Abstract
Background
Detecting asymptomatic and undiagnosed atrial fibrillation (AF) is increasingly important. Recently, we developed a wristwatch‐based pulse wave monitor (PWM; Seiko Epson, Japan) capable of long‐term recording, with an automatic diagnosis algorithm that uses frequency‐based pulse wave analysis. The aim of this study was to evaluate the validity of continuous pulse wave monitoring for detection of AF.
Methods
During the electrophysiological study (EPS) in patients with AF, simultaneous pulse wave monitoring and Holter electrocardiograms (ECG) were recorded (n = 136, mean age 62.7 ± 10.9 years). The diagnostic accuracy of the PWM for AF was compared to the Holter ECG diagnosis. Standard performance metrics (sensitivity [Se], specificity [Sp], positive predictive value [PPV], and negative predictive value [NPV]) were calculated. The duration‐based measurements were based on the diagnosis concordance ratios for the duration of time between diagnosis detected by the PWM and true diagnosis by the Holter ECG (AF or not AF). The episode‐based performance metrics were based on the proportion of episodes appropriately detected with the PWM relative to episodes determined by the Holter ECG.
Results
The total recording time was 1,542,770 s (AF: 270,945 s). A high diagnostic Sp (patient average: 96.4%, cumulative: 97.7%) and NPV (patient average: 95.1%, cumulative: 96.8%) were obtained in the duration‐based results. In the episode‐based metrics, all indices significantly improved with longer AF episode durations.
Conclusions
Continuous pulse wave monitoring can provide accurate and dependable information to aid in AF diagnosis. A high validity in confirming freedom from AF was shown by a high NPV.
Keywords: atrial fibrillation, monitoring, pulse wave
1. INTRODUCTION
Atrial fibrillation (AF) is one of the most common arrhythmias encountered in medical practice. The estimated number of patients with AF is over 30 million worldwide and is expected to grow in the coming years (Kirchhof et al., 2016). In Japan, AF is estimated to affect 1–2 million individuals. AF is associated with impaired quality of life, poorer cardiac hemodynamics, and an increased risk of heart failure and embolic events, including strokes, or vascular dementia, resulting in severe sequela. Such consequences also carry an economic burden. Antithrombotic therapy can prevent 60% of ischemic strokes in patients with AF (Hart, Pearce, & Aguilar, 2007). Therefore, early detection of AF and initiation of appropriate treatment with antithrombotic therapy are crucial. However, AF is often asymptomatic, so‐called “silent AF,” which can cause cryptogenic strokes as well as devastating large strokes. Among patients with AF‐associated stroke, about 25% of those had newly diagnosed AF only after the stroke event occurred (Friberg et al., 2014). The 2016 European Society of Cardiology (ESC) guidelines recommend screening with continuous electrocardiogram (ECG) monitoring for AF in high‐risk populations, those over the age of 65, and in all patients with a history of transient ischemic attack (Kirchhof et al., 2016).
Despite a need for early detection of AF, there remains no established screening device. Traditional Holter ECG monitoring for up to 72 hr does not sufficiently detect AF (Liao, Khalid, Scallan, Morillo, & O'Donnell, 2007). However, modern noninvasive devices are expected to be useful for AF screening. The effectiveness of patient‐activated event recorders, smartphone‐based applications using photoplethysmography (PPG) (Chan et al., 2016), and handheld recording single lead ECG has been reported (Klein‐Wiele et al., 2016) (Kaasenbrood et al., 2016). However, such devices are able to diagnose heart rhythms only at the precise moment when patients use them; therefore, these devices are unable to reliably detect silent AF. The performance of pulse wave technology as a long‐term recording method remains unknown. Longer term recording is known to have a better detection rate than short‐term recordings (Dussault et al., 2015). An implantable cardiac monitor is appealing with respect to longevity and diagnostic ability (Sanders et al., 2016), but remains costly and invasive, such that it is not suitable for general screening. Noninvasive, low cost, and long‐term screening devices are still needed.
A novel wristwatch‐based pulse wave monitor (PWM; Seiko Epson, Japan), with an automatic AF diagnosis algorithm, in which an analysis of the pulse wave frequency is used to identify AF, has been introduced in Japan. This noninvasive wearable device is capable of long‐term recording. However, the validity of the algorithm has not been well investigated. This study aimed to evaluate the diagnostic performance of a wristwatch‐based PWM.
2. METHODS
2.1. Study design
This was a prospective single‐center study coordinated by the National Cerebral and Cardiovascular Center, Japan. The study protocol was reviewed and approved by the institutional ethics committee (M26–134–5). Written informed consent was obtained from each participant.
