Abstract
Background
The cause of ischemic stroke or transient ischemic attack (TIA) remains unclear after initial cardiac monitoring in approximately one‐third of patients. Randomized controlled trials (RCTs) showed that the prolonged cardiac monitoring of patients with cryptogenic stroke or TIA increased detection of atrial fibrillation (AF). We aimed to perform a meta‐analysis of all RCTs that evaluated the prolonged monitoring ≥7 days in patients with cryptogenic stroke or TIA.
Methods
We searched PubMed, EMBASE, Cochrane CENTRAL, and relevant references for RCTs without language restriction (inception through December 2014) and performed meta‐analysis using random effects model. Detection of AF, use of anticoagulation at follow‐up, recurrent stroke or TIA, and mortality were major outcomes.
Results
Four RCTs with 1149 total patients were included in the meta‐analysis. Prolonged cardiac monitoring ≥7 days compared to shorter cardiac monitoring of ≤48 hours duration increased the detection of AF (≥30 seconds duration) in patients after cryptogenic stroke or TIA (13.8% vs. 2.5%; odds ratio [OR], 6.4; 95% confidence interval [CI], 3.50–11.73; P < 0.00001; I2, 0%]. It also increased the odds of AF detection of any duration (22.6% vs. 5.2%; 5.68[3.3–9.77]; P < 0.00001; I2, 0%). The patients who underwent prolonged monitoring were more likely to be on anticoagulation at follow‐up (2.21[1.52–3.21]; P < 0.0001; I2, 0%). No differences in recurrent stroke or TIA (0.78[0.40–1.55]; P = 0.48; I2, 0%) and mortality (1.33[0.29–6.00]; P = 0.71; I2, 0%] were observed between two strategies.
Conclusion
Prolonged cardiac monitoring improves detection of atrial fibrillation and anti‐coagulation use after cryptogenic stroke or TIA and therefore should be considered instead of shorter duration of cardiac monitoring.
Keywords: cardiac monitor, atrial fibrillation, stroke, TIA, meta‐analysis
Stroke leads to significant death and disability.1 Atrial fibrillation (AF) is a common cause of ischemic stroke and results in more recurrence and higher mortality after a stroke.2 It has been suggested that one third of the strokes and transient ischemic attack (TIA) are cryptogenic in origin and, therefore, require additional investigation and intervention.3 In patients with stroke or TIA, monitoring during hospital admission usually results in approximately 10% detection of atrial fibrillation4 while additional 11% can be detected with prolonged monitoring.5 Patients with a diagnosis of atrial fibrillation after a stroke or TIA are managed with anticoagulation to prevent future stroke. In absence of documented AF diagnosis, they are managed with antiplatelet agents that are inferior in preventing stroke but result in increased risks of bleeding compared to anticoagulants.6, 7, 8
The American Heart Association/American Stroke Association guidelines for stroke prevention recommend up to 1 month of cardiac monitoring in patients with cryptogenic stroke or TIA to investigate AF on the basis of observational studies.9 Two previous meta‐analyses with mostly heterogeneous observational studies found that the detection of atrial fibrillation increased significantly by longer monitoring.10, 11 However, no meta‐analysis has been published with the data from RCTs only. Therefore, to evaluate the efficacy of prolonged cardiac monitoring of ≥7 days in detecting AF in patients with cryptogenic stroke or TIA, and investigate its effects on anticoagulation use and mortality, we performed a meta‐analysis of all RCTs.
METHODS
Data Sources and Search Strategy
The meta‐analysis was performed in accordance with PRISMA statement. 12 We searched MEDLINE, EMBASE, and Cochrane CENTRAL register of clinical trials for RCTs without language restriction from inception through December 2014. The search terms were “cardiac monitor” or “loop monitor” or “implantable cardiac monitor” and “cryptogenic stroke” or “stroke” or “cerebrovascular accident” or “CVA” or “transient ischemic attack” or “TIA” with restriction to randomized designs. Two investigators (K.D. and B.C.) independently performed the database search and agreed on final study selection. We also searched ClinicalTrials.gov for past or ongoing trials of interest and performed manual search for all relevant references including reviews and meta‐analyses.
