Abstract
Objectives
Vitamin D (VitD) deficiency affects the cardiovascular system via endocrine, paracrine, and autocrine pathways. Limited data are available regarding cardiac autonomic dysfunction in VitD deficiency. The aim of this study was to assess the cardiac autonomic functions by using heart rate recovery index (HRRI) and heart rate variability (HRV) in apparently healthy subjects with VitD deficiency.
Methods
A total of 24 VitD deficient and 50 age‐, gender‐, and body mass index–matched VitD sufficient healthy participants who admitted to outpatient clinics at a tertiary centre were enrolled. All study participants underwent Treadmill exercise test and 24‐hour Holter recording to assess cardiac autonomic functions. HRRIs were calculated by subtracting first, second, and third minute heart rates during recovery period from maximal heart rate.
Results
Mean HRR1 (28.0 ± 8.3 vs 42.8 ± 6.4, P < 0.001), HRR2 (41.1 ± 11.2 vs 60.8 ± 10.4, P < 0.001), and HRR3 (44.9 ± 13.3 vs 65.9 ± 9.8, P < 0.001) were significantly higher in VitD sufficient group compared to VitD deficient group. HRV parameters as, SDNN (P = 0.040), SDANN (P < 0.001), RMSSD (P < 0.001), PNN50 (P < 0.001), and HF (P < 0.001) were significantly decreased in patients with VitD deficiency; but LF (P < 0.001) and LF/HF (P = 0.003) were significantly higher in VitD deficient group. Serum 25(OH)D level was positively correlated with HRRIs (P < 0.001), PNN50, RMSSD, SDANN, and HFnu; negatively correlated with LFnu and LF/HF (P < 0.05). Also, multivariate linear regression analysis showed that serum 25(OH)D level was significantly associated with HRRIs and HRV parameters (P < 0.001).
Conclusion
Our study results suggest that cardiac autonomic functions are impaired in patients with VitD deficiency despite the absence of overt cardiac involvement and symptoms. Further studies are needed to elucidate the prognostic significance and clinical implications of impaired autonomic functions in patients with VitD deficiency.
Keywords: vitamin D deficiency, cardiac autonomic function, heart rate recovery, heart rate variability
Besides its classical hormonal role in bone metabolism, vitamin D (VitD) metabolites also display physiological and pathological effects in nonskeletal tissues by paracrine and autocrine mechanisms.1 The 25‐hydroxyvitamin D (25(OH)D) is usually used as a circulating biomarker of VitD status.2 There were various studies investigating the cardiovascular effects of VitD deficiency.3, 4, 5, 6, 7 It has been known that cardiac autonomic tone was impaired both in cardiac and noncardiac conditions and it was linked to poor prognosis.8, 9, 10, 11 Heart rate recovery index (HRRI) and heart rate variability (HRV) are simplistic techniques to evaluate cardiac autonomic functions in daily practice.12 To our knowledge, there was no study evaluating the association of cardiac autonomic tone assessed by HRRI with VitD deficiency. Additionally, the association of HRV with VitD deficiency has been shown in a few studies.13, 14 Therefore, in this study we aimed to evaluate the cardiac autonomic functions by using HRRI and HRV in apparently healthy subjects with VitD deficiency.
METHODS
Study Population
In this cross‐sectional study, we enrolled a consecutive subset of 74 apparently healthy subjects (34 [45.9%] female; mean age 37.5 ± 6.7 years) who were admitted for routine check‐up and examined at outpatient clinics between June 2013 and August 2013 in Ankara. VitD deficiency is defined as a 25(OH)D below 20 ng/mL (50 nmol/L) according to The Endocrinology Society guidelines.15 Study population was divided into two as VitD sufficient (n = 50, 25(OH)D ≥30 ng/mL) and VitD deficient (n = 24, 25(OH)D <20 ng/mL) groups. Participants with hepatic, renal, or gastrointestinal dysfunction (inflammatory bowel disease and malabsorption), diabetes mellitus, hypertension, alcoholism, malignancy, pregnancy, rhythm abnormalities and structural heart disease including ischaemic heart disease and cardiomyopathy or patients taking drugs that can influence the autonomic nervous system, uncontrolled thyroid or parathyroid disease and individuals using calcium or VitD supplementation or therapies that interfere with VitD metabolism were excluded from the study. Informed consent was taken from each patient before enrolment. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by local ethics committe.