One hundred thirty‐six consecutive patients with AF were enrolled between May 2015 and October 2016 in this study. The patient inclusion criteria for enrollment were as follows: (a) a diagnosis of AF in accordance with the 2014 AHA/ACC/HRS guidelines on the management of patients with atrial fibrillation (January et al., 2014), and (b) patients who underwent an electrophysiological study (EPS) and radiofrequency catheter ablation or cryothermal energy balloon ablation for AF. The exclusion criteria were as follows: (a) structural heart disease and use of antiarrhythmic drugs, (b) use of implantable pacing device including pacemaker and ICD, and (c) a previous pulmonary vein ablation for atrial fibrillation. No age‐ or sex‐related criteria were set for this study.
2.2. Data collection from patients in electrophysiological study
Patients were treated with oral anticoagulants for at least 30 days before the procedure. Transesophageal echocardiography was performed to exclude the presence of atrial thrombi before ablation. During the procedure, intravenous heparin was used to maintain a target activated clotting time of >300 s. All patients underwent the EPS undersedation with a continuous infusion of propofol and dexmedetomidine. Surface electrocardiographic leads and bipolar intracardiac electrograms were recorded with a polygraph (EP‐lab, Bard Electrophysiology Division, Lowell, MA, USA). If sinus rhythm continued from the beginning of the procedure, arrhythmia was induced using atrial programmed stimulation or burst pacing with isoproterenol.
Before the procedure, we placed the PWM on the patient's left or right wrist and the Holter ECG monitor and started both recordings. For the Holter ECG recording, a Spiderview device (Ela Medical, Sorin Group, Italy) was used, which provides a digital ECG signal at a sampling frequency of 200 Hz. We recorded 2 channels using 5 electrodes in this study.
2.3. Annotation of Holter and PWM recordings
Two trained reviewers interpreted the Holter ECG for the presence of AF and marked it on the record. Continuous atrial flutter episodes were excluded, and short duration of atrial tachycardia and atrial flutter in AF episode were not distinguished. They were blinded to the patient demographics, clinical information, and pulse wave data. We compared PWM‐detected atrial tachycardia episodes to a reference diagnosis of AF made using Holter interpretation.
2.4. Device characteristics and AF detection algorithm
The wristwatch‐based PWM was capable of long‐term recording (5 days) with an automatic AF diagnosis algorithm using frequency analysis. The recorded pulse wave at 16 Hz was analyzed to obtain a series of pulse period (PP) values. Figure 1a shows the flow chart of the diagnostic algorithm. First, a pair of fast Fourier transforms (FFT/IFFTs) were applied to obtain a short‐term autocorrelation function (ACF) on the pulse waveforms every second over a sliding 4‐s time frame. The time lag of the ACF was defined as the PPi, which is assumed as the RR interval (RRI), in each time frame (Figure 1b). Subsequently, the PP series was normalized by the logarithmic ratios (ri) of the successive values (PPi and PPi − 1), as follows:
Figure 1.
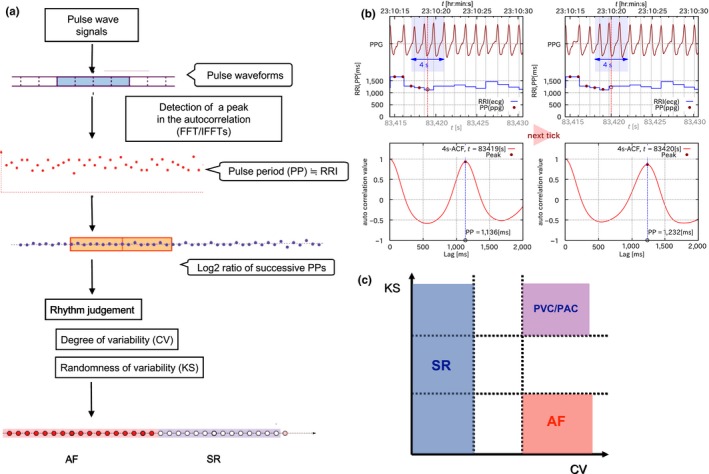
(a) Diagnostic algorithm: The logarithmic ratio of the successive pulse period (PP) values for each collected PP series was analyzed based on the standard deviation (SD), as an alternative to the coefficient of variation (CV) of the PP values and Kolmogorov‐Smirnov (KS) difference. (b) Pulse wave parameters: The time lag of the primary peak of the autocorrelation function (ACF) of the spectrum was regarded as the PP and assumed as the RR interval (RRI) in each time frame every 4 s. (c) Rhythm determination: When the pulse period series had low dispersion (low CV), the heart rhythm in that series was classified as sinus rhythm. When the pulse period series had both high variability (high CV) and high randomness (low KS), the heart rhythm was defined as atrial fibrillation (AF). The pulse period series with high variability (high CV) but low randomness (high KS) was assumed to have features of an atrial premature complex (PAC) or ventricular premature complex (PVC) with a constant coupling interval. In this study, we distinguished AF from non‐AF, which included SR and PAC or PVC
Finally, the N‐series of riwas analyzed for rhythm judgments by both the degree and randomness of variation. The standard deviation (SD) of ri was used as the relative degree of variation, as an alternative to the “CV” in substitution for the coefficient of variation (CV) of the original PP series. As for the index of randomness, the Kolmogorov‐Smirnov (KS) difference between the empirical distribution of riand its reference Gaussian distribution was used. The KS difference (D) in the N‐series was determined as follows:
In this case, EN (x) is the empirical distribution for rifound at x, and R (x) is the reference Gaussian distribution with the same average and variance. The KS difference was expected to be lower in the setting of AF due to a greater heart rate irregularity.
