Abstract
Background
Fragmented QRS evaluated in 12‐derivation electrocardiography has widely been accepted as a sign of myocardial fibrosis. The prognostic value of that marker has been demonstrated, particularly, in cardiac diseases that accompany myocardial scar and fibrosis. Myocardial fibrosis is also an issue in patients with aortic stenosis. In this study, we wanted to determine whether fragmented QRS could predict all‐cause mortality in aortic stenosis patients after transcatheter aortic valve replacement (TAVR).
Method
In this study, we evaluated a total of 116 eligible patients on whom we performed TAVR between 2014 and 2018. Patients' demographic and clinical findings, echocardiography results, in‐hospital and 30‐day mortality, long‐term survival statuses were noted. Patient's ECGs before the procedure were evaluated in regard to the occurrence of fragmented QRS. Predictors of mortality were evaluated using univariable and multivariable Cox regression analysis.
Results
The study population consisted of 116 patients of median age 79 (IQR 75–83), 64 females (55.2%). Mortality occurred in 27 (23%) patients; median follow‐up time was 319 (IQR 122–719) days. Fragmented QRS was observed in 44 out of 116 (37.9%) patients. The presence of a fragmented QRS (HR = 2.178, 95% CI 0.999–4.847, p = 0.050), a history of stroke (HR = 3.463, 95% CI 1.276–9.398, p = 0.015), and the creatinine levels at admission (HR = 2.198, 95% CI 1.068–4.520, p = 0.030) were associated with the long‐term mortality in multivariable Cox regression analysis.
Conclusion
Like in the case of the other diseases associated with myocardial fibrosis, fragmented QRS could also predict mortality in aortic stenosis patients after TAVR procedure.
Keywords: aortic stenosis, fragmented QRS, myocardial fibrosis, TAVR
1. INTRODUCTION
Aortic stenosis has become a more common health problem as the population ages (Thaden, Nkomo, & Enriquez‐Sarano, 2014). Transcatheter aortic valve replacement (TAVR) has widely been used for calcific aortic stenosis in patients with high surgery risk (Perrin, Frei, & Noble, 2018). Thus, the patients who are candidates for TAVR possess more risk factors and comorbidities. In advanced aortic stenosis, chronic pressure overload causes myocyte cell death and myocardial fibrosis, resulting in the transition from hypertrophy to heart failure (Dweck, Boon, & Newby, 2012). Some studies do exist showing myocardial fibrosis in patients with calcific aortic stenosis (AS) and worse clinical outcomes even after aortic valve replacement (Everett et al., 2018; Treibel et al., 2018; Weidemann et al., 2009). Fragmented QRS (fQRS) is an electrocardiographic finding related to conduction delay due to myocardial fibrosis. The prognostic significance of fQRS has mainly been studied in patients with myocardial infarction, ischemic, and non‐ischemic cardiomyopathies, arrhythmogenic right ventricular dysplasia and hypertrophic cardiomyopathies (Basaran et al., 2011; Bozbeyoğlu et al., 2016; Canpolat, Kabakç, Aytemir, & Dura, lM., Sahiner, L., Yorgun, H, Lenard, S., Oto, A., 2013; Das et al., 2009; Das, Khan, Jaco, Kumar, & Mahenthiran, 2006; Kang et al., 2014) Additionally, association between mortality and fQRS has been shown in various clinical conditions such as aortic dissection and pulmonary embolism (Çetin et al., 2016; Kalkan et al., 2015). Even though myocardial fibrosis plays an important role in the course of AS, there is a lack of data regarding the prognostic significance of fQRS in AS patients. In the present study, we sought to determine the prognostic significance of fQRS in patients who underwent TAVR for severe calcific aortic stenosis.
