalemia, ST‐segment ascent can occur but always the T wave in leads V2‐V3 is very tall (>7 mm) what is not seen in Type‐2 Brugada pattern.
If the base of the triangle is <4 mm, it is unlikely to correspond to a type‐2 Brugada pattern, there is no mismatch between leads V1/V2‐V5/V6 and the differential diagnosis include: (i) Incomplete RBBB (no ST‐segment ascent); (ii) Athletes (the ST‐segment ascent may exist but the Corrado index is <1; Fig. 10); (iii) Pectus excavatum (Fig. 5) the P wave is negative; (iv) Ventricular preexcitation syndrome with left accessory pathway (a delta wave can be seen).
Figure 5.
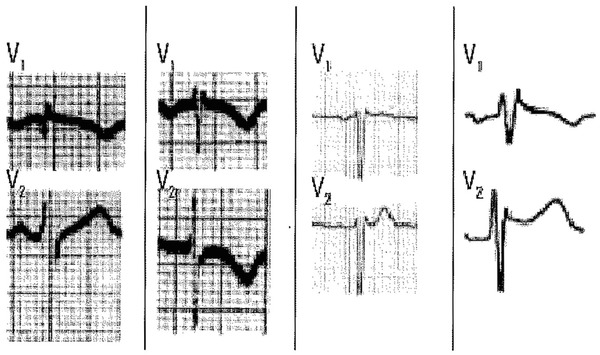
Different examples from patients with pectus excavatum. Note the negative P wave in lead V1.
CONCLUSIONS
The presence of rSr’ pattern in leads V1‐V2 that was considered in the past as a benign pattern; may also be expression of dangerous conditions such as Brugada syndrome or ARVD. This new electrocardiographic algorithm, based on the duration of the base of the triangle of r’, along with other criteria, may help on the diagnosis in the vast majority of the cases.
REFERENCES
- 1. Sodi Pallares D, Bisteni A, Medrano G. Electrocardiografia y Vectorcardiografia Deductiva. Mexico DF, Mexico: La Prensa Médica Mexicana, 1967. [Google Scholar]
- 2. Durrer D, Van Dam R, Freud G, et al. Total excitation of the isolated human heart. Circulation 1970;41:899–912. [DOI] [PubMed] [Google Scholar]
- 3. Bayes de Luna A. Textbook of Clinical ECG. Hoboken, NJ: Wiley‐Blackwell, 2012. [Google Scholar]
- 4. Liao YL, Emidy LA, Dyer A, et al. Characteristics and prognosis of incomplete right bundle branch block: An epidemiologic study. J Am Coll Cardiol 1986;7(3):492–499. [DOI] [PubMed] [Google Scholar]
- 5. Lau EW, Ng GA, Griffith MJ. A new sign of an accessory pathway in sinus rhythm: Pseudo partial right bundle branch block. Heart 1999;82:244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia Niebla J, Llontop‐Garcia P, Valle J, et al. Technical mistakes during the acquisition of the electrocardiogram. Ann Noninvasive Electrocardiol 2009;14:389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bussink BE, Holst AG, Jespersen L, et al. Right bundle branch block: Prevalence, risk factors, and outcome in the general population: Results from the Copenhagen City Heart Study. Eur Heart J 2013;34(2):138–146. [DOI] [PubMed] [Google Scholar]
- 8. Mauric AT, Samani NJ, de Bono DP. When should we diagnose incomplete right bundle branch block? Eur Heart J 1993;14(5):602–608. [DOI] [PubMed] [Google Scholar]
- 9. Rabkin SW, Mathewson FA, Tate RB. Long term followup of incomplete right bundle branch block: The risk of development of complete right bundle branch block. J Electrocardiol 1981;14(4):379–386. [DOI] [PubMed] [Google Scholar]
- 10. Schamroth L, Myburgh DP, Schamroth CL. The early signs of right bundle branch block. Chest 1985;87(2):180–185. [DOI] [PubMed] [Google Scholar]
- 11. Corrado D, Pelliccia A, Heidbuchel H, et al.; Section of Sports Cardiology, European Association of Cardiovascular Prevention and Rehabilitation . Recommendations for interpretation of 12‐lead electrocardiogram in the athlete. Eur Heart J 2010;31(2):243–259. [DOI] [PubMed] [Google Scholar]
- 12. Serra G, Baranchuk A, Bayés‐De‐Luna A, et al. New electrocardiographic criteria to differentiate the Type‐2 Brugada pattern from electrocardiogram of healthy athletes with r’‐wave in leads V1/V2 . Europace 2014;16:1639–1645. [DOI] [PubMed] [Google Scholar]
- 13. Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol 2012;45(5):433–442. [DOI] [PubMed] [Google Scholar]
- 14. Awad SFM, Barbosa‐Barros R, de Sousa Belem L, et al. Brugada Phenocopy in a patient with pectus excavatum: Systematic review of the ECG manifestations associated with pectus excavatum. Ann Noninv Eelctroacardiol 2013;18(5):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bayés de Luna A, García‐Niebla J, Baranchuk A. New electrocardiograpphic features in Brugada syndrome. Curr Cardiol Rev 2014;10(3):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chevallier S, Forclaz A, Tenkorang J, et al. New electrocardiographic criteria for discriminating between Brugada types 2 and 3 patterns and incomplete right bundle branch block. J Am Coll Cardiol 2011;58:2290–2298. [DOI] [PubMed] [Google Scholar]
- 17. Khairy P, Marelli AJ. Clinical use of electrocardiography in adults with congenital heart disease. Circulation 2007;116(23):2734–2746. [DOI] [PubMed] [Google Scholar]
- 18. Sung RJ, Tamer DM, Agha AS, et al. Etiology of the electrocardiographic pattern of “incomplete right bundle branch block” in atrial septal defect: An electrophysiologic study. J Pediatr 1975;87(6 Pt 2):1182–1186. [DOI] [PubMed] [Google Scholar]
- 19. Heller J, Hagege AA, Besse B, et al. “Crochetage” (notch) on R wave in inferior limb leads: A new independent electrocardiographic sign of atrial septal defect. J Am Coll Cardiol 1996;27:877–882. [DOI] [PubMed] [Google Scholar]
- 20. Yamaki S, Horiuchi T, Miura M, et al. Pulmonary vascular disease in secundum atrial septal defect with pulmonary hypertension. Chest 1986;89:694–698. [DOI] [PubMed] [Google Scholar]
- 21. Gottschalk B, Gysel M, Barbosa‐Barros R, et al. The use of Fontaine leads in the diagnosis of arrhythmogenic right ventricular dysplasia. Ann Noninv Electrocardiol 2014;19(3):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohkubo K, Watanabe I, Okumura Y, et al. A new criteria differentiating Type 2 and 3 Brugada patterns from ordinary incomplete right bundle branch block. Int Heart J 2011;52(3):159–163. [DOI] [PubMed] [Google Scholar]


