Abstract
Background
Magnetocardiographic mapping (MCG) provides quantitative assessment of the magnetic field (MF) induced by cardiac ionic currents, is more sensitive to tangential currents, and measures vortex currents undetectable by ECG, with higher reported sensitivity of MCG ventricular repolarization (VR) parameters for earlier detection of acute myocardial ischemia. Aims of this study were to validate the feasibility of in‐hospital unshielded MCG and to assess repeatability and reproducibility of quantitative VR parameters, considering also possible gender‐ and age‐related variability.
Methods
MCG of 204 healthy subjects [114 males—mean age 43.4 ± 17.3 and 90 females—mean age 40.2 ± 15.7] was retrospectively analyzed, with a patented proprietary software automatically estimating twelve VR parameters derived from the analysis of the dynamics of the T‐wave MF extrema (five parameters) and from the inverse solution with the effective magnetic dipole model giving the effective magnetic vector components (seven parameters). MCG repeatability was calculated as coefficient of variation (CV) ±standard error of the mean (SEM). Reproducibility was assessed as intraclass correlation coefficient (ICC).
Results
The repeatability of all MCG parameters was 16 ± 1.2 (%) (average CV ± SEM). Optimal (ICC > 0.7) reproducibility was found for 11/12 parameters (mean values) and in 8/12 parameters (single values). No significant gender‐related difference was observed; six parameters showed a strong/moderate correlation with age.
Conclusion
Reliable MCG can be performed into an unshielded hospital ambulatory, with repeatability and reproducibility of quantitative assessment of VR adequate for clinical purposes. Wider clinical use is foreseen with the development of multichannel optical magnetometry.
Keywords: healthy subjects, ischemic heart disease, repeatability, reproducibility, unshielded magnetocardiography, ventricular repolarization
1. INTRODUCTION
Multichannel magnetocardiographic mapping (MCG) is a noninvasive/noncontact and radiation‐free method detecting the cardiac magnetic field (CMF), through high‐sensitivity magnetometers such as the direct current superconducting quantum interference devices (DC‐SQUIDs) (Fenici, Brisinda, & Meloni, 2005; Fenici, Brisinda, Sorbo, & Venuti, 2011; Leder et al., 2001; Sosnytska, 2011; Tavarozzi, Comani, Del Gratta, Di Luzio et al., 2002) or, more recently, optical ones (Lau, Petkovic, & Haueisen, 2016; Morales et al., 2017). Compared to the electrocardiogram (ECG), MCG provides additional information, being CMF less affected by tissue conductivities, and is more sensitive to tangential currents, and capable to detect vortex currents too (Brockmeier et al., 1997; Fenici Brisinda, & Meloni 2005). Although mainly confined in research laboratories, MCG is increasingly used to study cardiac arrhythmias (Fenici, Brisinda, Venuti, & Sorbo, 2013; Fenici, Brisinda, & Meloni, 2005; Kwong, Leithäuser, Park, & Yu, 2013; Mäkijärvi et al., 2010) and ventricular repolarization (VR) abnormalities (VRa) due to different cardiopathy (Kawakami et al., 2017), especially ischemic heart disease (IHD) (Agarwal, Saini, Alyousef, & Umscheid, 2012; Brisinda, Bottelli, Napolitano, Priori, & Fenici, 2007; Fenici, Brisinda, Meloni, Sternickel, & Fenici, 2005; Hailer, Chaikovsky, Auth‐Eisernitz, Schäfer, & Van Leeuwen, 2005; Kandori et al., 2010; Kwon et al., 2010; Leithäuser, Park, Hill, Lam, & Jung, 2013; Li et al., 2015; Lim et al., 2007; Ogata et al., 2009; Park & Hill, 2005; Park, Leithäuser, Hill, & Jung, 2008; Park et al., 2015; Shin et al., 2015; Steinisch et al., 2013; Tolstrup et al., 2006; Wu et al., 2013).
MCG instrumentation is typically classified by the method used to improve the signal‐to‐noise ratio (S/Nr) (Fenici et al., 2011; Mäkijärvi et al., 2010; Tavarozzi, Comani, Del Gratta, Di Luzio et al., 2002). Although electromagnetic shielding provides a better S/Nr, it is unpractical for clinical use, impeding quick ambulatory screening and bedside MCG. Alternatively, instrumentations for unshielded MCG have been also developed (Chen, Thompson, Nolan, Clarke, & Bakharev, 2002; Chen, Thomson, Nolan, & Clarke, 2004; Fenici et al., 2013; Hailer, Van Leeuwen et al., 2005; Leder et al., 2001; Steinberg et al., 2005; Tolstrup et al., 2006); however, most of them feature a limited number of sensors (4–9 channels), implying sequential mapping (Chen et al., 2002, 2004; Leder et al., 2001; Quan et al., 2008; Steinberg et al., 2005), a potential possible source of error especially for dynamic evaluation of transient events (Smith et al., 2002).
Although a direct comparison among different MCG systems and standardization of analytic methods is still missing, some attempts to standardize MCG format and to assess reproducibility and age‐ or gender‐dependent variations of several MCG parameters (e.g., interval durations, waveforms, VR dipole motion, vector parameters) have been reported (Chen et al., 2004; Kandori, Ogata, Miyashita, Watanabe et al., 2008; Lim et al., 2007; Steinberg et al., 2005). Given the availability of a unique multichannel MCG instrumentation fully operational into our unshielded hospital laboratory for interventional electrophysiology since 2002 (Fenici, Brisinda, & Meloni 2005; Fenici, Brisinda, Meloni, Sternickel, & Fenici, 2005; Fenici et al., 2011, 2013), we have retrospectively selected and analyzed MCG data of 204 healthy subjects to assess the repeatability and reproducibility of automatically calculated VR parameters used for diagnosis of IHD (Bakharev, 2011; Kwong et al., 2013; Park & Jung, 2004; Steinberg et al., 2004, 2005; Tolstrup et al., 2006), to set up a normality database obtained with simultaneous mapping (Fenici, Brisinda, Meloni, & Fenici, 2003), from a larger cohort compared to previous studies (Chen et al., 2002, 2004; Hailer, Van Leeuwen et al., 2005; Tolstrup et al., 2006) and to evaluate gender‐ and/or age‐related variability.
2. METHODS
2.1. Subjects
MCG data of 204 healthy subjects selected among those consecutively screened between 2004 and 2016, mainly to assess fitness for sports activity, were retrospectively analyzed. MCG data were accepted only if averaged signals of all 36 channels were free from artifacts and with a stable baseline (thus, no interpolation of even one channel was required).
All subjects were free from cardiac risk factors and from use of any medication and had normal blood pressure and rest ECG. When appropriate, echocardiography and ECG stress test data were also available. Written informed consent to MCG and to retrospective use of de‐identified clinical and MCG data for research purpose had been obtained. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local review board.
2.2. Equipment and measurement technique
MCG was performed, into an unshielded laboratory for interventional electrophysiology, with a 36‐channel system featuring DC‐SQUIDs coupled to second‐order axial gradiometers (pickup coil 50–70 mm baseline), arranged in a square grid of 20 × 20 cm (pitch between sensors: 4 cm) (CardioMag Imaging, Inc., Schenectady, NY, USA) (Fenici et al., 2003) (Figure 1). The average intrinsic sensitivity of the system was about 30 fT/√Hz in the frequency range of clinical interest (1–100 Hz). MCG was recorded (bandwidth: DC‐250 Hz, sampling frequency 1 kHz, 24‐bit A/D conversion) in the supine position (after removal of metal objects), with the Dewar as close as possible to (but not touching) the anterior chest wall. MCG session lasted typically 90 seconds. ECG lead II was simultaneously recorded.