The study was performed in Ankara, Turkey which has a continental climate, with cold, snowy winters (December, January, February) and hot, dry summers (June, July, August). Rainfall occurs mostly during spring and autumn. The average temperature is 28°C (82°F) in summer and the temperatures as high as 42°C (108°F) were recorded.
Demographic and clinical information was recorded on the day of autonomic function assessment. The direct questioning of the symptoms which associated with dysautonomia were orthostatic hypotension, erectile disorder, decreased sweating, abnormal gastrointestinal motility, and impaired urine voiding. Body weight (kg) and height (m) were determined, and body mass index (BMI; kg/m2) was calculated. Additionally, in order to properly demonstrate other factors that may have a impact on VitD deficiency, all subjects were enquired about age, weight, height, wearing style, physical activity (by using subject responses in regard to hours spent on exercise, at work or leisure time), current smoking habits and sunlight exposure (by calculating the average time spent in the sun per day) by a questionnaire.
Assays
Blood samples were taken before the Treadmill test and at the same day through venipuncture and centrifuged within 2 hours after withdrawal. Serum was stored at −80°C. The 25(OH)D concentration was measured with direct ELISA (ImmunDiagnostiK, Bensheim, Germany) with intraassay and interassay coefficient of variations were <4.1% and <3.9%. The concentration of iPTH was determined by use of immunoradiometric assay (Immulite 2000, Diagnostic Products, Los Angeles, CA, USA) with a reference range of 9.5–75 pg/mL. The average intra‐ and interassay CV for iPTH were ≤4.6% and ≤3.8%, respectively.
Electrocardiography and Echocardiography
Twelve lead electrocardiography (ECG) at 25 mm/s (paper speed), treadmill exercise testing, and transthoracic echocardiography equipped with a 3.5‐MHz transducer (Vivid 7, GE Vingmed Ultrasound AS, Horten, Norway) were done in each patient.
Assessment of Autonomic Functions
Heart Rate Recovery Index
To calculate HRRIs, all patients underwent a symptom limited exercise stress test in accordance with the Bruce protocol reaching at least 85% of the age‐predicted heart rates. After achieving peak workload, all patients spent at least 3 minutes of recovery without cool‐down period. The participants maintain the standing position during the recovery period. Heart‐rate recovery indices were calculated by subtracting first‐, second‐, and third‐minute heart rates from the maximal heart rate obtained during stress testing; these results were designated HRR1, HRR2, and HRR3, respectively.16
Heart Rate Variability
Using the ELATEC Holter system, 24‐hour Holter recordings were obtained by using three‐channel analogue recorders. In the heart rate variability (HRV) analysis, the Standard parameters obtained from the time‐domain analysis of HRV including SDNN (Standard deviation of all NN intervals for a selected time period), SDANN (SD of the 5‐minute mean RR intervals tabulated over an entire day), RMSSD (square root of the mean of the sum of the squares of differences between adjacent RR intervals), and PNN50 (the proportion of differences in successive NN intervals greater than 50 ms) were used. Spectral analysis of HRV is obtained by summing powers of each frequency band; high‐frequency (HF) component (0.15–0.40 Hz); low‐frequency (LF) component (0.04–0.15 Hz); and very low‐frequency component (VLF) (0–0.04 Hz). In all patients the LF power/HF power was calculated.17 In the calculation and analysis of HRV parameters, ELATEC Holter software was used.
