Abstract
Introduction
Mortality in hemodialysis (HD) patients is high with significant proportion attributed to fatal arrhythmias. In a pilot study, we showed that intradialytic electrocardiographic (ECG) monitoring can yield stable profiles of selected repolarisation descriptors and heart rate variability (HRV) parameters. This study investigated the relationship of these ECG markers with major adverse cardiac events (MACE) and mortality.
Methods
Continuous ECGs were obtained during HD and repeated five times at 2‐week intervals. The QRS‐T angle calculated as Total Cosine R to T (TCRT) and T‐wave morphology dispersion (TMD) were calculated in overlapping 10 s ECG segments. High‐ (HF) and low (LF)‐frequency components and the LF/HF ratio of HRV were calculated every 5 min. These indices were averaged during the first hour of dialysis and subsequently overall recordings in each subject.
Results
All ECG parameters were available in 72 patients aged 61 ± 15, 23 (31.9%) females and 26 (36.1%) diabetics. After a median follow up of 54.8 months, 16 patients died, 20 were transplanted, and 9 suffered MACE. TCRT (in degrees) was higher and LF/HF was lower in patients who died compared to survivors (112 ± 30 vs. 73 ± 35, p = 0.000 and 0.222 ± 0.418 vs. 0.401 ± 0.274, p = 0.000, respectively) and in MACE positive compared to negative (117 ± 40 vs. 77 ± 34, p = 0.017 and 0.125 ± 0.333 vs.0.401 ± 0.274, p = 0.007 respectively). In multivariate Cox regression analysis of mortality risk adjusted for age, diabetes mellitus, and coronary artery disease, TCRT and LF/HF remained significant predictors (p < 0.05).
Conclusion
QRS‐T angle and HRV may serve risk assessment in future prospective studies in HD patients.
Keywords: arrhythmias, electrocardiogram, heart rate variability, mortality, QRS‐T angle, sudden cardiac death
1. INTRODUCTION
Patients on hemodialysis (HD) have higher mortality compared to Medicare populations with cancer (USRDS, 2017). Arrhythmia and sudden death comprise 40% of known deaths amongst these patients (USRDS, 2017). The underlying pathophysiology of cardiovascular disease in HD patients is related to unique mechanisms associated with advanced chronic kidney disease in addition to traditional risk factors that are commonly present in HD patients (Poulikakos, Banerjee, & Malik, 2013a,2013b). It is postulated that uraemic cardiomyopathy and autonomic imbalance contribute decisively to the heightened cardiovascular risk. Currently, however, there are no effective risk stratification strategies despite the fact that HD patients attend medical facilities regularly three times weekly for a 4‐hr HD treatment.
Research in noninvasive electrophysiology suggested tools for risk stratification purposes. These indices derived from the surface electrocardiogram (ECG) aimed at the characterization of arrhythmogenic substrate (Zabel et al., 2000) and cardiac autonomic dysregulation (Sassi et al., 2015). We have previously conducted a pilot study using continuous ECGs during five dialysis treatments in each HD participant and showed that selected repolarisation descriptors (Poulikakos et al., 2013a,2013b) and spectral heart rate variability (HRV) parameters (Poulikakos, Malik, & Banerjee, 2014) exhibited sufficient intrasubject stability making them suitable for risk stratification purposes.
In this study, we followed up the same cohort of patients for major cardiac events (MACE) and for total mortality in order to assess the predictive value of the selected ECG descriptors.
2. METHODS
2.1. Patients and Follow up
The characteristics of the study population have been previously described (Poulikakos et al., 2013a,2013b). In brief, patients established on maintenance thrice weekly HD for a minimum of 3 months who presented in sinus rhythm were recruited from the hospital and satellite HD units at St George's Healthcare NHS Trust. Patients were excluded if they had experienced a cardiovascular complication within the last year and/or if diagnosed with active malignancy or active infection. The study obtained ethical approval and all subjects provided informed written consent. Patients were followed up for total mortality and MACE defined as acute coronary syndrome, coronary revascularization, admission due to heart failure or arrhythmia, or sudden cardiac death.
2.2. ECG acquisition
Continuous Holter electrocardiograms (ECG) 12‐lead electrocardiograms recorded with the Mason‐Likar electrode configuration were obtained using CardioMem® CM 3000‐12 (Getemed, Teltow, Brandenburg Germany). The sampling rate for ECG digitization was 1,024 Hz. The recordings started at the beginning and stopped at the end of the 4‐hr HD session. The intradialytic ECGs were repeated every 2 weeks for altogether five times in each patient.