Study Inclusion and Exclusion Criteria
Randomized controlled trials comparing prolonged (≥7 days) versus shorter (≤48 Hours) cardiac monitoring in patients with cryptogenic stroke or TIA were included. Studies reporting any one of our major outcomes were included. We excluded the studies that were nonrandomized.
Data Extraction
Data were extracted by two investigators (K.D. and B.C.) in duplicate using standardized data‐extraction tables. We obtained data on study and patient characteristics, type and duration of cardiac monitoring, and outcomes of interest including adverse events.
Outcomes
The detection of atrial fibrillation was the primary outcome. The use of anticoagulant at follow‐up, recurrent stroke or TIA, and mortality were the secondary outcomes.
Statistical Analysis
Odds ratio (OR) with 95% confidence interval (CI) was used to pool the binary outcomes. Crude events from each study were used to compute OR with 95% CI. In addition, we calculated AF detection rates. We used DerSimonian‐Laird random effects model for meta‐analysis and considered P < 0.05 (two‐tailed) to be statistically significant for computed effects. Begg's funnel plot was used to visually examine publication bias at outcome level. We used Jadad scale13 to assess the quality of studies on the basis of randomization, blinding, and attrition of participants. For studies reporting different durations of follow‐up, we used the data with the longest follow‐up period. Study heterogeneity was evaluated with Cochran's Q and I 2 statistics with I 2 > 60% being significant heterogeneity, in which case sensitivity analyses were planned, although they were not performed as we did not observe significant heterogeneity in all outcomes of interest. We used Review Manager (RevMan 5.3, Cochrane Collaboration, Nordic Cochrane Center, Copenhagen, Denmark) for meta‐analysis.
RESULTS
Description of Included Studies
The flow diagram for study selection is shown in Figure 1. Initial electronic databases and manual search resulted in 167 citations with 11 duplicates. We reviewed 156 studies for eligibility and extracted nine studies for full‐text review. A total of four RCTs 14, 15, 16, 17 with 1149 patients (577 in longer monitoring and 572 in shorter monitoring) were included in the meta‐analysis.
Figure 1.
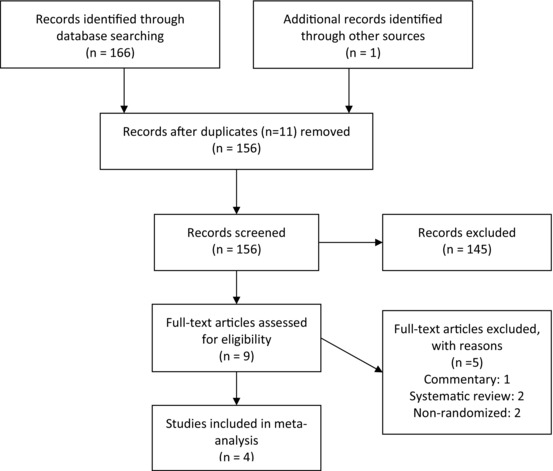
Flow diagram for study selection.
The individual study and patient characteristics are presented in Table 1. The duration of follow up was 14 days to 12 months. The patients were mostly Caucasian males and had stroke as index event.
Table 1.