Rhythm judgment was based on these 2 statistical values, CV and KS. When the pulse period series had low dispersion (low CV), the heart rhythm in that series was classified as sinus rhythm. When the pulse period series had both high variability (high CV) and high randomness (low KS), the heart rhythm was defined as atrial fibrillation (AF). The pulse period series with high variability (high CV) but low randomness (high KS) was assumed to have features of an atrial premature complex (PAC) or ventricular premature complex (PVC) with a constant coupling interval (Figure 1c). In this study, we distinguished AF from non‐AF, which included SR and PAC or PVC. If the evaluated data did not correspond with a particular heart rhythm, the preceding diagnosis continued until criteria were met for an alternate diagnosis. In this study, sinus rhythm and atrial or ventricular premature complexes were considered non‐AF periods. Figure 2 gives examples of recorded pulse waves and diagnoses.
Figure 2.
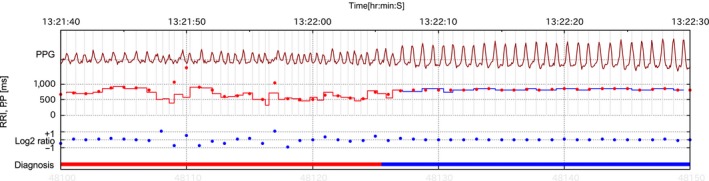
Examples of recorded pulse waves and diagnoses. Dark red line: Pulse wave; Red dot line: Calculated PP in the pulse wave; Step line: RR interval in ECG; Blue dot line: Log2 ratio of successive PPs; Red bar: AF diagnosis; Blue bar: Sinus rhythm diagnosis
2.5. Statistical analysis
This study included 2 phases of analysis. First, a pilot of 20 patients were randomly selected to identify the optimal threshold values for CV and KS. The kappa statistic (κ) was used to measure agreement. We evaluated the diagnostic accuracy of duration‐based measurements (sensitivity [Se], specificity [Sp], positive predictive value [PPV], and negative predictive value [NPV]). In the second phase, we assessed the reproducibility of diagnostic precision with another 116 patients. In this phase, the diagnostic accuracy of the duration‐based and episode‐based measurements was calculated.
The duration‐based measurements were evaluated according to the diagnosis concordance ratios for the duration of time between periods (AF or not AF) detected by the PWM, and the periods of true diagnosis (true AF or not AF) determined by the Holter ECG.
The episode‐based Se and Sp were evaluated according to the proportion of episodes (AF or not AF) appropriately detected with the PWM relative to true episodes (true AF or not AF) determined by the Holter ECG. The episode‐based PPV and NPV were defined as the proportion of true episodes, as determined by Holter ECG, detected with the PWM. For episode‐based Se and PPV, a true positive was defined as a true AF episode that overlapped for the AF duration. For episode‐based Sp and NPV, a true negative was defined as a true non‐AF episode that overlapped for the non‐AF duration. These values were calculated for episodes of >6 min, >10 min, and >30 min, respectively. A continuous episode of AF or non‐AF was counted as 1 episode.
We calculated cumulative results and patient‐based results in both duration‐ and episode‐ based measurements. In patient‐based results, we assessed the duration and episode results within each patient with AF episodes at least over 6 min and calculated patient average.
3. RESULTS
3.1. Study population
Patient characteristics are summarized in Table 1. The mean age was 62.7 ± 11.1 years, and 75.7% were male. A total of 98 patients (72.1%) had paroxysmal AF. There were no significant differences between the pilot and primary study groups with respect to demographics. All patients had AF during the procedure. Among them, 82 patients (the pilot study: n = 13, the primary study: n = 69) had AF episodes >6 min, who were included in patient‐based results. The total recording time, including 2 phases of analysis, was 1,542,770 s. Of that, a total AF duration of 270,945 s was identified.