2.3. ECG analysis
2.3.1. Heart rate variability
The spectral analysis was performed by fast Fourier transformation with Hanning window using the software of the Holter analyzer (Getemed, CardioDay®, 2006) and corresponded to previously published standards (Electrophysiology, 1996). The relative risk (RR) interval tachogram was derived from the RR interval time series and sampled at 1,024 Hz within individual 5‐min windows using linear interpolation. The low frequency (LF, 0.04 Hz to 0.15 Hz) and high frequency (HF, 0.15 Hz to 0.40 Hz) components were calculated from the derived spectrum. Average values of LF, HF, and LF/HF over the first hour of the recording were used for the analysis after decadic logarithmic transformation to normalize the distribution of the data.
2.3.2. Repolarization descriptors
All digital ECG files were automatically analyzed using custom written software package. The QRS‐T angle calculated as Total Cosine R to T (TCRT) and the so‐called T‐wave morphology dispersion (TMD) were calculated every 5 s in representative QRS‐T complexes of overlapping 10‐s ECG segments using a custom written software package that implemented previously published methods (Acar, Yi, Hnatkova, & Malik, 1999). TCRT is a measure of the vectorial deviation between depolarization and repolarization waves and is calculated as average of cosines of the angles between the three‐dimensional T wave and QRS loop vectors. The three‐dimensional vectorial representation of the electrical signal is accomplished with application of singular value decomposition to the eight independent surface ECG leads to produce a system of three independent orthogonal leads that contain 99% of the ECG energy. The TCRT technology has been shown to offer risk prediction advantages compare to the other possibilities of QRS‐T angle measurement (Hnatkova et al., 2017).
Mean values during the first hour and the last hour of the repeated recordings were used to confirm their intrasubject stability with repeated measures ANOVA (Poulikakos et al., 2013a,2013b). The average value over the first hour of all the repeated recordings for each patient was used for the outcome analysis for this study. The TCRT is an expression of spatial QRS‐T angle and reflects global repolarization heterogeneity and TMD reflects the morphologic differences of the T‐wave patterns between different ECG leads. For the purposes of this analysis TCRT was converted to degrees.
2.4. Statistical analysis
The intrasubject stability of repolarization descriptors and spectral HRV parameters averaged during the first and last hour of recordings was previously tested with Repeated Measures Anova and confirmed reproducibility of all measured parameters (Poulikakos et al., 2013a,2013b, 2014). For the purposes of this analysis, the average values over the first hour were included in the outcome analysis. Two sided independent t test and Mann–Whitney U test were used where appropriate for comparisons between dead and alive and MACE positive and negative subjects; p < 0.05 was considered statistically significant. Subsequently, every variable found statistically significantly different between the two groups was entered into univariate Cox regression analysis as categorical value dichotomized at the median to model its relationship with the timing of events for both mortality and MACE during follow up. Cox regression analysis for TCRT was also performed using 100 degrees as cut off value for dichotomization based on published data reporting a specificity of 85.0% to predict sudden cardiac death in the general population (Porthan et al., 2013). Univariable Cox Regression analysis was also performed to assess mortality risk prediction based on age, presence of diabetes mellitus, and history of coronary artery disease. Multivariable Cox regression analysis was subsequently performed with the variables that were statistically significant in the univariate analysis for total mortality but not for MACE because of the small number of events. In view of the previously reported correlation between TCRT and HRV (Poulikakos, Banerjee, & Malik, 2015) they were considered auto correlated values and were not entered together in the multivariable analysis.
Kaplan–Meier event probability curves were generated by dichotomizing the TCRT and LF/HF values at their population median as well as at 100 degrees of TCRT (Porthan et al., 2013) (10). The Kaplan–Meier event probability curves were computed together with their empirical inter‐quartile ranges and 10%–90% confidence intervals using bootstrap with 10,000 repetitions. Patients receiving kidney transplant were censored at the transplantation time. Statistical analysis was performed using IBM SPSS statistics 23.