Figure 1.
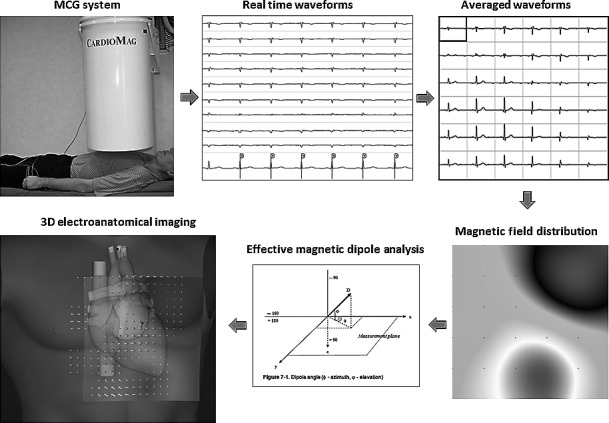
MCG recording setup, signal processing, and multimodal imaging
At least two MCGs were performed for each patient, the second one acquired by the same examiner few minutes after repositioning the subject on the examination table (to test for repeatability), or by different examiners in a different session (to test for reproducibility).
2.3. MCG processing and analysis
MCG parameters of VR were automatically calculated with a patented software tool (Bakharev, 2011), after digital low‐pass filtering, selective time averaging, and automatic computing of CMF distribution (CMFd). In case of residual artifacts at visual control, noisy and/or premature beats were manually removed before averaging. The reference baseline was automatically defined before the P wave, but was also manually selected, if necessary.
VR analysis consisted of:
Qualitative analysis, based on visual inspection of the CMFd dynamics, aimed to confirm stable dipolar morphology of CMFd during the ST segment and the T wave. If a multipolar pattern was present, in the time interval between 50 milliseconds after the J‐point and the T‐end (Figure 2), with CMF amplitude at least three times stronger than average noise in the TP interval, the VR pattern was considered abnormal and the record discarded.
-
Quantitative analysis, based on the solution of the inverse problem with the effective magnetic dipole (EMD) model and automatic calculation of the time‐variant dynamics of: (1) the three‐dimensional (3D) EMD vector (EMDV) components and (2) of the T‐wave magnetic field (MF) extrema:
-
EMDV parameters: The magnitude and strength of motion of the EMV can be described by seven predefined parameters T‐wave vector parameters calculated both before (Ascending limb, or A) and after (Descending limb, or D) the T‐wave peak:
Two azimuth mean: the angles between the projection of 3D average EMDV on XY plane and the x‐axis, being the origin of the axes the vertex (azimuth mean A normal if between −110° and −15 ° and azimuth mean D normal if between −100° and −22°);
Two Trajectory Length: EMDV trajectory lengths (Trajectory Length A normal if between 0 and 7.5 cm and Trajectory Length D normal if between 0 and 5 cm);
Two Angle Derivative Range: 3D EMDV angular deviations (between T‐start and T‐peak and between T‐peak and T‐end) defined through azimuth and elevation (i.e., the angle between the projection of 3D average EMV on XZ plane and the x‐axis, being the origin of the axes the vertex) (angle derivative range A normal if between 0 and 1.0 radians and angle derivative range D normal if between 0 and 0.7 radians);
The azimuth difference (A–D): angular displacement, with its own orientation, on XY plane, of the projection of 3D average EMDV (i.e., azimuth mean A minus azimuth mean D), normal if between −35° and 12°;
-
T‐wave extrema MF dynamics: five parameters automatically calculated during the first phase of VR, within a frame of 30 ms moving into an interval between the point where MF strength is equal to 1/3 of T‐wave peak MF and the T‐wave peak. Being generated, for each millisecond, an instant magnetic map with a positive and a negative pole (the highest and the lowest intensity of the MF) (Fenici, Brisinda, Meloni, Sternickel, & Fenici, 2005; Park & Jung, 2004; Steinberg et al., 2004, 2005), the following parameters were calculated:
Two angle extrema: maximum (angle extrema 1) and minimum (angle extrema 2) α angle between a line through the poles and a horizontal line, the origin set to plus pole (normal if between −110° and 20°);
The angle dynamics: α angle rotation in each interval of 30 ms (abnormal if >45°);
The distance dynamics: dynamic change of the distance between the poles ± (abnormal if >20 mm);
The ratio dynamics: MF strength ratio between the poles ± (abnormal if >0.3).
-
Figure 2.
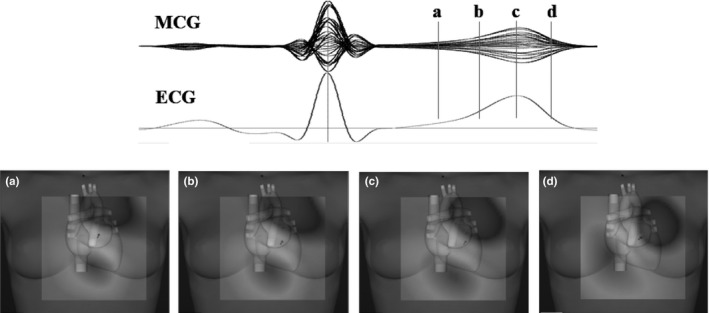
Example of stable dipolar configuration of the magnetic field distribution: during the ST segment (a), beginning (b), peak (c), and end (d) of T wave
2.4. Statistical analysis
Statistical analysis was carried out with SPSS (version 21.0). All MCG parameters were expressed as mean ± standard deviation (SD). A p‐value <.05 was considered statistically significant.
Repeatability (i.e., by definition, the capability of the instrumentation to reproduce results from two subsequent MCG performed by the same operator, under the same conditions and in a short period) was assessed through coefficient of variation (CV) between parameters for each subject. Data were subsequently expressed, for each parameter, as the average CV ± standard error of the mean (SEM). A small CV is an index of good repeatability. Graphically, a Bland–Altman plot for each parameter was created. Conventionally, repeatability must be made within maximum 72 hr; however, in this study we have chosen to analyze recordings taken subsequently within a time interval of few minutes.
Reproducibility (i.e., the degree of correlation between measurements performed at a distance of at least 72 hr by different operators) was tested with the one‐way random‐effects model intraclass correlation coefficient (ICC), accepting ICC >0.6 as index of a good and ICC >0.7 of optimal reproducibility.
To evaluate gender‐related variability of MCG parameters, parametric (t test) or nonparametric tests (Mann–Whitney U test—MW) were used.
To evaluate age‐related variability of MCG parameters, after dividing the whole population in eight age groups, p‐value of Kruskal–Wallis test and Pearson's correlation coefficient (r‐value, greater correlation if equal or close to ±1) were computed. Pearson's correlation coefficient was also calculated between single values and age. To correct for possible effect of obesity, the partial correlation coefficient was computed, using BMI and BSA as control variables.
3. RESULTS
The demographics of 204 investigated healthy volunteers [114 males (M), mean age 43.4 ± 17.3 years; and 90 females (F), mean age 40.2 ± 15.7 years] are summarized in Table 1. A graphic summary of overall results is shown in the Figure 2.
Table 1.