Baseline Study and Patient Characteristics
| Patients Characteristics at Baseline | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Age | Ischemic | Antiplatelet | ||||||||
| Study | Patients, | Device | Follow‐Up | (Years) | Female | White | Diabetes | HTN | Stroke | TIA | Agents |
| (Ref. No.) | n | Type | Duration | (mean ± SD) | (%) | (%) | (%) | (%) | (%) | (%) | Use (%) |
| Gladstone, 2014 16 | I:286 | 30‐day noninvasive ambulatory ECG monitor | 3 months | I:72.5 ± 8.5 | I:46.2 | I:89.9 | I:19.2 | I:71.3 | I:65.7 | I:34.3 | na |
| C:285 | C:73.2 ± 8.8 | C:43.9 | C:91.2 | C:19.3 | C:67.0 | C:60.4 | C:39.6 | ||||
| Higgins, 2013 14 | I:50 | 7‐day non‐invasive cardiac event monitor | 14 days and 3 months | I:67.1 ± 11.1 | I:52 | na | I:8 | I:56 | I:70 | I:30 | I:72 |
| C:50 | C:64.6 ± 13.3 | C:36 | C:22 | C:60 | C:66 | C:34 | C:78 | ||||
| Kamel, 2013 17 | I:20 | 21‐day mobile cardiac | 3 and 12 months | I:65 ± 12 | I:40 | Na | I:20 | I:75 | I:60 | I:40 | I:na |
| C:20 | Outpatient telemetry | C:69 ± 9 | C:45 | C:30 | C:70 | C:75 | C:25 | C:na | |||
| Sanna, 2014 15 | I:221 | Insertable cardiac monitor | 6 and 12 months | I:61.6 ± 11.4 | I:35.7 | I:87.8 | I:15.4 | I:65.2 | I:90.5 | I:9.5 | I:95.9 |
| C:220 | C:61.4 ± 11.3 | C:37.3 | C:86.8 | C:17.3 | C:57.7 | C:91.4 | C:8.6 | C:96.4 | |||
Primary Outcome: Detection of Atrial Fibrillation
Prolonged cardiac monitoring (≥7 days) increased the detection rate of ≥ 30 seconds atrial fibrillation in patients after cryptogenic stroke or TIA compared with shorter cardiac monitoring of ≤48 Hour duration (13.8% vs. 2.5%; OR, 6.4; 95% CI, 3.50‐11.73; P < 0.00001; I2, 0%) as shown in Figure 2. Kamel et al.’s study did not detect any atrial fibrillation at follow‐up in both intervention and control arms.17 Prolonged cardiac monitoring also increased the detection of any duration of atrial fibrillation (22.6% vs. 5.2%; 5.68 [3.3–9.77]; P < 0.00001; I2, 0%) compared to shorter monitoring.
Figure 2.
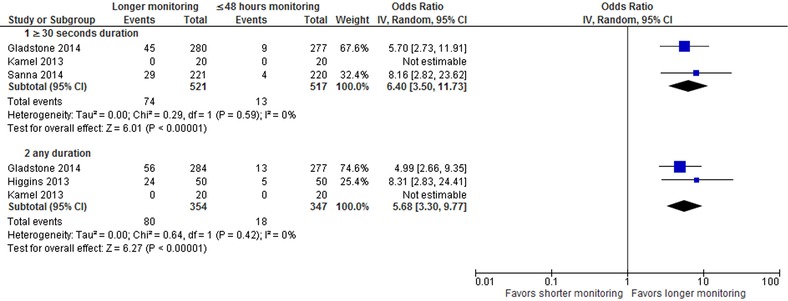
Forest plot for detection of atrial fibrillation.
SECONDARY OUTCOMES
Anticoagulation Use at Follow‐Up
The use of anticoagulation at follow‐up (Figure 3) was significantly higher in patients who underwent prolonged monitoring (2.21 [1.52–3.21]; P < 0.0001; I2, 0%] compared to shorter cardiac monitoring awing to increased AF detection. No patients received anticoagulation at follow‐up in the study by Kamel et al., as there was no detection of atrial fibrillation.
Figure 3.