Statistical Analysis
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) for Windows 20 (IBM SPSS Inc., Chicago, IL, USA). Normal distribution of variables were evaulated with Kolmogorov–Smirnov test. Numerical variables with a normal distribution were presented as the mean ± standard deviation and numerical variables with a skewed distribution were presented as the median (minimum and maximum) and categorical variables were presented as percentages. For numerical variables, an independent sample t test and Mann–Whitney U test were used for intergroup comparisons. Chi‐square test and Fisher's exact chi‐square test were used for comparisons of categorical variables. Univariate linear regression analysis was used to evaluate association of HRRIs and HRV parameters with several clinical and echocardiographic variables. Stepwise multivariate regression analysis was used to evaluate independent association of HRRIs and HRV parameters with VitD deficiency. Correlation was done using Pearson's correlation coefficient (r). A two‐tailed P < 0.05 was considered statistically significant.
RESULTS
Demographic Characteristics
Baseline characteristics of the participants according to VitD status were shown in Table 1. Both study groups were similar in regard to age, BMI, fasting plasma glucose, cholesterol levels, physical acitivity, sun exposure, and wearing clothes which restrict exposure to sunlight. All subjects were normotensives, and no significant differences were observed in resting systolic/diastolic blood pressures and resting heart rates between two groups. Echocardiographic parameters including biventricular and valvular functions were also in normal limits for both study group.
Table 1.
Baseline Clinical and Laboratory Characteristics of the Study Population (n = 74)
| VitD sufficient | VitD deficient | ||
|---|---|---|---|
| (n = 50) | (n = 24) | P | |
| Age, years | 37.2 ± 6.7 | 38.2 ± 6.6 | 0.540 |
| Gender, female, n (%) | 22 (44%) | 12 (50%) | 0.804 |
| BMI, kg/m2 | 22.4 ± 1.3 | 22.0 ± 1.8 | 0.350 |
| Current smokers, n (%) | 9 (18%) | 8 (25%) | 0.543 |
| Physical activity, n (%) | |||
| <15 min/day | 13 (26%) | 8 (33%) | 0.806 |
| 15–30 min/day | 21 (42%) | 9 (37.5%) | |
| >30 min/day | 16 (32%) | 7 (29.2%) | |
| Sun exposure (hours/week) | |||
| Summer | 1.44 ± 0.85 | 1.35 ± 0.85 | 0.687 |
| Wearing clothes which restrict exposure to sunlight, n (%) | 2 (4%) | 2 (4.2%) | 0.698 |
| Heart rate (bpm) | 85.5 ± 12.9 | 86.8 ± 10.9 | 0.670 |
| Systolic blood pressure (mmHg) | 115.6 ± 8.4 | 118.7 ± 7.0 | 0.123 |
| Diastolic blood pressure (mmHg) | 71.0 ± 8.2 | 73.3 ± 7.0 | 0.235 |
| LVEF (%) | 65.9 ± 3.4 | 66.9 ± 2.7 | 0.207 |
| LVEDD (mm) | 47.7 ± 3.5 | 48.1 ± 2.9 | 0.596 |
| LVPWT (mm) | 8.3±0.8 | 8.4±1.0 | 0.799 |
| LVSWT (mm) | 8.0 ± 0.7 | 8.1 ± 0.9 | 0.667 |
| Total cholesterol (mg/dL) | 177.3 ± 29.5 | 169 ± 9.6 | 0.182 |
| Triglyceride (mg/dL) | 131.5 ± 13.5 | 131.2 ± 18.3 | 0.957 |
| LDL‐cholesterol (mg/dL) | 111.4 ± 10.8 | 112.1 ± 8.4 | 0.780 |
| HDL‐cholesterol (mg/dL) | 45.7 ± 8.3 | 48.6 ± 6.3 | 0.130 |
| 25‐OH Vitamin D (ng/mL) | 34.0 ± 6.5 | 13.8 ± 2.3 | <0.001 |
| iPTH (pg/mL) | 32.3 ± 5.7 | 59.5 ± 7.6 | <0.001 |
Numerical variables were presented as the mean ± standard deviation or median (range), categorical variables were defined as percentage. BMI = body mass index; HDL = high‐density lipoprotein; iPTH = intact‐parathyroid hormone; LDL = low‐density lipoprotein; LVEDD = left ventricle end‐diastolic diameter; LVEF = left ventricle ejection fraction; LVPWT = left ventricle posterior wall thickness; LVSWT = left ventricle septal wall thickness.