2. METHODS
2.1. Study population
In this study, we included a total of 116 eligible patients who underwent transcatheter aortic valve implantation with a diagnosis of severe aortic stenosis in our center between 2013 and 2018. Patients presented with pace rhythm, bundle brunch block, and prior histories of myocardial infarction, coronary artery disease, and segmented wall motion abnormality detected with echocardiography, those whose pretreatment electrocardiography (ECG) results were missing and those patients without post‐procedural follow‐up data were excluded from the study. Patients' clinical and demographic findings, past medical history, prior medical treatments including beta‐blocker therapy, statin, angiotensin‐converting enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), and pre‐procedural creatinine and hemoglobin levels were recorded. The diagnosis of chronic obstructive lung disease (COPD) was made by a pulmonologist with aid of respiratory function test. Patients with noncritical coronary artery disease were included in the study cohort and depicted as having coronary artery disease. Pre‐procedural echocardiography results were recorded in all study patients, and left ventricular mass index (LVMI) was calculated using the previously mentioned formula (Lang, Bierig, & Devereux, 2005). Presence of paravalvular aortic regurgitation was classified as none/trace or mild and more than mild. Surgery risk of patients was estimated using the Society of Thoracic Surgeons (STS) risk score with the aid of an online calculator system. TAVR procedure was performed using standard techniques by the same primary cardiologist and the same team. TAVR was performed via the transfemoral route in most of the patients; however, it was performed through the subclavian artery in three patients and through the carotid artery in one patient. Valves manufactured by a total of five different companies were used; therefore, the type of valve was expressed as balloon expandable or self‐expandable. The study was approved by the local ethics committee and the study protocol complied with the Declaration of Helsinki.
2.2. ECG analysis
A standard 12‐lead ECG (filter range 0.15–150 Hz, 25 mm/s, 10 mm/mV) was obtained from all patients before the procedure. Fragmented QRS was defined as QRS complex with duration <120 ms with additional R wave (R′), notched R wave, notched S wave, or presence of >1R′ in at least two contiguous leads corresponding to coronary artery region without the characteristic of bundle branch block, in accordance with previously reported data (Das et al., 2006). Coronary artery regions were defined as anterior: leads V1–V5; inferior: leads II, III, and aVF; and lateral: leads V6, I, and aVL. ECGs were evaluated by two experienced readers blinded to the data. In cases of different assessment, the readers made their final decision by mutual agreement.
2.3. Statistical analyses
In order to present the numerical variables, the median value and the interquartile ranges were used. The categorical variables were presented in numbers (n) and percentages.
The primary outcome of the study: It was defined as the mortality occurring in the long‐term follow‐up of the patients consecutively included in the study.
The candidate predictors: The candidate predictors, to be included in the model created to evaluate the long‐term mortality after TAVR, should be plausible both clinically and biologically. It is also of a remarkable importance that the candidate predictors should have been demonstrated to be associated with the long‐term mortality. Therefore, we selected the variables according to these criteria to be included in the model. The candidate predictors included the age, gender, history of stroke, NYHA‐IV at admission, the emergence of paravalvular aortic regurgitation after the procedure, the creatinine level, and the presence of a fragmented QRS. The values of the variables were not included in the model if they were at the very low levels or if they were presented at very high frequencies. Finally, the final model included seven candidate variables.
Sample size calculation and statistical modeling: In order to develop a clinically meaningful model, the sample size should be of a sufficient size, however, the number of predictors should be defined conservatively. To explain it more specifically, in the model, there must be at least 10–15 patients positive for the primary outcome compared to the number of selected candidate predictors for the model (outcome/variable>10–15). In our clinical model, 27 patients were positive for the primary outcome, and a total of seven candidate predictors were selected (27/7 = 4). Therefore, the least absolute shrinkage and the selection operator (Lasso) penalized estimation methods were used in combination with the traditional Cox regression so that the risk of overfitting would be reduced (model‐1). The Lasso penalized regression method was used to examine the associations between the primary outcome and the candidate predictors due to the risk of overfitting. The assumption for the proportional hazard was evaluated by the Schoenfeld residuals. The rate of cumulative events during the follow‐up period was calculated using the Kaplan–Meier method, and the difference between the groups was tested by the log‐rank test. In addition, a second model was developed to include the STS scores, the presence of fQRS, and the paravalvular aortic insufficiency. This second model did not include the variables of age, gender, creatinine levels, NYHA, and the history of stroke since all of these variables were involved in calculating the STS risk score. These two models were compared with the likelihood ratios.