Subject characteristics
| Male (n = 114) | Female (n = 90) | p‐value | |
|---|---|---|---|
| Age (years) | |||
| Minimum | 4 | 4 | |
| Maximum | 82 | 79 | |
| Average value | 43.4 | 40.2 | .17 |
| Standard deviation | ±17.3 | ±15.7 | |
| Weight (kg) | |||
| Minimum | 17 | 19 | |
| Maximum | 110 | 98 | |
| Average value | 78.2 | 58.4 | <.05 |
| Standard deviation | ±15 | ±11.7 | |
| Height (cm) | |||
| Minimum | 101 | 108 | |
| Maximum | 193 | 176 | |
| Average value | 174.6 | 161.1 | <.05 |
| Standard deviation | ±11.8 | ±8.6 | |
| BMI (kg/m2) | |||
| Minimum | 16.67 | 14.7 | |
| Maximum | 36.75 | 40.79 | |
| Average value | 25.43 | 22.39 | <.05 |
| Standard deviation | ±3.72 | ±3.79 | |
| BSA (m2) | |||
| Minimum | 0.69 | 0.75 | |
| Maximum | 2.41 | 2.06 | |
| Average value | 1.94 | 1.61 | <.05 |
| Standard deviation | ±0.24 | ±0.19 | |
BMI, body mass index; BSA, body surface area.
3.1. Repeatability
Two MCG recordings of 138 subjects (52% males), subsequently acquired by the same examiner, were analyzed. Average CV ± SEM for each MCG parameter are shown in Table 2. For azimuth difference (A minus D), the absolute value was used. Correspondent Bland–Altman plots are shown in Figure 3.
Table 2.
Repeatability
| MCG parameters | Ml AV | M2 AV | Average CV (%) | SEM (%) |
|---|---|---|---|---|
| Trajectory length range A (cm) | 2.46 | 2.35 | 15.83 | 1.18 |
| Angle derivative range A (radians) | 0.33 | 0.32 | 15.17 | 1.28 |
| Azimuth mean A (degree) | −57.83 | −57.78 | 4.78 | 0.47 |
| Trajectory length range D (cm) | 1.97 | 2 | 18.41 | 1.11 |
| Angle derivative range D (radians) | 0.28 | 0.28 | 15.69 | 1.05 |
| Azimuth mean D (degree) | −50.64 | −50.27 | 4.37 | 0.41 |
| Azimuth diff AD (degree) | −7.19 | −7.51 | 25.39 | 2.58 |
| Angle extrema 1 (degree) | −72.54 | −71.78 | 4.4 | 0.41 |
| Angle extrema 2 (degree) | −62.92 | −62.37 | 3.69 | 0.45 |
| Angle dynamics (degree) | 6.32 | 5.99 | 22.53 | 1.77 |
| Distance dynamics (mm) | 8.27 | 7.78 | 27.94 | 2.15 |
| Ratio dynamics | 0.16 | 0.14 | 33.91 | 2.51 |
CV, coefficient of variation; M1 AV, measurement 1 average value; M2 AV, measurement 2 average value; SEM, standard error of the mean.
Figure 3.
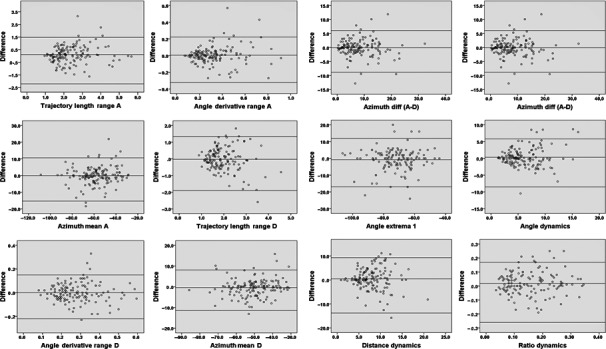
Bland–Altman Plots of VR parameters. The average value of two measures and their difference are plotted, respectively, on the x‐ and on the y‐axis. Central horizontal line corresponds to the average of differences while the line above and the line below represent the average of differences ±1.96 SD: more points fall within the two outside lines is greater repeatability
3.2. Reproducibility
One hundred and thirteen healthy subjects (52% males) had two MCG acquired by different examiners at a distance of at least 72 hr. For each MCG parameter, one‐way random‐effects model ICC between the first and second groups of measurements was calculated, considering the examiners as random effects (as sampled from a larger pool of potential raters). Reproducibility, in terms of average raters absolute ICC, was optimal for 11 of 12 parameters. Single raters absolute ICC, instead, was optimal for 8 and good for 3 of the 12 parameters (Table 3). Only MF extrema distance dynamics showed a low reproducibility which, however, increases considering its log10 (0.35 for single raters absolute ICC and 0.52 for average raters absolute ICC).
Table 3.
Reproducibility
| MCG parameters | Single raters ICC | Average raters ICC |
|---|---|---|
| Trajectory length range A (cm) | 0.71 | 0.82 |
| Angle derivative range A (radians) | 0.78 | 0.87 |
| Azimuth mean A (degree) | 0.93 | 0.96 |
| Trajectory length range D (cm) | 0.55 | 0.71 |
| Angle derivative range D (radians) | 0.77 | 0.87 |
| Azimuth mean D (degree) | 0.90 | 0.95 |
| Azimuth diff AD (degree) | 0.87 | 0.93 |
| Angle extrema 1 (degree) | 0.83 | 0.9 |
| Angle extrema 2 (degree) | 0.83 | 0.91 |
| Angle dynamics (degree) | 0.65 | 0.79 |
| Distance dynamics (mm) | 0.22 | 0.37 |
| Ratio dynamics | 0.61 | 0.75 |
ICC, intraclass correlation coefficient.
3.3. Creation of the database of normality
Overall, MCG data of 403 recordings of the whole studied population were used to obtain the database of normal values. To define maximum and minimum values of each parameter, we used all 403 files individually. To define mean values and their SD, single subject's data were obtained from the average of all individual recordings. All parameters (Table 4) were within the previously published normality range (Park & Jung, 2004; Shin et al., 2017; Steinberg et al., 2005; Tolstrup et al., 2006) except ratio dynamics, which was >0.3 in 7.6% of cases and whose highest value was 0.4, for both males and females.
Table 4.
MCG ventricular repolarization parameters values
| MCG parameters | Male (n = 114) | Female (n = 90) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | AV | ±SD | Min | Max | AV | ±SD | p‐value | Test | |
| Trajectory length range A (cm) | 0.95 | 7.01 | 2.52 | 1.03 | 0.93 | 5.9 | 2.57 | 1.10 | .94 | MWa |
| Angle derivative range A (radians) | 0.07 | 0.93 | 0.32 | 0.15 | 0.09 | 1 | 0.37 | 0.18 | .04 | MWa |
| Azimuth mean A (degree) | −95.47 | −25.37 | −57.48 | 15.61 | −108.93 | −28.65 | −61.29 | 15.65 | .09 | t test |
| Trajectory length range D (cm) | 0.78 | 4.81 | 2.08 | 0.70 | 0.81 | 4.96 | 2.14 | 0.82 | .86 | MWa |
| Angle derivative range D (radians) | 0.07 | 0.7 | 0.27 | 0.13 | 0.1 | 0.65 | 0.32 | 0.12 | .00 | MWa |
| Azimuth mean D (degree) | −82.59 | −30.03 | −51.39 | 10.68 | −86.65 | −26.34 | −52.96 | 11.51 | .32 | t test |
| Azimuth diff AD (degree) | −31.58 | 11.73 | −6.26 | 8.11 | −33.05 | 7.95 | −8.33 | 7.25 | .06 | t test |
| Angle extrema 1 (degree) | −108 | 8 | −74.06 | 14.74 | −108 | −44 | −73.54 | 13.48 | .80 | t test |
| Angle extrema 2 (degree) | −104 | 73 | −63.28 | 13.13 | −101 | −41 | −64.31 | 10.40 | .54 | t test |
| Angle dynamics (degree) | 1 | 20 | 6.54 | 3.37 | 0.22 | 27.11 | 6.30 | 3.68 | .36 | MWa |
| Distance dynamics (mm) | 0.88 | 24.97 | 8.18 | 3.82 | 0.9 | 20.37 | 8.07 | 3.22 | .99 | MWa |
| Ratio dynamics | 0.01 | 0.4 | 0.15 | 0.09 | 0.01 | 0.4 | 0.17 | 0.09 | .05 | MWa |
AV, average value; Max, maximum; Min, minimum; SD, standard deviation.