Table 1.
Baseline characteristics of the study subjects
| Pilot (n = 20) | Primary (n = 116) | |
|---|---|---|
| Age, mean ± SD, years | 64.5 ± 9.9 | 62.4 ± 11.3 |
| Sex, male, n (%) | 15 (75.0) | 88 (75.9) |
| Paroxysmal AF, n (%) | 15 (75.0) | 83 (71.6) |
| Medical history, n (%) | ||
| Hypertension | 8 (53.3) | 45 (38.8) |
| Diabetes mellitus | 3 (15) | 11 (9.5) |
| Previous stroke | 1 (0.5) | 11 (9.5) |
| EF (%) | 63.5 ± 4.6 | 60.3 ± 7.9 |
| Left atrial diameter (mm) | 37.9 ± 6.6 | 39.4 ± 7.6 |
| CHADS2 score, n (%) | ||
| 0–1 | 16 (80.0) | 92 (79.3) |
| ≧2 | 4 (20.0) | 24 (20.7) |
| CHA2DS2‐VASc score, n (%) | ||
| 0–1 | 9 (45.0) | 62 (53.4) |
| ≥2 | 11 (55.0) | 54 (46.6) |
AF: atrial fibrillation; EF: ejection fraction; CHADS2: congestive heart failure, hypertension, age ≥75 years, diabetes, stroke (doubled); CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65–75 years, sex category (female).
3.2. Duration‐based results from the pilot study
The total recording time was 216,750 s from the 20 subjects in the pilot study. The average recording time was 11,344 s. True AF duration with the Holter ECG was 49,374 s. The PWM accurately detected 83.8% (41,400/49,374 s) of the total AF duration (Se), and 94.6% (158,262/167,376 s) of the non‐AF duration (Sp). The AF duration detected with the PWM (including true AF and false‐positive AF) was 50,514 s; the Holter ECG confirmed true AF for 82.0% (41,400 s) of this duration (PPV). Of the period of time absent from AF, as detected by the PWM, 95.2% (158,262 s) was confirmed to be non‐AF with the Holter ECG (NPV). A κ statistic of 0.78 was obtained with reference KS and CV values of 0.15 and 0.25, respectively.
3.3. Results from the primary study
The primary study recorded 1,326,020 s of data, including 221,571 s of AF in 116 patients. The duration‐based results from the primary cohort were comparable to those from the pilot cohort (Table 2).
Table 2.
Duration‐based results
| Pilot (n = 20) | Primary (n = 116) | |
|---|---|---|
| Sensitivity (%) | ||
| Cumulative | 83.8 | 84.1 |
| Patient average | 81.6 | 81.0 |
| Specificity (%) | ||
| Cumulative | 94.6 | 97.7 |
| Patient average | 94.4 | 96.4 |
| Positive predictive value (%) | ||
| Cumulative | 82.0 | 88.0 |
| Patient average | 67.3 | 86.6 |
| Negative predictive value (%) | ||
| Cumulative | 95.2 | 96.8 |
| Patient average | 95.1 | 95.1 |
| κ | 0.78 | 0.83 |
PWM recordings included 45 AF episodes of >30 min, 66 of >10 min, and 91 of >6 min. Figure 3 shows the cumulative and patient average episode‐based results. Longer episode durations were associated with improved PWM diagnostic capacity.
Figure 3.
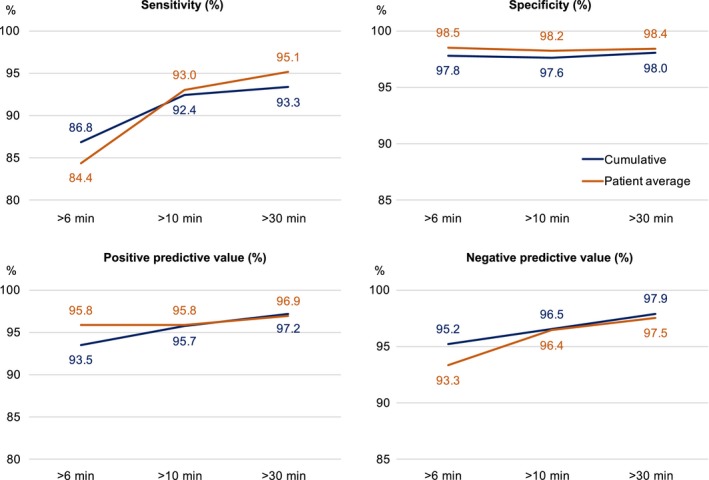
Episode‐based results. The horizontal line shows the duration of episodes. As episode duration increased, the diagnostic capacity improved
When we focused on the diagnosis breakdown of false negative, the judgment of PVC/PAC accounted for a larger part of false‐negative duration than SR (Figure S1). In accordance, NPV at patient average where non‐AF diagnosis was SR was higher than that where it was PVC/PAC. These results might suggest the presence of some AF rhythms with high variability but low randomness, which are hardly distinguishable from PVC/PAC with this algorithm.