All statistical analyses were performed with the “glmnet”, “survminer”, and “survival” packages using R‐software v. 3.5.1 (R statistical software, Institute for Statistics and Mathematics, Vienna, Austria).
3. RESULTS
From December 2013 to January 2018, a total of 116 consecutive patients, who underwent a TAVR, were included in the study [median age 79 (75–83), 64 (55.2%) females]. The presence of a fragmented QRS was detected in 44 (% 37.9) patients. In‐hospital and the 30‐day mortalities occurred in 9 (7%) patients while the total mortality rate in the study was 23%, occurring in 27 patients. The median duration of the follow‐up period was 319 (IQR: 122–719) days.
The baseline clinical features of the patients who died and who survived were presented in Table 1 together with the results of the univariable Cox regression analysis. The Model‐1 was developed by including the age, gender, a history of stroke, NYHA‐IV at admission, the presence of paravalvular aortic regurgitation after the procedure, the creatinine levels, and the presence of fragmented QRS in the traditional multivariable Cox regression model. The presence of a fragmented QRS (HR=2.178, 95% CI 0.999–4.847, p = 0.050), a history of stroke (HR=3.463, 95% CI 1.276–9.398, p = 0.015), and the creatinine levels at admission (HR=2.198, 95% CI 1.068–4.520, p = 0.030) were associated with the long‐term mortality. A Lasso regression analysis method was also performed due to the risk of overfitting. The three predictors of long‐term mortality listed above, which are the presence of a fragmented QRS, a history of stroke, and the creatinine levels at admission, were also valid for the traditional model with the respective Lasso coefficients of 1.637, 2.846, and 1.637 (Table 2). A cross‐validated error plot of the Lasso model is shown in Figure 1a,b. The most regularized and parsimonious model with a cross‐validated error within 1 standard error of the minimum included 3 variables. The path of all coefficients included in the model at varying levels of log‐transformed lambda is shown in Figure 1a. In addition to the Model‐1, a second model, Model‐2, was developed by including only the following variables including the STS scores, the presence of a fQRS, and the presence of a paravalvular aortic insufficiency. The modeled fQRS variable and the STS score were found out to be associated with the long‐term mortality (Table 3). However, the likelihood ratio of the Model‐1 was 17 while this value was 10.5 for the Model‐2. Figures 2 and 3 show the survival curves in patients grouped according to the presence of fQRS and a history of stroke. There was a significant difference in the survival rates between the 2 groups (p = 0.010 at log‐rank analysis), with the Kaplan–Meier estimates of one‐year survival rates of 84% and 67% in fQRS(−) and fQRS(+) patients, respectively (Figure 2). Similarly, there was a significant difference in the survival rates between the 2 groups (p < 0.001 at log‐rank analysis), with Kaplan–Meier estimates of one‐year survival rates of 82% and 43% in the patients with and without a history of stroke, respectively (Figure 3).
Table 1.