Mann–Whitney U test.
3.4. Gender‐related differences
To check for gender‐related difference of VR parameters, the Student t test was used when data distribution was normal (five of 12 parameters), while the Mann–Whitney U test was used for non‐normally distributed parameters. Only angle derivative range (A and D) and the ratio dynamics were significantly different (Table 4).
3.5. Evaluation of age‐related variability
To evaluate age‐related variability, the whole study population was arbitrarily divided into 8 age decades. Average values of 8 of the 12 parameters MCG parameters were significantly different among groups (Table 5). Average values of each MCG parameter [azimuth difference (A minus D) calculated using absolute values] of each decade were correlated with age progression (Table 6). An optimal (r‐value >.7) direct correlation for five parameters and a good (r‐value −.5) reverse correlation for a sixth one (angle derivative range D) were found (Figure 4). Instead, the correlation between each MCG parameter and individual age was weak (r‐value <.4), even if corrected for BMI and BSA (Table 7).
Table 5.
Average values of ventricular repolarization parameters of the eight age groups
| Age groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | <10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | >70 | |
| No of subjects | 5 | 12 | 42 | 38 | 42 | 35 | 21 | 9 | p‐value |
| MCG parameters (average values) | |||||||||
| Trajectory length range A (cm) | 1.84 | 1.75 | 2.21 | 2.39 | 2.65 | 3.34 | 3.34 | 2.5 | <.001 |
| Angle derivative range A (radians) | 0.32 | 0.25 | 0.32 | 0.37 | 0.34 | 0.36 | 0.36 | 0.38 | n.s. |
| Azimuth mean A (degree) | −82.25 | −49.58 | −55.88 | −64.08 | −56.12 | −60.3 | −60.3 | −67.85 | <.05 |
| Trajectory length range D (cm) | 2.03 | 1.96 | 1.97 | 1.97 | 2.14 | 2.54 | 2.54 | 2.46 | n.s. |
| Angle derivative range D (radians) | 0.47 | 0.27 | 0.29 | 0.32 | 0.26 | 0.29 | 0.29 | 0.3 | n.s. |
| Azimuth mean D (degree) | −71.08 | −46.56 | −49.55 | −54.2 | −49.07 | −51.79 | −51.79 | −62.35 | <.01 |
| Azimuth diff AD (degree) | 11.18 | 4.41 | 7.65 | 11.01 | 7.99 | 8.51 | 8.51 | 8.65 | n.s. |
| Angle extrema 1 (degree) | −98.2 | −66.6 | −69.3 | −76.7 | −71.5 | −77.8 | −77.8 | −84.2 | <.001 |
| Angle extrema 2 (degree) | −90.2 | −58.7 | −61.3 | −65.9 | −61.5 | −65.3 | −65.3 | −69.5 | <.01 |
| Angle dynamics (degree) | 5.999 | 5.364 | 5.049 | 6.988 | 6.13 | 7.435 | 7.435 | 8.668 | <.001 |
| Distance dynamics (mm) | 3.54 | 7.92 | 6.68 | 7.91 | 8.43 | 10.2 | 10.2 | 9.41 | <.05 |
| Ratio dynamics | 0.196 | 0.1171 | 0.1307 | 0.1461 | 0.1675 | 0.1882 | 0.1882 | 0.1789 | <.05 |
n.s., not significant.
Table 6.
Pearson's correlation between age groups and average values of ventricular repolarization parameters
| MCG parameters (average values) | r‐value |
|---|---|
| Trajectory length range A (cm) | .82 |
| Angle derivative range A (radians) | .75 |
| Azimuth mean A (degree) | .14 |
| Trajectory length range D (cm) | .83 |
| Angle derivative range D (radians) | −.50 |
| Azimuth mean D (degree) | .11 |
| Azimuth diff AD (degree) | −.01 |
| Angle extrema 1 (degree) | .10 |
| Angle extrema 2 (degree) | .33 |
| Angle dynamics (degree) | .84 |
| Distance dynamics (mm) | .85 |
| Ratio dynamics | .39 |
Figure 4.
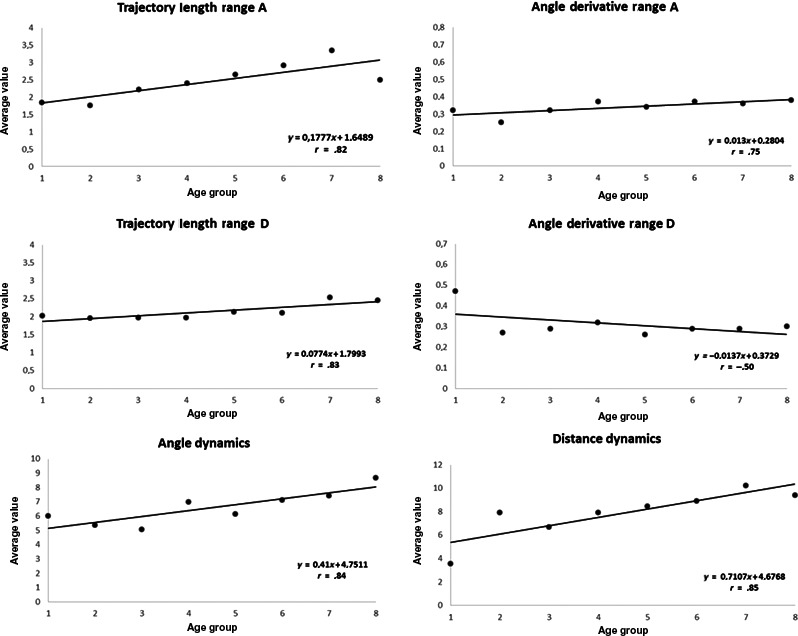
Scatter plot of the Pearson's correlation between age groups (x‐axis) and average values of 6 VR parameters (y‐axis)
Table 7.
Correlation between individual age and ventricular repolarization parameters
| Control variable | None | BMI | BSA |
|---|---|---|---|
| MCG parameters | Pearson's r‐value | PCC r‐value | PCC r‐value |
| Trajectory length range A (cm) | .35 | .24 | .29 |
| Angle derivative range A (radians) | .15 | .21 | .21 |
| Azimuth mean A (degree) | −.03 | −.03 | −.10 |
| Trajectory length range D (cm) | .20 | .12 | .19 |
| Angle derivative range D (radians) | −.10 | −.05 | −.01 |
| Azimuth mean D (degree) | −.06 | −.05 | −.13 |
| Azimuth diff AD (degree) | .01 | .03 | .05 |
| Angle extrema 1 (degree) | −.08 | −.08 | −.12 |
| Angle extrema 2 (degree) | .03 | .01 | −.04 |
| Angle dynamics (degree) | .23 | .19 | .22 |
| Distance dynamics (mm) | .32 | .23 | .26 |
| Ratio dynamics | .19 | .11 | .19 |
BMI, body mass index; BSA, body surface area; PCC, partial correlation coefficient.