Exercise Test Parameters
All participants were in sinus rhythm and normal 12‐lead ECG results at rest. Study participants completed the Treadmill exercise test without any complications, ischemic changes and rhythm abnormalities. The parameters used in Treadmill exercise test including HRR indices of the study groups were shown in Table 2. Mean HRR1 (28.0 ± 8.3 vs 42.8 ± 6.4, P < 0.001), HRR2 (41.1 ± 11.2 vs 60.8 ± 10.4, P < 0.001) and HRR3 (44.9 ± 13.3 vs 65.9 ± 9.8, P < 0.001) were significantly higher in VitD sufficient group compared to VitD deficient group (Fig. 1). Also, all study participants were asymptomatic during the postexercise period.
Table 2.
Comparison of Cardiac Autonomic Function Parameters between Study Groups (n = 74)
| VitD sufficient | VitD deficient | ||
|---|---|---|---|
| (n = 50) | (n = 24) | P | |
| Treadmill exercise test parameters | |||
| Baseline heart rate (bpm) | 85.5 ± 12.9 | 86.8 ± 10.9 | 0.670 |
| Maximum SBP (mmHg) | 157.3 ± 12.8 | 153.3 ± 14.5 | 0.227 |
| Maximum DBP (mmHg) | 81.2 ± 6.3 | 83.5 ± 11.1 | 0.250 |
| Exercise test duration (min) | 7.4 ± 2.2 | 7.4 ± 1.5 | 0.990 |
| Maximum heart rate (bpm) | 155.2 ± 9.8 | 157.6 ± 9.4 | 0.325 |
| Peak exercise capacity (METS) | 9.3 ± 2.5 | 9.0 ± 1.8 | 0.646 |
| HRR1 | 42.8 ± 6.4 | 28.0 ± 8.3 | <0.001 |
| HRR2 | 60.8 ± 10.4 | 41.1 ± 11.2 | <0.001 |
| HRR3 | 65.9 ± 9.8 | 44.9 ± 13.3 | <0.001 |
| HRV parameters | |||
| Mean RR | 758 ± 78 | 777 ± 106 | 0.389 |
| Mean heart rate (bpm) | 70.3 ± 9.5 | 71.4 ± 10.5 | 0.656 |
| SDNN (ms) | 83.1 ± 18.4 | 58.3 ± 15.2 | 0.040 |
| SDANN (ms) | 179.2 ± 33.2 | 86.2 ± 36.1 | <0.001 |
| RMSDD (ms) | 70.3 ± 18.4 | 19.9 ± 6.2 | <0.001 |
| PNN50 (%) | 33.4 ± 9.8 | 3.5 ± 1.1 | <0.001 |
| LF n.u. (%) | 55.6 ± 12.7 | 68.6 ± 10.8 | <0.001 |
| HF n.u. (%) | 36.6 ± 13.4 | 18.8 ± 9.6 | <0.001 |
| LF/HF | 1.91 ± 1.15 | 6.37 ± 2.14 | 0.003 |
Numerical variables with a normal distribution were presented as the mean ± standard deviation.
DBP = diastolic blood pressure before exercise; HF = high‐frequency component; HRR = heart rate recovery; LF = low‐frequency component; pNN50 = the number of pairs of adjacent NN intervals differing by more than 50 ms divided by the total number of all NN intervals; RMSSD = the square root of the mean of the sum of the squares of ifferences between adjacent NN intervals; SBP = systolic blood pressure before exercise; SDNN = standard deviations of all NN intervals; SDANN = standard deviation of the averages of NN intervals in all 5‐minute segments of the entire recording.