Univariable Cox regression analysis of study patients
| All (n = 116) | Death Occurred (n = 27) | Survived (n = 89) | Univariable Cox regression Unadjusted HR, 95% CI | p Value | |
|---|---|---|---|---|---|
| Age (years) (IQR) | 79 (75–83) | 80 (72–85) | 79 (75–83) | 1.042 (0.980‐(1.107) | 0.190 |
| Female Gender n (%) | 64 (55.2) | 13 (48.1) | 51 (57.3) | 1.354 (0.636–2.882) | 0.432 |
| BMI (kg/m2) (IQR) | 26.7 (24.5–30.4) | 26.6 (25.3–27.7) | 26.7 (24.5–30.4) | 0,944 (0,864–1.031) | 0.201 |
| NYHA‐IV n (%) | 64 (55.2) | 17 (63) | 47 (52.8) | 1.436 (0.657–3.141) | 0.364 |
| DM n (%) | 33 (28.4) | 6 (22.2) | 27 (30.3) | 0.534 (0.212–1.341) | 0.182 |
| Stroke n (%) | 13 (11.2) | 8 (29.6) | 5 (5.6) | 4.048 (1.766–9.279) | 0.001 |
| COPD n (%) | 38 (32.8) | 10 (37) | 28 (31.5) | 1.067 (0.486–2.343) | 0,871 |
| HT n (%) | 68 (58.6) | 14 (51.9) | 54 (60.7) | 0.677 (0.317–1.447) | 0.314 |
| STS score (IQR) | 8.4 (6.4–9.3) | 8.9 (7.9–9.9) | 8.1 (6.2–9.1) | 1.148 (1.039–1.268) | 0.007 |
| CAD n (%) | 19 (16.4) | 2 (7.4) | 17 (19.1) | 0.832 (0.193–3.591) | 0.805 |
| Paravalvular regurgitation n (%) | 14 (12.1) | 5 (18.5) | 9 (10.1) | 1.557 (0.588–4.128) | 0.373 |
| Atrial fibrillation n (%) | 13 (11.2) | 5 (18.5) | 8 (9) | 1,657 (0.625–4.391) | 0,310 |
| Creatinine concentration (mg/dl) (IQR) | 0.9 (0.8–1.2) | 0.98 (0.8–1.4) | 0.8 (0.7–0.9) | 2.134 (1.123–4.053) | 0.020 |
| Hemoglobin concentration (mg/dl) (IQR) | 12 (11–13) | 12 (11–13) | 12 (11–13) | 1.050 (0.829–1.330) | 0.684 |
| EF (IQR) | 60 (55–60) | 60 (55–60) | 60 (55–60) | 0.995 (0.956–1.036) | 0.823 |
| LVMI (IQR) | 136.2 (117.7–159.9) | 139.4 (132.4–165.7) | 134.6 (116–157.4) | 1.008 (0.997–1.019) | 0.155 |
| LA (cm) (IQR) | 4.1 (3.8–4.5) | 4.4 (3.8–4.8) | 4.1 (3.8–4.5) | 1.358 (0.766–2.406) | 0.295 |
| Mean gradient (mmHg) (IQR) | 45 (44–51) | 45 (40–53) | 45 (44–52) | 0.996 (0.952–1.042) | 0.852 |
| Balloon expendable valve n (%) | 45 (38.8) | 12 (44.4) | 33 (37.1) | 1.296 (0.606–2.770) | 0.504 |
| Fragmented QRS n (%) | 44 (37.9) | 16 (59.3) | 28 (31.5) | 2.619 (1.213–5.664) | 0.014 |
Values are n (%), mean (SD), or median (25th to 75th interquartile range).
BMI: body mass index; CAD: Coronary artery disease; COPD: chronic pulmonary lung disease; DM: diabetes mellitus; EF: ejection fraction; HT: hypertension; LA: left atrium; LVMI: left ventricular mass index; STS: The Society of Thoracic Surgery.
Table 2.
Multivariable traditional Cox regression and Lasso penalized regression for model‐1
| Cox regression HR 95% CI | Lasso regression HR | p Value | |
|---|---|---|---|
| fQRS | 2.178 (0.999–4.847) | 1.637 | 0.050 |
| Creatinine level | 2.198 (1.068–4.520) | 1.645 | 0.030 |
| Stroke history | 3.463 (1.276–9.398) | 2.846 | 0.015 |
| NHYA‐IV | 1.291 (0.571–2.923) | ‐ | 0.539 |
| Paravalvular regurgitation | 0.891 (0.306–2.593) | ‐ | 0.833 |
| Age | 1.001 (0.945–1.061) | ‐ | 0.957 |
| Female gender | 1.121 (0.520–2.416) | ‐ | 0.769 |
Figure 1.