4. DISCUSSION
Mass screening of healthy young people and sports practitioners is not considered cost‐effective even if performed simply with ECG recording (Maron et al., 2014). Body surface potential mapping (Taccardi & Punske, 2004) recently improved with modern multielectrode vests is still an expensive and complex procedure, which requires multimodal integration with CT scan to obtain pre‐interventional electro‐anatomical imaging (Dubois et al., 2015; Zhou, Jin, Yu, Wu, & He, 2016).
MCG is a contactless alternative for faster mass screening (Mäkijärvi et al., 2010; Malmivuo, 1995; Steinhoff et al., 2004; Tavarozzi, Comani, Del Gratta, Romani et al., 2002; Wu et al., 2013) and radiation‐ and risk‐free method for three‐dimensional electro‐anatomical imaging through passive detection of CMF (Agarwal et al., 2012; Brisinda et al., 2007; Brockmeier et al., 1997; Fenici, Brisinda, Meloni, Sternickel, & Fenici, 2005; Hailer, Van Leeuwen et al., 2005; Kandori et al., 2010; Kwon et al., 2010; Kwong et al., 2013; Leithäuser et al., 2013; Li et al., 2015; Lim et al., 2007; Ogata et al., 2009; Park & Hill, 2005; Park et al., 2008, 2015; Shin et al., 2015; Steinisch et al., 2013; Tolstrup et al., 2006; Wu et al., 2013), which is directly related primary electrophysiological source (the impressed current), thus containing information undetectable with ECG (Fenici, Brisinda, & Meloni, 2005; Tavarozzi, Comani, Del Gratta, Di Luzio et al., 2002). VR analysis is considered one of the most useful applications of MCG, mainly to recognize ischemic, inflammatory, or degenerative abnormalities (Tavarozzi, Comani, Del Gratta, Di Luzio et al., 2002). However, although some attempts to assess the reproducibility and comparability of MCG parameters gathered with different instrumentations have been reported (Burghoff, Nenonen, Trahms, & Katila, 2000; Nalbach et al., 2002; Pesola et al., 2000; Smith et al., 2006; Takala et al., 2001), a standardization adequate for clinical application is still missing, especially taking into account the potential source of error due to different S/Nr and/or sequential data acquisition (Bang et al., 2016; Hänninen et al., 2002; Kawakami et al., 2017; Mäkijärvi et al., 2010; Shin et al., 2015) as occurs with most instrumentations for unshielded MCG (Fenici, Brisinda, & Meloni, 2005; Leder et al., 2001) due to their limited number of sensors.
This study aimed at evaluating (and to the best of our knowledge is the first reporting) the normality range of twelve VR parameters automatically calculated from MCG data acquired with an unshielded 36‐channel instrumentation in a hospital environment (Fenici et al., 2003). As the repeatability and reproducibility of conventional time domain parameters (QRS duration, QT interval, etc.) were largely evaluated in the past (Fenici, Brisinda, & Meloni, 2005; Tavarozzi, Comani, Del Gratta, Romani et al., 2002), this study focused only on parameters automatically obtained from the inverse solution and the analysis of the time‐variant CMF dynamics.
Compared to previous studies (Chen et al., 2002, 2004; Steinberg et al., 2004, 2005; Tolstrup et al., 2006), a larger cohort, 204 normal subjects, has been investigated. Moreover, all subjects underwent at least two MCG recordings, carried out under variable conditions (i.e., different hours of the days, implying different intensity of the background electromagnetic noise) to check for repeatability and for reproducibility of data analysis performed with signals acquired in the “real‐life” of an unshielded hospital environment.
Morphological analysis of CMF distribution during VR confirmed a stable dipolar homogeneous pattern during the whole ST interval and the T wave (Figure 2). The range of quantitative T‐wave parameters, automatically calculated after solution of the inverse problem, was consistent with previously applied criteria, except for the ratio dynamics, which exceeded the upper level of 0.3 in 7.6% of investigated cases, without gender‐related difference. As values above 0.4 were not observed, being the studied population certainly normal, we suggest elevating to 0.4 the upper limit of normality for this parameter.
Although significant age‐ and gender‐related differences were previously reported for parameters of normal subjects investigated with a 9‐channel unshielded MCG system (Chen et al., 2004) and with shielded multichannel mapping (Kandori, Ogata, Miyashita, Takaki et al., 2008), no gender‐related differences of the automatically calculated parameters were found in the present study, except for the angle derivative range A and D (p < .05 and .001, respectively).
As concerns the effect of age, after dividing our cohort into eight age groups, differences of distribution by age class resulted significant for 8 of 12 parameters; five parameters (trajectory length range A and D, angle derivative range A, angle dynamics, and distance dynamics) showed high direct correlation (r‐value >.7), while angle derivative range D moderate reverse correlation with decades groups (Figure 2). Instead, the correlations between individual MCG parameters and age, even if corrected for BMI and BSA, were all weak (Table 7).
Repeatability and reproducibility of estimated parameters ranged from optimal to good, thus adequate for clinical application (Bakharev, 2011; Leder et al., 2001; Lim et al., 2007; Steinberg et al., 2005). However, our results were better than previously reported (Steinberg et al., 2005), probably because of the larger cohort analyzed.
4.1. Study limitations
A first limitation of this study is that, to avoid any subjective bias in the interpretation, only the T‐wave parameters automatically calculated by the proprietary software were evaluated. Thus, our results can be applied as normality reference only when using the same or equivalent automatic analysis methods (Steinisch et al., 2013). On the other hand, this does not detract the value of the study because so far, the normality values available in previous investigation were obtained from limited cohorts of local volunteers (Steinberg et al., 2005; Tolstrup et al., 2006) or unpublished. Second, although our sample is relatively large, it was probably insufficient to evaluate gender‐related variability from numerically homogeneous groups. The same could explain the lack of correlation between age and VR parameters when analyzing individual values and not age groups. Thus, to assess gender‐ and age‐related variability, further investigation on larger number of subjects may be required.
Finally, interobserver reproducibility was not evaluated, due to the lack of a systematic collection of data by at least two different operators on the same group of subjects.
5. CONCLUSION
This study demonstrates that unshielded MCG is feasible in hospital environments, with repeatability and reproducibility for VR parameters adequate for clinical application of the method, thus confirming the reliability of previous measurements carried out with smaller systems in noisy emergency departments and other clinical settings (Fenici et al., 2013; Hailer, Van Leeuwen et al., 2005; Kwong et al., 2013; Steinberg et al., 2005; Tolstrup et al., 2006).