Figure 1.
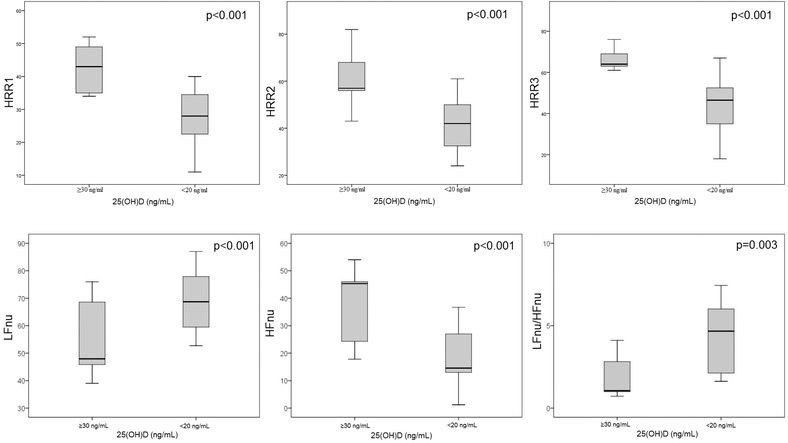
Box plot graph showing the comparison of HRRIs and frequency‐domain analysis of HRV between VitD deficient and sufficient groups.
There were remarkably positive correlations between the 25(OH)D and HRR1 (r = 0.851, P < 0.001), HRR2 (r = 0.787, P < 0.001) and HRR3 (r = 0.685, P < 0.001) (Fig. 2). Additionally, there was no correlation between 25(OH)D and HRRI with BMI (P > 0.05).
Figure 2.
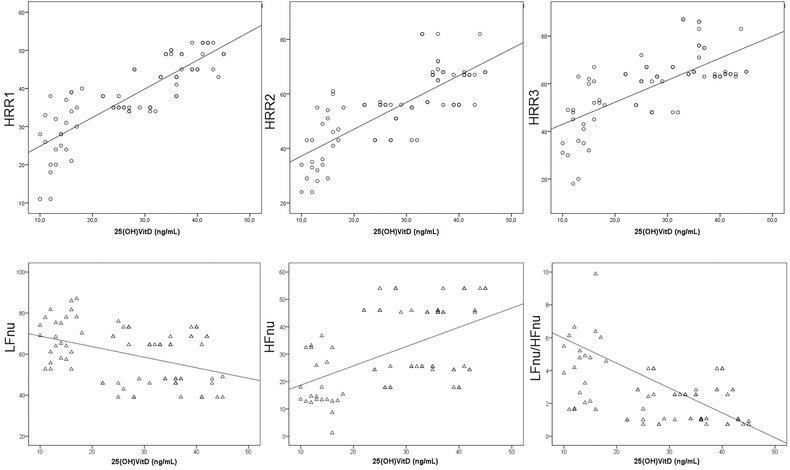
Correlation analysis showing a significantly positive correlation of serum 25(OH)D levels with HRRIs and HFnu, negative correlation of serum 25(OH)D levels with LFnu and LFnu/HFnu.
HRV Parameters
The time‐domain and frequency‐domain parameters of HRV were compared between the VitD deficient and VitD sufficient groups; SDNN (58.3 ± 15.2 vs 83.1 ± 18.4, P = 0.04), SDANN (86.2 ± 36.1 vs 179.2 ± 33.2, P < 0.001), RMSSD (19.9 ± 6.2 vs 70.3 ± 18.4, P < 0.001), PNN50 (3.5 ± 1.1 vs 33.4 ± 9.8, P < 0.001), and HFnu (18.8 ± 9.6 vs 36.6 ± 13.4, P < 0.001) were significantly decreased in patients with VitD deficiency compared with VitD sufficient group, as illustrated in Table 2. However, LFnu (68.6 ± 10.8 vs 55.6 ± 12.7, P < 0.001) and LFnu/HFnu (6.37 ± 2.14 vs 1.91 ± 1.15, P = 0.003) were significantly higher in VitD deficient patients (Fig. 1).