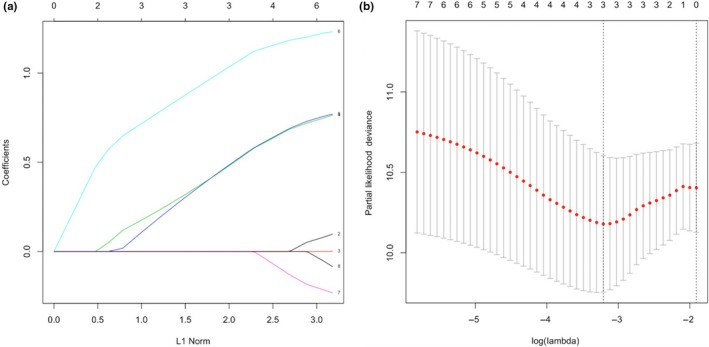
Cross‐validated error plot of the lasso model
Table 3.
Multivariable traditional Cox regression for model‐2
|
Cox regression HR 95% CI |
p Value | |
|---|---|---|
| fQRS | 2.428 (1.104–5.337) | 0.027 |
| STS score | 1.120 (1.014–1.237) | 0.024 |
| Paravalvular regurgitation | 1.093 (0.389–3.067) | 0.865 |
Figure 2.
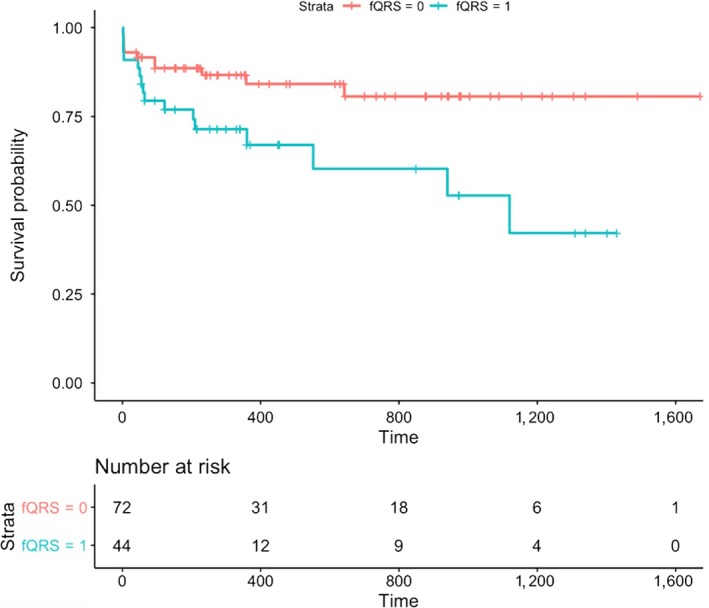
Survival curves in patients grouped according to fragmented QRS
Figure 3.