3.4. Rapid/regular tachycardia zone
In the pilot data, we found that AF with high ventricular response tended to deviate from reference AF episodes, because both the calculated PP variability and randomness tended to be low in the case of AF with short RR intervals (Figure 4a). Figure 4b shows that the SD frequency of the 20‐successive log2‐based ECG‐RRI ratio during AF from the pilot data had a bimodal distribution. The first peak of the low log2‐based RRI ratio included AF with a rapid ventricular response and short duration of atrial tachycardia or atrial flutter. Excluding rapid/regular tachycardia with a regular RR, defined as an AF zone with both (a) a standard deviation (SD) of the log2 ratio of successive RRIs <0.12 (mean SD 0.073) and (b) heart rate >100 bpm (mean heart rate 140 bpm), the duration‐ and episode‐based Se (>6 min) improved to 85.8% and 92.0%, respectively.
Figure 4.
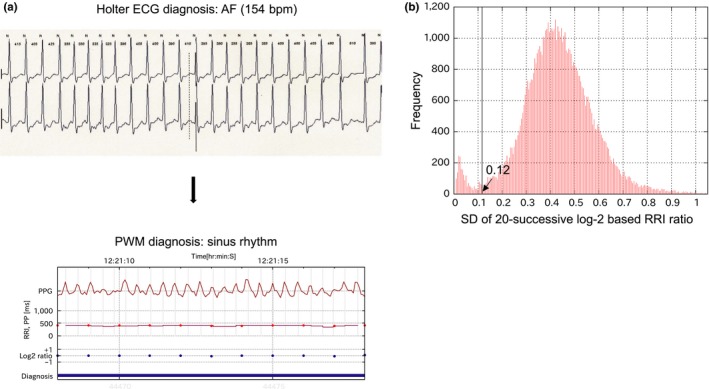
(a) Example of recording AF with a high ventricular response. The upper figure is holter ECG showing AF (heart rate 154 bpm). In the lower figure, pulse period (RR interval) series had low dispersion, as shown by red dot line and blue dot line (standard deviation [SD] of 20‐successive log2‐based RR interval [RRI] ratios: 0.11). The diagnosis from the pulse wave monitor was falsely sinus rhythm, indicated with blue bar at the bottom. (b) The frequency of the SD of 20‐successive log2‐based RRI ratios during AF exhibited a bimodal distribution (pilot data). AF with a very low SD of 20‐successive log2‐based RRI ratios was likely to be regarded as sinus rhythm
4. DISCUSSION
In this study, we evaluated the diagnostic performance of a novel algorithm using frequency‐based pulse wave analysis. Our results showed a high NPV (96.8%), suggesting excellent capacity to identify non‐AF rhythms. Reproducibility in the primary cohort validated the reliability of this algorithm. This noninvasive PWM could accurately discriminate AF from sinus rhythm with an Se of 84.1% and a Sp of 97.7%. This diagnostic performance is consistent among patients with AF >6 min.
4.1. Long‐term recording with the PWM
Previous studies have revealed the efficacy of finger PPG using a smartphone camera or finger probe, with a high detection Se (97%–100%) and Sp (92%–94%) (Chan et al., 2016) (Lewis, Parker, Weston, & Bowes, 2011) (Mc et al., 2016). However, these studies are limited by the short duration of pulse wave capture. Little evidence is available regarding the efficacy of continuous long‐term pulse wave monitoring for AF. The present study yielded a comparable performance to previous studies using the given algorithm in a wristwatch‐based PWM for long‐term recording.
Long‐term monitoring is known to improve detection of AF. The detection rate for undiagnosed AF with a single‐time‐point screen in the general population is 1.1%–1.4% (Kaasenbrood et al., 2016)(Lowres, Neubeck, Redfern, & Freedman, 2013), which is lower than the true rates of AF. In the STUDY‐AF trial, screening with 14‐day recordings in a population aged >55 years with 2 AF risk factors detected AF in 5.3% of patients. The rate of atrial high‐rate episodes in patients with cardiovascular implantable devices rises to 30%–50%, depending on the population and detection algorithm (Healey et al., 2012; Swiryn et al., 2016; Ziegler et al., 2010). After a recent stroke, long‐term monitoring improves the detection of AF (Dussault et al., 2015; Gladstone et al., 2014; Sanna et al., 2014; Sposato et al., 2015).