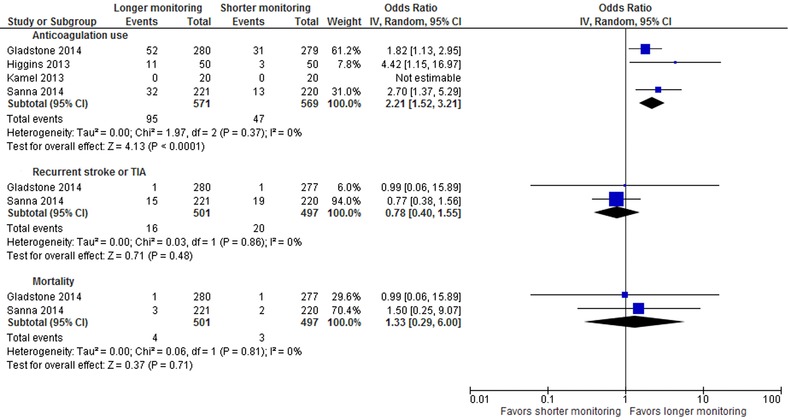
Forest plot for anticoagulation use, recurrent stroke or TIA and mortality.
Recurrent Stroke or TIA and Mortality
At follow‐up (Figure 3), prolonged monitoring compared to shorter monitoring did not show any difference in recurrent stroke or TIA (0.78 [0.40–1.55]; P = 0.48; I2, 0%) and mortality (1.33 [0.29–6.00]; P = 0.71; I2, 0%). None of the studies were powered to detect a difference in mortality. Only two studies reported on these outcomes.15, 16 In Higgins et al.’s study, there were a total of four events of stroke, TIA, MI, or death in each arm.
Study Quality and Publication Bias
Jadad score was three for all studies. Funnel plots were symmetrical for all outcomes indicating that there was no publication bias that was confirmed by Egger's regression intercept and Begg's rank correlation. No heterogeneity was observed for all outcomes as indicated by I2 statistics.
DISCUSSION
The major findings of the current meta‐analysis were in patients with cryptogenic stroke or TIA, prolonged cardiac monitoring (≥7 days) compared to shorter monitoring (≤48 hours) increased the detection of atrial fibrillation and the use of anticoagulation at follow‐up. It, however, resulted in no differences in recurrent stroke or TIA and mortality.
A prior meta‐analysis that included mostly single‐arm observational studies and only one RCT showed that atrial fibrillation detection rate was 11.5% with longer cardiac monitoring. 10 The meta‐analysis, however, was limited by small sample sizes and significant heterogeneity observed as noted by the authors. Current AHA/American Stroke Association guidelines recommend prolonged cardiac monitoring up to 30 days in patients with cryptogenic stroke or TIA on the basis of observational studies. 5, 9 The strength of current meta‐analysis lies on the fact that it includes all available RCTs on this topic of great interest and we performed analyses on the use of anticoagulation at follow‐up, recurrent stroke or TIA, and mortality.
A higher detection rate of atrial fibrillation has important clinical implications as it increases the rate of being initiated on anticoagulation as evidenced in the current meta‐analysis. These patients otherwise would just be getting antiplatelet agents which are inferior to antithrombotic agents in stroke prevention in atrial fibrillation. In addition, device‐detected atrial fibrillation significantly increases the risks of future stroke.18, 19 Although the study did not find any advantage on recurrent stroke or TIA and mortality outcomes, it should be noted that the studies were not designed to detect difference in such outcomes.
Study Limitations
The current meta‐analysis has several limitations including lack of patient‐level data, different duration of follow‐up among studies, and variations in type of device. It was not possible to ascertain which device and follow‐up duration worked better compared to the others. It must be noted that there were not enough studies to define the exact duration of monitoring required although longer the duration of monitoring, higher the detection rate was. Since the shortest device monitoring was for 1 week duration, it may be suggested that a minimum of one week monitoring is beneficial but depending on patients’ interests and choices, a longer duration of monitoring should be recommended. Despite all these limitations, the current meta‐analysis is strengthened by inclusion of randomized trials and absence of detectable heterogeneity in all outcomes.