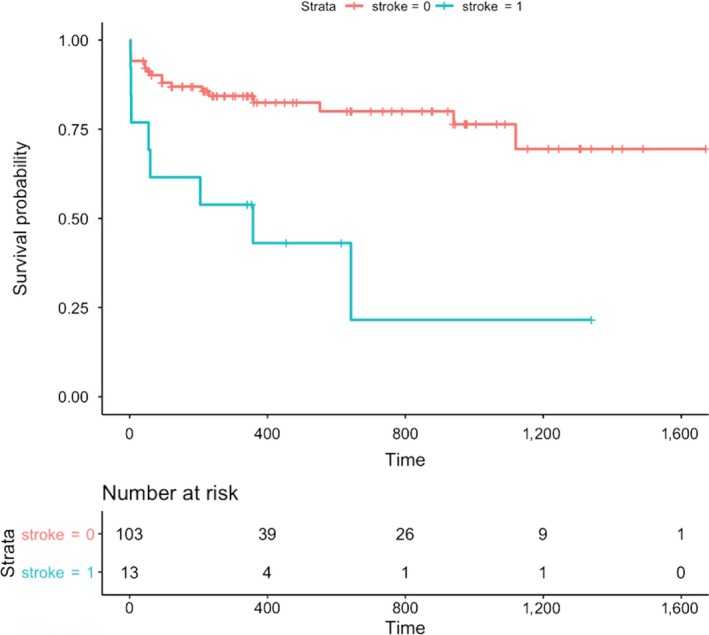
Survival curves in patients grouped according to stroke history
4. DISCUSSION
In this present study, our main finding is that fragmented QRS on ECG evaluated before TAVR procedure is an independent predictor of mortality. In our study population, a history of stroke and initial creatinine concentration emerged as other independent predictors of mortality. Fragmented QRS is an electrocardiographic marker of myocardial fibrosis the diagnostic usefulness of which has been demonstrated before. The underlying pathophysiology for fQRS is delayed depolarization caused by electrically inactive fibrotic tissue. This altered activation leads inhomogeneous conduction and multiple spikes within the QRS complexes. Das et al. demonstrated that fQRS is a better predictor than Q wave to show regional scarring in patients with coronary artery disease using single photon emission tomography (SPECT; Das et al., 2006). Lorgis at al. proved in a magnetic resonance imaging (MRI) study that fQRS could indicate larger infarct size and lower ejection fraction in patients with ST elevating myocardial infarction (Lorgis et al., 2014). The prognostic importance of myocardial fibrosis in patients with severe aortic stenosis has been demonstrated in several studies. Weidemann et al. showed that as the degree of myocardial fibrosis increases in patients with severe AS after aortic valve replacement, the prognosis worsens (Weidemann et al., 2009). They demonstrated myocardial fibrosis using MRI and biopsy obtained during valve surgery. In this article, the authors suggest that in the early stage of the disease, left ventricular ejection fraction (LVEF) was not affected as fibrosis begins at the endocardium. In the same study, aortic replacement failed to improve myocardial fibrosis after nine‐month follow‐up. In our study, patients with fQRS had larger left ventricular diastolic diameter and higher LVMSI with similar LVEF when compared with patients without fQRS; but those factors did not predict mortality. These findings may indicate that in the advanced stage of aortic stenosis, left ventricular enlargement accompanies myocardial fibrosis but the worsening of prognosis is much more related to myocardial fibrosis than altered left ventricular functions. In another study by Everett R.J. and Tastet L. et al, myocardial fibrosis was evaluated using MRI in patients with the different severities of AS. They demonstrated that as myocardial fibrosis increases progressively during the course of AS, within midwall involvement, the progression of fibrosis accelerates. Thus, since the patients who are candidates for TAVR are older and in an advanced stage of the disease, they are more likely to have fibrosis. The use of an ACE inhibitor and/or ARB may theoretically inhibit the fibrosis process. However, in our study population, prior use of these drugs was similar in both the fQRS group and non‐fQRS group (31.8% and 40.2%, respectively, p = 0.360).
The occurrences of fQRS in AS patients were demonstrated in a study by Ağaç et al. (2014) previously. In their study, they found out that fQRS occurred in 40% of the severe AS patients compared to the 18% rate of fQRS observed in the patients without AS. In our study, we observed a fQRS rate of 37.9% in 116 patients. In another study, Acikgöz et al. found out that AS patients with fQRS had a lower LVEF and a higher mean and peak systolic transaortic gradients (Açıkgöz et al., 2015). In our study patients, the values of LVEF were found out to be similar when the two groups of patients, patients with fQRS and without fQRS, were compared. Furthermore, the values of LVEF did not predict the mortality. Our finding is compatible with data reported by Weideman et al., saying that the accumulation of fibrosis starts before the apparent reduction in LVEF. Thus, estimating LVEF using classical methods can be misleading; strain and strain rate analysis may give more accurate results. In our study, as the data were collected retrospectively, we did not have any chance to do strain analysis. The fibrotic substrate in the myocardium can induce malignant ventricular arrhythmias. Malignant ventricular arrhythmias and fQRS association have been demonstrated in studies performed in various patient populations. Also, in a study, the Tpe/QT ratio, as an indicator of ventricular arrhythmia, was found out to be increased in severe AS patients (Yayla et al., 2016). Therefore, increased mortality in AS patients with fQRS could hypothetically be attributed to malignant arrhythmias. In our study, the lack of data regarding the cause of death in follow‐up, or the absence of rhythm holter monitoring limits comment in this regard. Since the association between fQRS and myocardial fibrosis has not been validated using imaging modalities in AS patients before, our results should be interpreted cautiously. Conversely, when it is considered that fQRS is functionally a sign of myocardial fibrosis and that the prognostic value of fQRS in this field has been studied extensively before the results of our work are notable. The relatively small sample size and its retrospective nature are other limitations of our study.