4.2. Screening for AF episodes over 6 min
The present study showed that a PWM is effective at diagnosing AF lasting over 6 min, with performance improving with duration. Although the prognostic significance of brief episodes remains unknown, the association between screened AF burden and embolic events has been well evaluated. AF >24 hr duration is independently associated with a threefold risk of embolic events (Capucci et al., 2005). An analysis of patients with implanted devices from the SOS project (Stroke preventiOn Strategies based on atrial fibrillation information from implanted devices) demonstrated that an AF burden >1 hr was associated with a 2‐fold risk of ischemic stroke (Boriani et al., 2014). In patients with a history of hypertension and without prior AF, atrial tachyarrhythmia >6 min is associated with a twofold risk of ischemic stroke or systemic embolism (Healey et al., 2012). In accordance, ESC guidelines recommend further assessment for oral anticoagulation in patients presenting with atrial high‐rate episodes >5–6 min with implanted devices (Kirchhof et al., 2016). Evidently, there is ongoing need for the ability to reliably screen patients for AF episodes >6 min.
4.3. Frequency domain autocorrelation analysis for pulse periodicity detection
In most devices using pulse wave monitoring, each peak of the pulse wave is regarded as an R wave. However, pulse waves often have mild peaks, unstable amplitude, or noises due to body movements, which make it difficult to obtain reliable peak detection results in active states. This novel algorithm used in this PWM takes short‐term autocorrelation in place of the RR interval. Autocorrelation is a method of evaluating the period of a signal by integrating the whole waveform instead of using only the part around the peak. It is more tolerant of the instabilities than the local peak detection method. Autocorrelation has been proposed for wearable ECG monitoring, due to its noise‐tolerant heart rate detection performance (Fujii et al., 2013). Our method would be equally appropriate for long‐term recording of pulse waves, given the highly efficient reduction in noise. Furthermore, the reliability of the randomness index has been improved. Past reports have shown excellent performance (sensitivity: 96%, specificity: 98%) with an algorithm that detects AF only at the moment when the patients using a smartphone‐based application using the root mean square of successive RR differences (RMSSD) for the index of variation, and Shannon entropy for the index of randomness, (McManus et al., 2013). In this study, we identified for the first time 2 statistical methods, CV and KS, that reliably distinguish AF in long‐term recording.
4.4. Wristwatch‐based PWM
Screening is especially necessary for populations with an underlying risk factor for stroke or systemic embolism, such as hypertension, diabetes mellitus (Kishimoto et al., 2016), or sleep apnea (Hendrikx et al., 2017). The ESC guidelines recommend AF screening in accordance with the CHA2DS2‐VASc score (Kirchhof et al., 2016). Screening high‐risk populations improves AF diagnosis rates (Davis et al., 2012), facilitating early therapeutic intervention, and improving cost‐effectiveness (Aronsson et al., 2015). The wristwatch‐based PWM is user‐friendly and provides an easy screening method for patients, including the elderly and those without symptoms. Moreover, this wearable PWM is capable of repeated use and intermittent recording with an unlimited capacity at a low cost and does not require additional hardware.
4.5. Limitations
A limitation of this study is the PWM was installed on the patients during bed rest. Effect of noise, motion artifact, or improper fitting were not considered in this study. Assessment of the acquisition of pulse waveforms during dynamic states will be needed.
We were unable to consider the effect of atrial tachycardia or flutter in the present study. Consequently, there is a possibility of underestimating the prevalence of atrial tachycardia or atrial flutter with a regular ventricular response. Patients on antiarrhythmic medications, who tend to have atrial tachycardia or atrial flutter, were excluded in the present study.
5. CONCLUSIONS
The AF detection algorithm described in this paper has a high diagnostic accuracy when used in a PWM. Such monitoring allows for long‐term or extended intermittent recordings capable of detecting clinically important AF. This study suggests that further research should focus on translating these findings into rigorous clinical applications.
CONFLICT OF INTEREST
None.
Supporting information
ACKNOWLEDGMENTS
We wish to thank Hiroko Sakai, Chikako Tokudome, Masako Kotera, Yoshiko Takenobu, Mami Niimi, Kazuko Shigehira for their secretarial assistance, and Masashi Inagaki, Masaru Sugimachi, Aiko Koda, Yoshiki Yanagi, and Koji Ogawa as scientific advisors.