Given the theoretical and technical advantages of MCG in detecting ischemic electrophysiological events and their DC components noninvasively (Cohen, Savard, & Rifkin, 1983), with reported predictive accuracy comparable with that of SPECT (Bakharev, 2011; Shin et al., 2017; Steinberg et al., 2005; Steinisch et al., 2013; Tolstrup et al., 2006), once the drawbacks and complexity of cryogenic instrumentations and/or of expensive heavy electromagnetic shielding will be overcome, (Kwong et al., 2013; Shin et al., 2017; Steinisch et al., 2013), its use in emergency departments for quick and early triage of chest pain patients with still normal 12‐lead ECG and high‐sensitivity cardiac troponin assays is foreseen as an attractive radiation‐free option to rule out acute coronary syndrome (ACS) and to reduce indication to stress SPECT and CT coronary angiography (Bakharev, 2011; Shin et al., 2017; Steinberg et al., 2005; Steinisch et al., 2013; Tolstrup et al., 2006). Still, the need of liquid helium and related costs of cryogenic instrumentations may be a limitation. However, recent development of reliable optical magnetometers working at room temperature is foreseen as an important cornerstone for the construction of innovative portable biomagnetic devices, usable in emergency room, at patient's bedside (Morales et al., 2017; Shah, 2013) or even for dynamic monitoring (Lau et al., 2016). Other novel technologies, such as magnetometry with nitrogen‐vacancy defects in diamond are under investigation with advanced testing for biomagnetic measurements (Barry et al., 2016).
Sorbo AR, Lombardi G, La Brocca L, Guida G, Fenici R, Brisinda D. Unshielded magnetocardiography: Repeatability and reproducibility of automatically estimated ventricular repolarization parameters in 204 healthy subjects. Ann Noninvasive Electrocardiol. 2018;23:e12526 10.1111/anec.12526
REFERENCES
- Agarwal, R. , Saini, A. , Alyousef, T. , & Umscheid, C. A. (2012). Magnetocardiography for the diagnosis of coronary artery disease: A systematic review and meta‐analysis. Annals of Noninvasive Electrocardiology, 17(4), 291–298. 10.1111/j.1542-474x.2012.00538.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakharev, A . (2011). Ischemia identification, quantification and partial localization in MCG ‐ EP 1 349 494 B1.
- Bang, W. , Kim, K. , Lee, Y. , Kwon, H. , Park, Y. , Pak, H. , … Joung, B. (2016). Repolarization heterogeneity of magnetocardiography predicts long‐term prognosis in patients with acute myocardial infarction. Yonsei Medical Journal, 57(6), 1339–1346. 10.3349/ymj.2016.57.6.1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, J. F. , Turner, M. J. , Schloss, J. M. , Glenn, D. R. , Song, Y. , Lukin, M. D. , … Walsworth, R. L. (2016). Optical magnetic detection of single‐neuron action potentials using quantum defects in diamond. Proceedings of the National Academy of Sciences, 113(49), 14133 10.1073/pnas.1601513113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisinda, D. , Bottelli, G. , Napolitano, C. , Priori, S. G. , & Fenici, R. (2007). Magnetocardiographic findings and follow‐up in an asymptomatic Brugada patient. Effects of Flecainide and of exercise tests. International Congress Series, 1300, 459–462. 10.1016/j.ics.2006.12.079 [DOI] [Google Scholar]
- Brockmeier, K. , Schmitz, L. , Bobadilla Chavez, J. D. , Burghoff, M. , Koch, H. , Zimmermann, R. , & Trahms, L. (1997). Magnetocardiography and 32‐Lead Potential Mapping: Repolarization in normal subjects during pharmacologically induced stress. Journal of Cardiovascular Electrophysiology, 8(6), 615–626. 10.1111/j.1540-8167.1997.tb01824.x [DOI] [PubMed] [Google Scholar]
- Burghoff, M. , Nenonen, J. , Trahms, L. , & Katila, T. (2000). Conversion of magnetocardiographic recordings between two different multichannel SQUID devices. IEEE Transactions on Biomedical Engineering, 47(7), 869–875. 10.1109/10.846680 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Thompson, P. D. , Nolan, V. , Clarke, J , & Bakharev, A . (2002). The normal magnetocardiogram at rest and post‐exercise in healthy volunteers in an unshielded clinical environment. In Biomag 2002, Proceedings of 13th International Conference on Biomagnetism (p. 533).
- Chen, J. , Thomson, P. D. , Nolan, V. , & Clarke, J. (2004). Age and sex dependent variations in the normal magnetocardiogram compared with changes associated with ischemia. Annals of Biomedical Engineering, 32(8), 1088–1099. 10.1114/B:ABME.0000036645.35013.ad [DOI] [PubMed] [Google Scholar]
- Cohen, D. , Savard, P. , & Rifkin, R. D. (1983). Magnetic measurements of S‐T and T‐Q segment shifts in humans. Part II: exercise‐induced S‐T segment depression. Circulation Research, 53(2), 274–279. http://www.scopus.com/inward/record.url?eid=2-s2.0-0020643212%26partnerID=40&md5=b3dec616e6532a6995fcb55a602b10e8 [DOI] [PubMed] [Google Scholar]
- Dubois, R. , Shah, A. J. , Hocini, M. , Denis, A. , Derval, N. , Cochet, H. , … Haissaguerre, M. (2015). Non‐invasive cardiac mapping in clinical practice: Application to the ablation of cardiac arrhythmias. Journal of Electrocardiology, 48(6), 966–974. 10.1016/j.jelectrocard.2015.08.028 [DOI] [PubMed] [Google Scholar]
- Fenici, R. , Brisinda, D. , & Meloni, A. M. (2005). Clinical application of magnetocardiography. Expert Review of Molecular Diagnostics, 5(3), 291–313. 10.1586/14737159.5.3.291 [DOI] [PubMed] [Google Scholar]
- Fenici, R. , Brisinda, D. , Meloni, A. M. , & Fenici, P. (2003). First 36‐channel system for clinical magnetocardiography in unshielded hospital laboratory for cardiac electrophysiology. International Journal of Bioelectromagnetism, 5(1), 80–83. http://hdl.handle.net/10807/16136 [Google Scholar]
- Fenici, R. , Brisinda, D. , Meloni, A. M. , Sternickel, K. , & Fenici, P . (2005). Clinical validation of machine learning for automatic analysis of multichannel Magnetocardiography In Frangi A. F., Radeva P. I., Santos A., Hernandez M. (Eds.), Functional Imaging and Modeling of the Heart (pp. 143–152). Berlin, D: Springer Science & Business Media; 10.1007/11494621_15 [DOI] [Google Scholar]
- Fenici, R. , Brisinda, D. , Sorbo, A.R. , & Venuti, A . (2011). MCG instrumentation and application In Rogalla H. & Kes P. H. (Eds.), Medical Application. 100 Years of Superconductivity (pp. 598–599). Boca Raton, FL: CRC Press. [Google Scholar]
- Fenici, R. , Brisinda, D. , Venuti, A. , & Sorbo, A. R. (2013). Thirty years of clinical magnetocardiography at the Catholic University of Rome: Diagnostic value and new perspectives for the treatment of cardiac arrhythmias. International Journal of Cardiology, 168(5), 5113–5115. 10.1016/j.ijcard.2013.07.238 [DOI] [PubMed] [Google Scholar]