5. CONCLUSION
As a reflection of myocardial fibrosis on ECG, fQRS can predict mortality in severe aortic stenosis patients after TAVR procedure. The relationship between fQRS and degree of fibrosis evaluated with imaging modalities, and malignant arrhythmic events in patients with AS and fQRS warrants further investigation.
CONFLICT OF INTEREST
The authors have no conflict of interests to disclose.
ACKNOWLEDGEMENTS
The authors thank Ibrahim Halil Tanboga (Hisar Intercontinental Hospital, Istanbul) for editorial assistance and data analysis.
Gulsen K, Ince O, Kum G, Ozkalayci F, Sahin I, Okuyan E. Could fragmented QRS predict mortality in aortic stenosis patients after transcatheter aortic valve replacement? Ann Noninvasive Electrocardiol. 2019;24:e12618 10.1111/anec.12618
REFERENCES
- Açıkgöz, E. , Yaman, B. , Açıkgöz, S. K. , Topal, S. , Tavil, Y. , & Boyacı, N. B. (2015). Fragmented QRS can predict severity of aortic stenosis. Annals of Noninvasive Electrocardiology, 20(1), 37–42. 10.1111/anec.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ağaç, M. T. , Korkmaz, L. , Bektas, H. , Acar, Z. , Erkan, H. , Kurt, I. H. , … Celik, S. (2014). Increased frequency of fragmented QRS in patients with severe aortic valve stenosis. Medical Principles and Practice, 23(1), 66–69. 10.1159/000355474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaran, Y. , Tigen, K. , Karaahmet, T. , Isiklar, I. , Cevik, C. , Gurel, E. , … Basaran, O. (2011). Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography, 28(1), 62–68. 10.1111/j.1540-8175.2010.01242.x [DOI] [PubMed] [Google Scholar]
- Bozbeyoğlu, E. , Yıldırımtürk, Ö. , Yazıcı, S. , Ceylan, U. S. , Erdem, A. , Kaya, A. , … Çetin, M. (2016). Fragmented QRS on Admission Electrocardiography Predicts Long‐Term Mortality in Patients with Non‐ST‐Segment Elevation Myocardial Infarction. Annals of Noninvasive Electrocardiology, 21(4), 352–357. 10.1111/anec.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canpolat, U. , Kabakç, I. G. , Aytemir, K. , & Dura, lM., Sahiner, L., Yorgun, H, Lenard, S., Oto, A, (2013). Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Journal of Cardiovascular Electrophysiology, 24(11), 1260–1266. [DOI] [PubMed] [Google Scholar]
- Çetin, M. S. , Ozcan Cetin, E. H. , Arisoy, F. , Kuyumcu, M. S. , Topaloglu, S. , Aras, D. , & Temizhan, A. (2016). Fragmented QRS complex predicts in‐hospital adverse events and long‐term mortality in patients with acute pulmonary embolism. Annals of Noninvasive Electrocardiology, 21(5), 470–478. 10.1111/anec.12332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, M. K. , Khan, B. , Jaco, B. S. , Kumar, A. , & Mahenthiran, J. (2006). Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation, 113(21), 2495–2501. 10.1161/CIRCULATIONAHA.105.595892 [DOI] [PubMed] [Google Scholar]
- Das, M. K. , Michael, M. A. , Suradi, H. , Peng, J. , Sinha, A. , Shen, C. , … Kovac, s R.J., (2009). Usefulness of fragmented QRS on a 12‐lead electrocardiogram in acute coronary syndrome for predicting mortality. American Journal of Cardiology, 104(12), 1631–1637. 10.1016/j.amjcard.2009.07.046 [DOI] [PubMed] [Google Scholar]