Kashiwa A, Koyama F, Miyamoto K, et al. Performance of an atrial fibrillation detection algorithm using continuous pulse wave monitoring. Ann Noninvasive Electrocardiol. 2019;24:e12615 10.1111/anec.12615
Funding information
This work was supported by the Practical Research Project for Life‐Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus, from the Japan Agency for Medical Research and Development (AMED) (grant no. H26‐019, 16ek0210035h0103) and intramural research funds (25‐4‐7) for cardiovascular disease from the National Cerebral and Cardiovascular Center.
REFERENCES
- Aronsson, M. , Svennberg, E. , Rosenqvist, M. , Engdahl, J. , Al‐Khalili, F. , Friberg, L. , … Levin, L. A. (2015). Cost‐effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace, 17(7), 1023–1029. 10.1093/europace/euv083. [DOI] [PubMed] [Google Scholar]
- Boriani, G. , Glotzer, T. V. , Santini, M. , West, T. M. , De Melis, M. , Sepsi, M. , … Singer, D. E. (2014). Device‐detected atrial fibrillation and risk for stroke: An analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). European Heart Journal, 35(8), 508–516. 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capucci, A. , Santini, M. , Padeletti, L. , Gulizia, M. , Botto, G. , Boriani, G. , … Grammatico, A. (2005). Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. Journal of the American College of Cardiology, 46(10), 1913–1920. 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- Chan, P. H. , Wong, C. K. , Poh, Y. C. , Pun, L. , Leung, W. W. , Wong, Y. F. , … Siu, C. W. (2016). Diagnostic performance of a smartphone‐based photoplethysmographic application for atrial fibrillation screening in a primary care setting. Journal of the American Heart Association, 5(7), e003428. 10.1161/jaha.116.003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. C. , Hobbs, F. D. , Kenkre, J. E. , Roalfe, A. K. , Iles, R. , Lip, G. Y. , & Davies, M. K. (2012). Prevalence of atrial fibrillation in the general population and in high‐risk groups: The ECHOES study. Europace, 14(11), 1553–1559. 10.1093/europace/eus087. [DOI] [PubMed] [Google Scholar]
- Dussault, C. , Toeg, H. , Nathan, M. , Wang, Z. J. , Roux, J. F. , & Secemsky, E. (2015). Electrocardiographic monitoring for detecting atrial fibrillation after ischemic stroke or transient ischemic attack: Systematic review and meta‐analysis. Circulation: Arrhythmia and Electrophysiology, 8(2), 263–269. 10.1161/circep.114.002521. [DOI] [PubMed] [Google Scholar]
- Friberg, L. , Rosenqvist, M. , Lindgren, A. , Terent, A. , Norrving, B. , & Asplund, K. (2014). High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke, 45(9), 2599–2605. 10.1161/strokeaha.114.006070. [DOI] [PubMed] [Google Scholar]
- Fujii, T. , Nakano, M. , Yamashita, K. , Konishi, T. , Izumi, S. , Kawaguchi, H. , & Yoshimoto, M. (2013). Noise‐tolerant instantaneous heart rate and R‐peak detection using short‐term autocorrelation for wearable healthcare systems. Conference Proceedings IEEE Engineering in Medicine and Biology Society, 2013, 7330–7333. 10.1109/embc.2013.6611251. [DOI] [PubMed] [Google Scholar]
- Gladstone, D. J. , Spring, M. , Dorian, P. , Panzov, V. , Thorpe, K. E. , Hall, J. , … Mamdani, M. (2014). Atrial fibrillation in patients with cryptogenic stroke. New England Journal of Medicine, 370(26), 2467–2477. 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- Hart, R. G. , Pearce, L. A. , & Aguilar, M. I. (2007). Meta‐analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Annals of Internal Medicine, 146(12), 857–867. 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- Healey, J. S. , Connolly, S. J. , Gold, M. R. , Israel, C. W. , Van Gelder, I. C. , Capucci, A. , … Hohnloser, S. H. (2012). Subclinical atrial fibrillation and the risk of stroke. New England Journal of Medicine, 366(2), 120–129. 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- Hendrikx, T. , Sundqvist, M. , Sandstrom, H. , Sahlin, C. , Rohani, M. , Al‐Khalili, F. , … Franklin, K. A. (2017). Atrial fibrillation among patients under investigation for suspected obstructive sleep apnea. PLoS ONE, 12(2), e0171575 10.1371/journal.pone.0171575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January, C. T. , Wann, L. S. , Alpert, J. S. , Calkins, H. , Cigarroa, J. E. , Cleveland, J. C. Jr , … Yancy, C. W. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology, 64(21), e1–e76. 