- Hailer, B. , Chaikovsky, I. , Auth‐Eisernitz, S. , Schäfer, H. , & Van Leeuwen, P. (2005). The value of magnetocardiography in patients with and without relevant stenoses of the coronary arteries using an unshielded system. PACE ‐ Pacing and Clinical Electrophysiology, 28(1), 8–16. 10.1111/j.1540-8159.2005.09318.x [DOI] [PubMed] [Google Scholar]
- Hailer, B. , Van Leeuwen, P. , Chaikovsky, I. , Auth‐Eisernitz, S. , Schäfer, H. , & Grönemeyer, D. (2005). The value of magnetocardiography in the course of coronary intervention. Annals of Noninvasive Electrocardiology, 10(2), 188–196. 10.1111/j.1542-474X.2005.05625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen, H. , Takala, P. , Korhonen, P. , Oikarinen, L. , Mäkijärvi, M. , Nenonen, J. , … Toivonen, L. (2002). Features of ST segment and T‐wave in exercise‐induced myocardial ischemia evaluated with multichannel magnetocardiography. Annals of Medicine, 34(2), 120–129. 10.1080/07853890252953518 [DOI] [PubMed] [Google Scholar]
- Kandori, A. , Ogata, K. , Miyashita, T. , Takaki, H. , Kanzaki, H. , Hashimoto, S. , … Aonuma, K. (2010). Subtraction magnetocardiogram for detecting coronary heart disease. Annals of Noninvasive Electrocardiology, 15(4), 360–368. 10.1111/j.1542-474X.2010.00392.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandori, A. , Ogata, K. , Miyashita, T. , Watanabe, Y. , Tanaka, K. , Murakami, M. , … Yamaguchi, I. (2008). Standard Template of Adult Magnetocardiogram. Annals of Noninvasive Electrocardiology, 13(4), 391–400. 10.1111/j.1542-474x.2008.00246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandori, A. , Ogata, K. , Watanabe, Y. , Takuma, N. , Tanaka, K. , Murakami, M. , … Oka, Y. (2008). Space‐time database for standardization of adult magnetocardiogram‐making standard MCG parameters. Pacing and Clinical Electrophysiology, 31(4), 422–431. 10.1111/j.1540-8159.2008.01011.x [DOI] [PubMed] [Google Scholar]
- Kawakami, S. , Takaki, H. , Hashimoto, S. , Kimura, Y. , Nakashima, T. , Aiba, T. , … Sugimachi, M. (2017). Utility of high‐resolution magnetocardiography to predict later cardiac events in nonischemic cardiomyopathy patients with normal QRS duration. Circulation Journal, 81(1), 44–51. 10.1253/circj.CJ-16-0683 [DOI] [PubMed] [Google Scholar]
- Kwon, H. , Kim, K. , Lee, Y.‐H. , Kim, J.‐M. , Yu, K. K. , Chung, N. , & Ko, Y.‐G. (2010). Non‐invasive magnetocardiography for the early diagnosis of coronary artery disease in patients presenting with acute chest pain. Circulation Journal, 74(7), 1424–1430. 10.1253/circj.CJ-09-0975 [DOI] [PubMed] [Google Scholar]
- Kwong, J. S. W. , Leithäuser, B. , Park, J. W. , & Yu, C. M. (2013). Diagnostic value of magnetocardiography in coronary artery disease and cardiac arrhythmias: A review of clinical data. International Journal of Cardiology, 167(5), 1835–1842. 10.1016/j.ijcard.2012.12.056 [DOI] [PubMed] [Google Scholar]
- Lau, S. , Petkovic, B. , & Haueisen, J. (2016). Optimal magnetic sensor vests for cardiac source imaging. Sensors (Switzerland), 16(6), 1–17. 10.3390/s16060754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder, U. , Schrey, F. , Haueisen, J. , Dörrer, L. , Schreiber, J. , Liehr, M. , … Seidel, P. (2001). Reproducibility of HTS‐SQUID magnetocardiography in an unshielded clinical environment. International Journal of Cardiology, 79(2–3), 237–243. 10.1016/S0167-5273(01)00440-5 [DOI] [PubMed] [Google Scholar]
- Leithäuser, B. , Park, J.‐W. , Hill, P. , Lam, Y.‐Y. , & Jung, F. (2013). Magnetocardiography in patients with acute chest pain and bundle branch block. International Journal of Cardiology, 168(1), 582–583. 10.1016/j.ijcard.2013.01.254 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Che, Z. , Quan, W. , Yuan, R. , Shen, Y. , Liu, Z. , & Wang, W. (2015). Diagnostic outcomes of magnetocardiography in patients with coronary artery disease. International Journal of Clinical and Experimental Medicine, 8(2), 2441–2446. [PMC free article] [PubMed] [Google Scholar]
- Lim, H. K. , Chung, N. , Kim, K. , Ko, Y. G. , Kwon, H. , Lee, Y. H. , … Park, Y. K. (2007). Reproducibility of quantitative estimate of magnetocardiographic ventricular depolarization and repolarization parameters in healthy subjects and patients with coronary artery disease. Annals of Biomedical Engineering, 35(1), 59–68. 10.1007/s10439-006-9210-9 [DOI] [PubMed] [Google Scholar]
- Mäkijärvi, M. , Korhonen, P. , Jurkko, R. , Väänänen, H. , Siltanen, P. , & Hänninen, H . (2010). Magnetocardiography In Macfarlane P. W., van Oosterom A., Pahlm O., Kligfield P., Janse M., & Camm J. (Eds.), Comprehensive electrocardiology (pp. 2007–2028). London, UK: Springer; 10.1007/978-1-84882-046-3_44 [DOI] [Google Scholar]
- Malmivuo, J. P. R. (1995). Bioelectromagnetism Oxford, UK: Oxford University Press. [Google Scholar]
- Maron, B. J. , Friedman, R. A. , Kligfield, P. , Levine, B. D. , Viskin, S. , Chaitman, B. R. , … Thompson, P. D. (2014). Assessment of the 12‐lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people. Journal of the American College of Cardiology, 64(14), 1479–1514. 10.1016/j.jacc.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Morales, S. , Corsi, M. C. , Fourcault, W. , Bertrand, F. , Cauffet, G. , Gobbo, C. , … Labyt, E. (2017). Magnetocardiography measurements with 4He vector optically pumped magnetometers at room temperature. Physics in Medicine and Biology., 62, 7267–7279. Retrieved from http://iopscience.iop.org/10.1088/1361-6560/aa6459 [DOI] [PubMed] [Google Scholar]
- Nalbach, M. , Skipa, O. , Trahms, L. , Nenonen, J. , Kosch, O. , Steinhoff, U. , & Dössel, O. (2002). Imaging characteristics of different multichannel magnetocardiographic systems. Biomedizinische Technik. Biomedical Engineering, 2002(47), 445–448. 10.1515/bmte.2002.47.s1a.445 [DOI] [PubMed] [Google Scholar]
- Ogata, K. , Kandori, A. , Watanabe, Y. , Suzuki, A. , Tanaka, K. , Oka, Y. , … Kamakura, S. (2009). Repolarization spatial‐time current abnormalities in patients with coronary heart disease. Pacing and Clinical Electrophysiology, 32(4), 516–524. 10.1111/j.1540-8159.2009.02313.x [DOI] [PubMed] [Google Scholar]
- Park, J. W. , & Hill, P. (2005). Magnetocardiography predicts coronary artery disease in patients with acute chest pain. Annals of Noninvasive Electrocardiology, 49, 312–323. http://onlinelibrary.wiley.com/doi/10.1111/j.1542-474X.2005.00634.x/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. W. , & Jung, F. (2004). Qualitative and quantitative description of myocardial ischemia by means of Magnetocardiography. Biomedizinische Technik/Biomedical Engineering, 49(10), 266–272. 10.1515/bmt.2004.050 [DOI] [PubMed] [Google Scholar]