- Dweck, M. R. , Boon, N. A. , & Newby, D. E. (2012). Calcific aortic stenosis: A disease of the valve and the myocardium. Journal of the American College of Cardiology, 60(19), 1854–1863. 10.1016/j.jacc.2012.02.093 [DOI] [PubMed] [Google Scholar]
- Everett, R. , Tastet, L. , Clavel, M. A. , Chin, C. W. L. , Capoulade, R. , & Vassiliou, V. S. (2018). Progression of hypertrophy and myocardial fibrosis in aortic stenosis: a Multicenter Cardiac Magnetic Resonance Study. Circulation: Cardiovascular Imaging, 11(6), e007451 10.1161/CIRCIMAGING.117.007451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkan, A. K. , Cakmak, H. A. , Kalkan, M. E. , Tuncer, M. A. , Aydin, E. , Yanartas, M. , … Kirali, M. K. (2015). The predictive value of admission fragmented QRS complex for in‐hospital cardiovascular mortality of patients with type 1 acute aortic dissection. Annals of Noninvasive Electrocardiology, 20(5), 454–463. 10.1111/anec.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, K. W. , Janardhan, A. H. , Jung, K. T. , Lee, H. S. , Lee, M. H. , & Hwang, H. J. (2014). Fragmented QRS as a candidate marker for high‐risk assessment in hypertrophic cardiomyopathy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 11(8), 1433–1440. [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Bierig, M. , & Devereux, R. R. (2005). Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. Journal of the American Society of Echocardiography, 18, 1440–1463. 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Lorgis, L. , Cochet, A. , Chevallier, O. , Angue, M. , Gudjoncik, A. , Lalande, A. , … Cottin, Y. (2014). Relationship between fragmented QRS and no‐reflow, infarct size, and peri‐infarct zone assessed using cardiac magnetic resonance in patients with myocardial infarction. Canadian Journal of Cardiology, 30(2), 204–210. 10.1016/j.cjca.2013.11.026 [DOI] [PubMed] [Google Scholar]
- Perrin, N. , Frei, A. , & Noble, S. (2018). Transcatheter aortic valve implantation: Update in 2018. European Journal of Internal Medicine, 55, 12–19. 10.1016/j.ejim.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Thaden, J. J. , Nkomo, V. T. , & Enriquez‐Sarano, M. (2014). The global burden of aortic stenosis. Progress in Cardiovascular Diseases, 56(6), 565–571. 10.1016/j.pcad.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Treibel, T. A. , Kozor, R. , Schofield, R. , Benedetti, G. , Fontana, M. , Bhuva, A. N. , … Moon, J. C. (2018). Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. Journal of the American College of Cardiology, 71(8), 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann, F. , Herrmann, S. , Störk, S. , Niemann, M. , Frantz, S. , Lange, V. , … Strotmann, J. M. (2009). Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation, 120(7), 577–584. 10.1161/CIRCULATIONAHA.108.847772 [DOI] [PubMed] [Google Scholar]
- Yayla, Ç. , Bilgin, M. , Akboğa, M. K. , Gayretli Yayla, K. , Canpolat, U. , Dinç Asarcikli, L. , & Aydoğdu, S. (2016). Evaluation of Tp‐E interval and Tp‐E/QT ratio in patients with aortic stenosis. Annals of Noninvasive Electrocardiology, 21(3), 287–293. 10.1111/anec.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]