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Kaasenbrood, F. , Hollander, M. , Rutten, F. H. , Gerhards, L. J. , Hoes, A. W. , & Tieleman, R. G. (2016). Yield of screening for atrial fibrillation in primary care with a hand‐held, single‐lead electrocardiogram device during influenza vaccination. Europace, 18(10), 1514–1520. 10.1093/europace/euv426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof, P. , Benussi, S. , Kotecha, D. , Ahlsson, A. , Atar, D. , Casadei, B. , … Zeppenfeld, K. (2016). 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace, 18(11), 1609–1678. 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- Kishimoto, I. , Makino, H. , Ohata, Y. , Tamanaha, T. , Tochiya, M. , Kusano, K. , … Ogawa, H. (2016). Impact of B‐type natriuretic peptide (BNP) on development of atrial fibrillation in people with Type 2 diabetes. Diabetic Medicine, 33(8), 1118–1124. 10.1111/dme.12856. [DOI] [PubMed] [Google Scholar]
- Klein‐Wiele, O. , Faghih, M. , Dreesen, S. , Urbien, R. , Abdelghafor, M. , Kara, K. , … Hailer, B. (2016). A novel cross‐sector telemedical approach to detect arrhythmia in primary care patients with palpitations using a patient‐activated event recorder. Cardiology Journal, 23(4), 422–428. 10.5603/CJ.a2016.0033. [DOI] [PubMed] [Google Scholar]
- Lewis, M. , Parker, D. , Weston, C. , & Bowes, M. (2011). Screening for atrial fibrillation: Sensitivity and specificity of a new methodology. British Journal of General Practice, 61(582), 38–39. 10.3399/bjgp11X548956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, J. , Khalid, Z. , Scallan, C. , Morillo, C. , & O'Donnell, M. (2007). Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: A systematic review. Stroke, 38(11), 2935–2940. 10.1161/strokeaha.106.478685. [DOI] [PubMed] [Google Scholar]
- Lowres, N. , Neubeck, L. , Redfern, J. , & Freedman, S. B. (2013). Screening to identify unknown atrial fibrillation. A Systematic Review. Thrombosis and Haemostasis, 110(2), 213–222. 10.1160/th13-02-0165. [DOI] [PubMed] [Google Scholar]
- McManus, M. D. , Chong, J. W. , Soni, A. , Saczynski, J. S. , Esa, N. , Napolitano, C. , … Chon, K. H. (2016). PULSE‐SMART: Pulse‐based arrhythmia discrimination using a novel smartphone application. Journal of Cardiovascular Electrophysiology, 27(1), 51–57. 10.1111/jce.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, D. D. , Lee, J. , Maitas, O. , Esa, N. , Pidikiti, R. , Carlucci, A. , … Chon, K. H. (2013). A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm: the Official Journal of the Heart Rhythm SocietyThe Official Journal of the Heart Rhythm Society, 10(3), 315–319. 10.1016/j.hrthm.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, P. , Purerfellner, H. , Pokushalov, E. , Sarkar, S. , Di Bacco, M. , Maus, B. , & Dekker, L. R. (2016). Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: Results from the Reveal LINQ Usability Study. Heart Rhythm: the Official Journal of the Heart Rhythm SocietyThe Official Journal of the Heart Rhythm Society, 13(7), 1425–1430. 10.1016/j.hrthm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Sanna, T. , Diener, H. C. , Passman, R. S. , Di Lazzaro, V. , Bernstein, R. A. , Morillo, C. A. , … Brachmann, J. (2014). Cryptogenic stroke and underlying atrial fibrillation. New England Journal of Medicine, 370(26), 2478–2486. 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- Sposato, L. A. , Cipriano, L. E. , Saposnik, G. , Ruiz Vargas, E. , Riccio, P. M. , & Hachinski, V. (2015). Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta‐analysis. The Lancet Neurology, 14(4), 377–387. 10.1016/s1474-4422(15)70027-x. [DOI] [PubMed] [Google Scholar]
- Swiryn, S. , Orlov, M. V. , Benditt, D. G. , DiMarco, J. P. , Lloyd‐Jones, D. M. , Karst, E. , … Waldo, A. L. (2016). Clinical implications of brief device‐detected atrial tachyarrhythmias in a cardiac rhythm management device population: Results from the registry of atrial tachycardia and atrial fibrillation episodes. Circulation, 134(16), 1130–1140. 10.1161/circulationaha.115.020252. [DOI] [PubMed] [Google Scholar]
- Ziegler, P. D. , Glotzer, T. V. , Daoud, E. G. , Wyse, D. G. , Singer, D. E. , Ezekowitz, M. D. , … Hilker, C. E. (2010). Incidence of newly detected atrial arrhythmias via implantable devices in patients with a history of thromboembolic events. Stroke, 41(2), 256–260. 10.1161/strokeaha.109.571455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