- Park, J. W. , Leithäuser, B. , Hill, P. , & Jung, F. (2008). Resting Magnetocardiography predicts 3‐year mortality in patients presenting with acute chest pain without ST segment elevation. Annals of Noninvasive Electrocardiology, 13(2), 171–179. 10.1111/j.1542-474X.2008.00217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. W. , Shin, E. S. , Ann, S. H. , Gödde, M. , Park, L. S. , Brachmann, J. , … Jung, F. (2015). Validation of magnetocardiography versus fractional flow reserve for detection of coronary artery disease. Clin Hemorheol Microcirc, 59(3), 267–281. https://doi.org/doi: 10.3233/CH‐141912. [DOI] [PubMed] [Google Scholar]
- Pesola, K. , Lojtjonen, J. , Nenonen, J. , Magnin, I. E. , Lauerma, K. , Fenici, R. , & Katila, T. (2000). The effect of geometric and topologic differences in boundary element models on magnetocardiographic localization accuracy. Transactions on Biomedical Engineering, 47(9), 1237–1247. 10.1109/10.867958 [DOI] [PubMed] [Google Scholar]
- Quan, W. W. , Lu, G. P. , Qi, W. H. , Li, Y. M. , Shen, Y. , & Yuan, R. (2008). Diagnostic value of magnetocardiography in patients with coronary heart disease and in‐stent restenosis. Chinese Medical Journal, 121(1), 22–26. 10.1016/j.ijcard.2012.12.056 [DOI] [PubMed] [Google Scholar]
- Shah, V. W. R. (2013). A compact, high performance atomic magnetometer for biomedical applications. Physics in Medicine & Biology, 58(22), 8153–8161. 10.1016/j.pestbp.2011.02.012.Investigations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, E. S. , Ann, S. H. , Brachmann, J. , Lam, Y. Y. , Jung, F. , & Park, J. W. (2015). Noninvasive detection of myocardial ischemia: A case of magnetocardiography. Clinical Hemorheology and Microcirculation, 60(1), 163–169. 10.3233/CH-151945 [DOI] [PubMed] [Google Scholar]
- Shin, E. S. , Lam, Y. Y. , Her, A. Y. , Brachmann, J. , Jung, F. , & Park, J. W. (2017). Incremental diagnostic value of combined quantitative and qualitative parameters of magnetocardiography to detect coronary artery disease. International Journal of Cardiology, 228, 948–952. 10.1016/j.ijcard.2016.11.165 [DOI] [PubMed] [Google Scholar]
- Smith, F. E. , Langley, P. , Trahms, L. , Steinhoff, U. , Bourke, J. P. , & Murray, A. (2002). Errors in repolarization measurement using magnetocardiography. Pacing and Clinical Electrophysiology, 25(8), 1223–1229. https://doi.org/doi: 10.1046/j.1460‐9592.2002.01223.x [DOI] [PubMed] [Google Scholar]
- Smith, F. E. , Langley, P. , van Leeuwen, P. , Hailer, B. , Trahms, L. , Steinhoff, U. , … Murray, A. (2006). Comparison of magnetocardiography and electrocardiography: A study of automatic measurement of dispersion of ventricular repolarization. Europace, 8, 887–893. 10.1093/europace/eul070 [DOI] [PubMed] [Google Scholar]
- Sosnytska, T. V. (2011). Clinical application of magnetic mapping. Lik Sprava, 1‐2, 29–47. [PubMed] [Google Scholar]
- Steinberg, B. A. , Roguin, A. , Allen, E. , Wahl, D. R. , Smith, C. S. , John, M. St , … Resar, J. R. (2004). Reproducibility and interpretation of magneto‐cardio‐gram maps in detecting ischemia. Journal of the American College of Cardiology, 43(5), A149 10.1016/s0735-1097(04)90634-1 [DOI] [Google Scholar]
- Steinberg, B. A. , Roguin, A. , Watkins, S. P. , Hill, P. , Fernando, D. , & Resar, J. R. (2005). Magnetocardiogram recordings in a nonshielded environment ‐ Reproducibility and ischemia detection. Annals of Noninvasive Electrocardiology, 10(2), 152–160. 10.1111/j.1542-474X.2005.05611.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff, U. , Knappe‐Grueneberg, S. , Schnabel, A. , Trahms, L. , Smith, F. , Langley, P. , … Koch, H. (2004). Magnetocardiography for pharmacology safety studies requiring high patient throughput and reliability. Journal of Electrocardiology, 37, 187–192. 10.1016/j.jelectrocard.2004.08.055 [DOI] [PubMed] [Google Scholar]
- Steinisch, M. , Torke, P. R. , Haueisen, J. , Hailer, B. , Grönemeyer, D. , Van Leeuwen, P. , & Comani, S. (2013). Early detection of coronary artery disease in patients studied with magnetocardiography: An automatic classification system based on signal entropy. Computers in Biology and Medicine, 43(2), 144–153. 10.1016/j.compbiomed.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Taccardi, B. , & Punske, B. B . (2004). Body surface potential mapping In Zipes D., & Jalife J. (Eds.), Cardiac electrophysiology ‐ Fourth Edition (pp. 803–811). Amsterdam, NL: Elsevier; 10.1016/b0-7216-0323-8/50090-7 [DOI] [Google Scholar]
- Takala, P. , Hänninen, H. , Montonen, J. , Mäkijärvi, M. , Nenonen, J. , Oikarinen, L. , … Katila, T. (2001). Magnetocardiographic and electrocardiographic exercise mapping in healthy subjects. Annals of Biomedical Engineering, 29(6), 501–509. 10.1114/1.1376388 [DOI] [PubMed] [Google Scholar]
- Tavarozzi, I. , Comani, S. , Del Gratta, C. , Di Luzio, S. , Romani, G. L. , Gallina, S. , … De Caterina, R. (2002). Magnetocardiography: Current status and perspectives. Part II: Clinical applications. Italian Heart Journal, 3(3), 151–165. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11974660 [PubMed] [Google Scholar]
- Tavarozzi, I. , Comani, S. , Del Gratta, C. , Romani, G. L. , Di Luzio, S. , Brisinda, D. , … De Caterina, R. (2002). Magnetocardiography: Current status and perspectives. Part I: Physical principles and instrumentation. Italian Heart Journal, 3(2), 75–85. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11926016 [PubMed] [Google Scholar]
- Tolstrup, K. , Madsen, B. E. , Ruiz, J. A. , Greenwood, S. D. , Camacho, J. , Siegel, R. J. , … Smars, P. A. (2006). Non‐invasive resting magnetocardiographic imaging for the rapid detection of ischemia in subjects presenting with chest pain. Cardiology, 106(4), 270–276. 10.1159/000093490 [DOI] [PubMed] [Google Scholar]
- Wu, Y. W. , Lee, C. M. , Liu, Y. B. , Wang, S. S. , Huang, H. C. , Tseng, W. K. , … Wu, C. C. (2013). Usefulness of magnetocardiography to detect coronary artery disease and cardiac allograft vasculopathy. Circulation Journal, 77(7), 1783–1790. 10.1253/circj.CJ-12-1170 [DOI] [PubMed] [Google Scholar]
- Zhou, Z. , Jin, Q. , Yu, L. , Wu, L. , & He, B. (2016). Non‐invasive imaging of human atrial activation during atrial flutter and normal rhythm from body surface potential maps. PLoS ONE, 11(10), 1–14. 10.1371/journal.pone.0163445 [DOI] [PMC free article] [PubMed] [Google Scholar]