CONCLUSIONS
The current meta‐analysis of randomized trials shows that prolonged cardiac monitoring improves detection of atrial fibrillation and anticoagulation use after cryptogenic stroke or TIA and therefore supports the updated AHA/American Stroke Association guidelines 2014. Further randomized trials should be designed to define the optimum duration of follow‐up and the effects on the risks of recurrent stroke or mortality.
Authors’ Contributions
Drs. K. Dahal and B. Chapagain had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: K. Dahal. Acquisition, analysis, or interpretation of data: K. Dahal, B. Chapagain, R. Maharjan, H.H. Farah, A. Nazeer, R.J. Lootens, A. Rosenfeld. Drafting of the manuscript: K. Dahal, B. Chapagain. Critical revision of the manuscript for important intellectual content: R. Maharjan, H.H. Farah, A. Nazeer, R.J. Lootens, A. Rosenfeld. Statistical analysis: K. Dahal. Study supervision: B. Chapagain, R. Maharjan, H.H. Farah, A. Nazeer, R.J. Lootens, A. Rosenfeld.
[Correction added on March 14, 2017, after first online publication: The second author name was corrected to Bikash Chapagain and the fourth author name was corrected to Husam H. Farah.]
Conflicts of Interest Disclosures: None.
Funding/Support: This research was self‐funded.
REFERENCES
- 1. Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 2. Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population‐based study. Stroke 2005;36(6):1115–1119. [DOI] [PubMed] [Google Scholar]
- 3. Khan M, Miller DJ. Detection of paroxysmal atrial fibrillation in stroke/tia patients. Stroke Res Treat 2013;2013:840265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizos T, Guntner J, Jenetzky E, et al. Continuous stroke unit electrocardiographic monitoring versus 24‐hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke 2012;43(10):2689–2694. [DOI] [PubMed] [Google Scholar]
- 5. Flint AC, Banki NM, Ren X, Rao VA, Go AS. Detection of paroxysmal atrial fibrillation by 30‐day event monitoring in cryptogenic ischemic stroke: the Stroke and Monitoring for PAF in Real Time (SMART) Registry. Stroke 2012;43(10):2788–2790. [DOI] [PubMed] [Google Scholar]
- 6. Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non‐valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2007(3):CD006186. [DOI] [PubMed] [Google Scholar]
- 7. Cameron C, Coyle D, Richter T, et al. Systematic review and network meta‐analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open 2014;4(6):e004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146(12):857–867. [DOI] [PubMed] [Google Scholar]
- 9. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45(7):2160–2236. [DOI] [PubMed] [Google Scholar]
- 10. Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta‐analysis. Stroke 2014;45(2):520–526. [DOI] [PubMed] [Google Scholar]
- 11. Dussault C, Toeg H, Nathan M, Wang ZJ, Roux JF, Secemsky E. Electrocardiographic Monitoring for Detecting Atrial Fibrillation after Ischemic Stroke or Transient Ischemic Attack: A Systematic Review and Meta‐Analysis. Circ Arrhythm Electrophysiol 2015;C8(2):263–269. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 14. Higgins P, MacFarlane PW, Dawson J, McInnes GT, Langhorne P, Lees KR. Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke 2013;44(9):2525–2531. [DOI] [PubMed] [Google Scholar]
- 15. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370(26):2478–2486. [DOI] [PubMed] [Google Scholar]
- 16. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370(26):2467–2477. [DOI] [PubMed] [Google Scholar]
- 17. Kamel H, Navi BB, Elijovich L, et al. Pilot randomized trial of outpatient cardiac monitoring after cryptogenic stroke. Stroke 2013;44(2):528–530. [DOI] [PubMed] [Google Scholar]
- 18. Boriani G, Glotzer TV, Santini M, et al. Device‐detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014;35(8):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santini M, Gasparini M, Landolina M, et al. Device‐detected atrial tachyarrhythmias predict adverse outcome in real‐world patients with implantable biventricular defibrillators. J Am Coll Cardiol 2011;57(2):167–172. [DOI] [PubMed] [Google Scholar]