3. RESULTS
From a total of 81 recruited patients, four did not have available data for repolarization descriptors and five developed atrial fibrillation and were excluded from the HRV analysis. There were 72 patients with available ECG data for both HRV and repolarization descriptors. They were aged 61 ± 15, 23 (31.9%) were females, 26 (36.1%) were diabetics and 16 (22.2%) had a history of coronary artery disease. The median time on dialysis was 24 months (range 5–190 months). After a median follow up of 54.8 months (range 5.2–72.1 months) 16 patients died, 20 were transplanted, and nine suffered MACE. TCRT expressed in degrees was higher and LF/HF was lower in dead and MACE positive patients compared to alive and MACE negative patients and TMD was higher in MACE positive patients compared MACE negative (59 ± 25 vs. 26 ± 18 respectively, p = 0.033; Table 1). There was no difference in LF and HF between those who died and survived and between MACE positive and negative patients. Comparison between patients who received kidney transplantation and those who died showed that transplanted patients had lower TCRT and TMD (70 ± 27 vs. 110 ± 31 p = 0.000 and 16 ± 11 vs. 45 ± 27, p = 0.000 respectively) and higher LF/HF (0.489 ± 0.22 vs. 0.145 ± 0.226 p = 0.000) but there was no statistical difference for LF and HF (−5.21 ± 0.42 vs. −5.26 ± 0.44, p = 0.752 and −5.59 ± 0.44 vs. −5.34 ± 0.49, p = 0.127 respectively). On univariable Cox regression analysis for mortality TCRT and LF/HF dichotomised at their median values and TCRT dichotomized at 100 degrees were statistically significant. Kaplan–Meier survival curves for patients stratified by TCRT and LF/HF above and below median are presented in Figures 1 and 2 respectively. Univariable Cox regression analysis for all‐cause mortality including demographic and clinical data were statistically significant for age with RR 1.06 confidence Interval (CI), 1.01–1.10, p = 0.004, for diabetes with RR of 3.44 (CI 1.25–9.47, p = 0.017) and for history of coronary artery disease with RR of 3.21 (CI 1.19–8.63, p = 0.021). In multivariable Cox regression analysis for all cause mortality including age, presence of diabetes, coronary artery disease and TCRT dichotomized at median value, TCRT and age remained significant (RR 4.71, CI 1.01–21.87, p = 0.048 and RR 1.05, CI 1.00–1.10, p = 0.22, respectively). In multivariable Cox regression analysis for mortality including age, presence of diabetes, coronary artery disease and LF/HF dichotomized at median value, LF/HF and age remained significant (RR 0.205, CI 0.04–0.98, p = 0.048 and RR 1.05 CI 1.00–1.10, p = 0.029, respectively).
Table 1.
Comparisons of T‐wave morphology parameters and spectral indices of Heart Rate Variability between dead and alive patients and event positive and negative patients for major cardiac events
| Mortality | Major cardiac events | |||||
|---|---|---|---|---|---|---|
| ECG variable | Alive (n = 56) | Dead (n = 16) | p value | No (n = 63) | Yes (n = 9) | p value |
| TCRT | 73 ± 35 | 112 ± 30 | 0.000 | 77 ± 34 | 117 ± 40 | 0.017 |
| TMD | 25 ± 18 | 47 ± 27 | 0.156 | 26 ± 18 | 59 ± 25 | 0.033 |
| LF | −5.19 ± 0.44 | −5.24 ± 0.44 | 0.744 | −5.18 ± 0.43 | −5.37 ± 0.46 | 0.224 |
| HF | −5.53 ± 0.51 | −5.34 ± 0.49 | 0.098 | −5.51 ± 0.52 | −5.43 ± 0.53 | 0.703 |
| LF/HF | 0.4013 ± 0.274 | 0.222 ± 0.418 | 0.000 | 0.401 ± 0.274 | 0.125 ± 0.333 | 0.007 |
ECG: Electrocardiogram; HF: high frequency component (0.15 Hz to 0.40 Hz) of Heart Rate Variability (HRV) in absolute values; LF: low frequency component (0.04 Hz to 0.15 Hz) of HRV in absolute values; LF/HF: ratio between LF and HF; n: number of subjects; TCRT: Total Cosine of R to T; TMD: T‐wave Morphology Dispersion.
LF, HF, and LF/HF values are presented after decadic logarithmic transformation. All numerical data are expressed as mean ± SD. Shaded areas highlight statistically significant differences.
Figure 1.
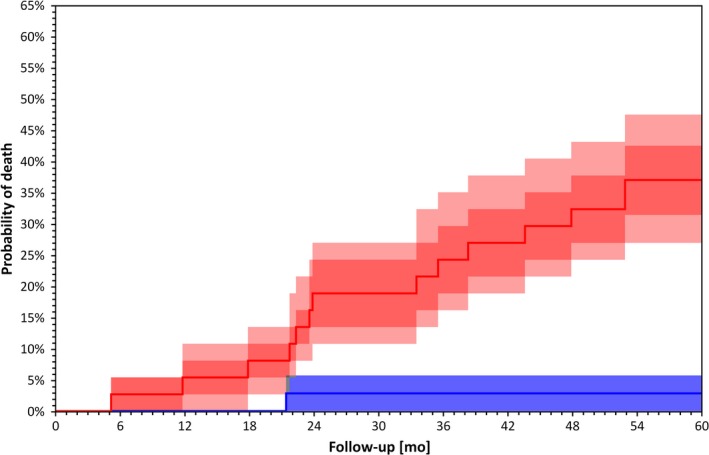
Kaplan–Meier survival curves for patients stratified by TCRT below (blue) and above (red) median value (p = 0.001 by Log Rank test). Darker bands are interquartile ranges, the lighter bands are the ranges between 10th and 90th percentile. These confidence intervals were calculated using bootstrap with 10,000 repetitions
Figure 2.