Additionally, there was significantly positive correlation of 25(OH)D with PNN50 (r = 0.719, P < 0.001), RMSSD (r = 0.674, P < 0.001), SDANN (r = 0.617, P < 0.001), and HFnu (r = 0.522, P < 0.001); negative correlation with LFnu (r = −0.419, P < 0.001) and LF/HF (r = −0.278, P = 0.018) (Fig. 2).
Predictors of HRRI and HRV
In univariate linear regression analysis, age, maximum systolic blood pressure and 25(OH)D levels were associated with HRRI and HRV parameters (P < 0.05). BMI was not associated with HRRI and HRV parameters (P > 0.05). However, in stepwise multivariate linear regression analysis, only 25(OH)D level was significantly associated with HRR1 (β = 0.835, P < 0.001), HRR2 (β = 0.769, P < 0.001), and HRR3 (β = 0.689, P < 0.001). For HRV parameters, only 25(OH)D level was significantly associated with LFnu (β = −0.445, P < 0.001), HFnu (β = 0.539, P < 0.001), and LFnu/HFnu (β = −0.255, P = 0.030), age and 25(OH)D level were significantly associated with PNN50, SDANN, and RMSDD (P < 0.05)
Discussion
Main findings of our study included; (a) cardiac autonomic tone assessed by using HRRI and HRV parameters was significantly impaired in apparently healthy subjects with VitD deficiency, (b) serum 25(OH)D levels were positively correlated with HRRIs, PNN50, RMSDD, SDANN, and HFnu; there was significantly negative correlation of serum 25(OH)D levels with LFnu and LFnu/HFnu, (c) serum 25(OH)D level was found as the significant predictor of HRRIs and HRV parameters in the study population. To the best of our knowledge, this is the first study in the literature evaluating cardiac autonomic functions by using HRRI and HRV parameters as a combination in patients with VitD deficiency.
Autonomic nervous system plays a pivotal role in the regulation of cardiovascular functions. It has been known that increased sympathetic and decreased parasympathetic activities are associated with increased risk of sudden death and ventricular arrhythmias. Therefore, assessment of autonomic tone is a potential method for identifying patients at high risk for sudden death. It is difficult to measure and quantify the autonomic modulation. Heart rate, HRV, HRRI, baroreflex sensitivity, heart rate turbulence, and plasma/coronary sinus catecholamine levels can be used to reflect autonomic activity.12 Analysis of HRRI and HRV are noninvasive and inexpensive methods for measurement of cardiac autonomic tone.
Heart rate recovery derived from Treadmill exercise test parameters is one of the commonly used techniques reflecting cardiac autonomic tone. The period of recovery after exercise is accompanied by further dynamic changes in autonomic tone which are clinically characterized by the gradual return of HR to its previous resting level. This period of HRR results from a combination of sympathetic withdrawal and parasympathetic reactivation.12 It has been shown that an abnormal HRRI which was defined as a failure of heart rate to decrease 12 beats or more during the first minute after peak exercise, is an independent predictor of adverse cardiovascular outcomes.18, 19 Additionally Jouven et al.20 found that HRRI less than 25 beats/min at first minute of the recovery period had 2.1 times the risk of sudden death, 0.9 times the risk of nonsudden death, and 1.3 times the risk of death from any cause. Besides HRRIs, also HRV analysi is convenient and safe method used for evaluation of the autonomic nervous system functions. In the time‐domain analysis, SDNN reflects the general measurement of autonomic nervous system balance and PNN50 predominantly reflects the parasympathetic activity. On the other hand, in the frequency‐domain analysis, HF is modulated predominantly by the parasympathetic nervous system, whereas LF is under the influence of both parasympathetic and sympathetic nervous systems. The HF/LF ratio is an indicator of the sympathovagal balance.17
VitD plays a significant role in mineral homeostasis and skeletal health. However, VitD metabolites also exhibit integral physiological roles in nonskeletal tissues including cardiovascular system. Several pathways and cell types that are relevant to cardiovascular physiology and pathology are influenced by VitD metabolites. VitD metabolites act via endocrine, autocrine, and paracrine pathways and modulate vascular endothelial cell function, arterial/myocardial remodelling and maintain immune and inflammatory responses at vascular tissues. It has been shown that there were significant associations between VitD deficiency and cardiovascular pathologies including coronary heart disease, hypertension, left ventricular hypertrophy.1 Also, previous studies showed that patients with low 25(OH)D levels were at increased risk of sudden arrhythmic death compared to other subjects.3, 4 In an animal study, rats fed a VitD deficient diet demonstrated markedly increased ventricular and vascular muscle contractile function with increased sensitivity to norepinephrine, the principal sympathetic neurotransmitter, compared to controls.21. However, there was little evidence for the effects of VitD deficiency on cardiac autonomic functions in clinical practice. Activated VitD is proposed to have the ability to diffuse across the blood–brain barrier, implicating a role for 1,25‐dihydroxy VitD in augmenting autonomic vagal control by binding directly to nuclear VitD receptors in the adrenergic neurons located centrally in the spinal cord and brain tissue.22 In a small sized study, Mann et al.14 showed that participants in the lowest 1,25‐dihydroxy VitD quartile experienced significant withdrawal of inhibitory vagal control, as well as altered overall sympathovagal balance throughout AngII challenge. Also in a recent study, Mann et al.13 reported that VitD supplementation in a 13 healthy subjects VitD deficiency has significantly improved sympathovagal balance. Although the study results were promising for significant effects of VitD deficiency and supplementation on cardiac autonomic functions in healthy subjects, the study findings were limited with very small sample sizes. Although our study results were confirmatory to previous two studies, we included relatively larger sample size and evaluated cardiac autonomic tone by using Treadmill exercise test derived HRRI and 24‐hour Holter analysis derived HRV parameters. In our study, we showed that patients with VitD deficieny had decreased HRRIs, PNN50, SDANN, RMSDD, and HFnu, and increased LFnu and LFnu/HFnu which were indicators of enhanced sympathetic limb activity and reduced parasympathetic limb activity in participants with VitD deficiency.
The main strength of our study is the demonstration of cardiac autonomic dysfunction with several indices in apparently healthy subjects by using HRRI and HRV which were widely available, simple and noninvasive techniques. However, our study has some limitations. First, our study group was consisted of relatively small number apparently healthy participants. Though the study results could not be generalized to whole populations. Second, we did not evaluate the effect of VitD supplementation on impaired HRRIs and HRV in participants with VitD deficiency. Third, the measurement of VitD level was only performed once. We do not know if the results will be consistently the same if two or three samples (in different days of course) would have been taken. Finally, despite the reports showing impairment in cardiac autonomic dysfunctions, clinical implication of those findings was not evaluated in our study. Further prospective studies are needed to elucidate the exact pathophysiologic mechanisms underlying VitD deficiency and clinical implications of impaired autonomic functions in those patients.
In conclusion, VitD deficiency is significantly associated with impaired cardiac autonomic functions assessed by using HRRI and HRV in apparently healthy subjects. When the prognostic significance of HRRI and HRV is considered, patients with VitD deficiency should be followed closely for adverse cardiovascular outcomes. Indices of cardiac autonomic dysfunction may be useful in order to identify high risk patients with VitD deficiency.
This study was not funded by any institution. Authors do not have any conflict of interest.
Disclosure: None.
REFERENCES
- 1. Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res 2014;114(2):379–393. [DOI] [PubMed] [Google Scholar]
- 2. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 3. Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25‐hydroxyvitamin d and 1,25‐dihydroxyvitamin d levels with all‐cause and cardiovascular mortality. Arch Intern Med 2008;168(12):1340–1349. [DOI] [PubMed] [Google Scholar]
- 4. Deo R, Katz R, Shlipak MG, et al. Vitamin D, parathyroid hormone, and sudden cardiac death: Results from the Cardiovascular Health Study. Hypertension 2011;58(6):1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab 2009;94(10):4023–4030. [DOI] [PubMed] [Google Scholar]
- 6. Giallauria F, Milaneschi Y, Tanaka T, et al. Arterial stiffness and vitamin D levels: The Baltimore longitudinal study of aging. J Clin Endocrinol Metab 2012;97(10):3717–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ari H, Ari S, Koca V, et al. A rare cause of reversible dilated cardiomyopathy: Hypocalcemia. Turk Kardiyol Dern Ars 2009;37(4):266–268. [PubMed] [Google Scholar]
- 8. Lazzerini PE, Capecchi PL, Guideri F, et al. Connective tissue diseases and cardiac rhythm disorders: An overview. Autoimmun Rev 2006;5(5):306–313. [DOI] [PubMed] [Google Scholar]
- 9. Bulur S, Turan H, Aslantas Y, et al. Heart rate recovery index in patients with psoriasis. Turk Kardiyol Dern Ars 2012;40(5):400–404. [DOI] [PubMed] [Google Scholar]
- 10. Canpolat U, Dural M, Aytemir K, et al. Evaluation of various cardiac autonomic indices in patients with familial Mediterranean fever on colchicine treatment. Auton Neurosci 2012;167(1–2):70–74. [DOI] [PubMed] [Google Scholar]
- 11. Yorgun H, Canpolat U, Aytemir K, et al. Evaluation of cardiac autonomic functions in patients with systemic lupus erythematosus. Lupus 2012;21(4):373–379. [DOI] [PubMed] [Google Scholar]
- 12. Lahiri MK, Kannankeril PJ, Goldberger JJ Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J Am Coll Cardiol 2008;51(18):1725–1733. [DOI] [PubMed] [Google Scholar]
- 13. Mann MC, Exner DV, Hemmelgarn BR, et al. Vitamin D supplementation is associated with improved modulation of cardiac autonomic tone in healthy humans. Int J Cardiol 2014;172(2):506–508. [DOI] [PubMed] [Google Scholar]
- 14. Mann MC, Exner DV, Hemmelgarn BR, et al. Vitamin D levels are associated with cardiac autonomic activity in healthy humans. Nutrients 2013;5(6):2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holick MF, Gordon CM The Hormone Foundation's: Patient guide to vitamin D deficiency. J Clin Endocrinol Metab 2011;96(7):1–2. [DOI] [PubMed] [Google Scholar]
- 16. Cole CR, Blackstone EH, Pashkow FJ, et al. Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999;341(18):1351–1357. [DOI] [PubMed] [Google Scholar]
- 17. Heart rate variability: standards of measurement, physiological interpretation and clinical use . Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 18. Morshedi‐Meibodi A, Larson MG, Levy D, et al. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). Am J Cardiol 2002;90(8):848–852. [DOI] [PubMed] [Google Scholar]
- 19. Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all‐cause death in asymptomatic women: A 20‐year follow‐up of the lipid research clinics prevalence study. J Am Med Acad 2003;290(12):1600–1607. [DOI] [PubMed] [Google Scholar]
- 20. Jouven X, Empana JP, Schwartz PJ, et al. Heart‐rate profile during exercise as a predictor of sudden death. N Engl J Med 2005;352(19):1951–1958. [DOI] [PubMed] [Google Scholar]
- 21. Weishaar RE, Simpson RU Vitamin D3 and cardiovascular function in rats. J Clin Invest 1987;79(6):1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sternberg Z Autonomic dysfunction: A unifying multiple sclerosis theory, linking chronic cerebrospinal venous insufficiency, vitamin D(3), and Epstein‐Barr virus. Autoimmun Rev 2012;12(2):250–259. [DOI] [PubMed] [Google Scholar]