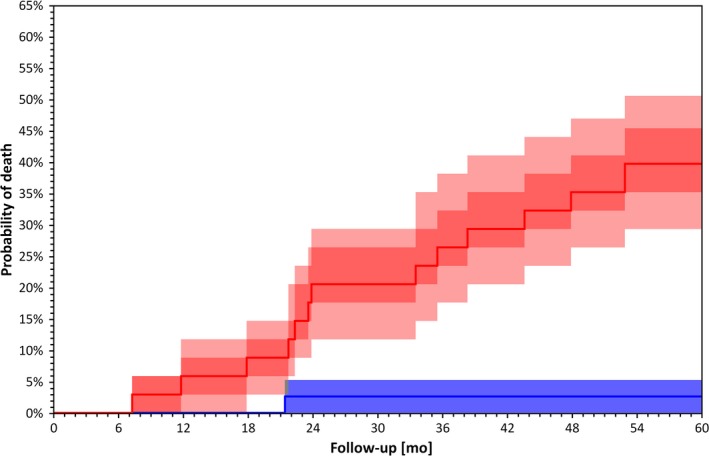
Kaplan–Meier survival curves for patients stratified by LF/HF above (blue) and below (red) median value (p = 0.000 by Log Rank test). Darker bands are interquartile ranges, the lighter bands are the ranges between 10th and 90th percentile. These confidence intervals were calculated using bootstrap with 10,000 repetitions
On univariable Cox regression analysis for MACE, TCRT was statistically significant when dichotomized at 100 degrees but was not significant when dichotomized at median value. Kaplan–Meier event probability curves for MACE for patients stratified by TCRT above and below 100° is shown in Figure 3. On univariate Cox regression analysis, TMD and LF/HF dichotomised at median values were not statistically significant for MACE (Table 2).
Figure 3.

Kaplan–Meier event probability curves for Major Cardiac Event (MACE) for patients stratified by TCRT above (red) and below (blue) 100° (p = 0.001 by Log Rank test). Darker bands are interquartile ranges, the lighter bands are the ranges between 10th and 90th percentile. These confidence intervals were calculated using bootstrap with 10,000 repetitions
Table 2.
TCRT and LF/HF and risk for all‐cause mortality and major cardiac events from Univariate Cox Regression Analysis
| Mortality | Major cardiac events | |||
|---|---|---|---|---|
| ECG Variable | Relative risk (95% CI) | p value | Relative risk (95% CI) | p value |
| TCRT (median)a | 7.68 (1.74–33.85) | 0.007 | 7.91 (0.97–64.45) | 0.053 |
| TCRT (100°)b | 4.60 (1.68–12.70) | 0.003 | 9.02 (1.80–45.02) | 0.007 |
| LF/HFc | 0.110 (0.025–0.786) | 0.004 | 0.255 (0.051–1.265) | 0.94 |
| TMD | N/A | 7.84 (0.96–63.78) | 0.54 | |
CI: Confidence Interval; ECG: Electrocardiogram; HF: high frequency component (0.15 Hz to 0.40 Hz) of Heart Rate Variability (HRV) in absolute values; LF: low frequency component (0.04 Hz to 0.15 Hz) of HRV in absolute values; LF/HF: ratio between LF and HF; TCRT: Total Cosine of R to T; TMD: T‐wave morphology dispersion. Shaded areas highlight statistically significant differences.
TCRT dichotomized at median value.
TCRT dichotomized at 100°.
LF/HF dichotomized at median value.
4. DISCUSSION
This follow‐up of the stability pilot study shows that QRS‐T angle calculated as TCRT and LF/HF from intradialytic ECG monitoring predicted risk of death and major cardiovascular events in our cohort of HD patients. The ECG recordings were performed during the regular dialysis treatment and did not require an additional hospital visit.
The predictive value of TCRT is in line with a previous retrospective study that calculated the QRS‐T angle in 277 incident Caucasians HD patients and showed an association between abnormal QRS‐T angle and mortality and sudden cardiac death (De Bie et al., 2012) and with a recent prospective study in 358 incident HD patients from US that showed an association between abnormal QRS‐T angle and cardiovascular mortality and sudden cardiac death (Tereshchenko et al., 2016). In the first study (De Bie et al., 2012), the investigators applied software that used inverse Dower matrix to construct the vectorcardiogram from routinely collected snapshot digital ECGs, calculated the angle between the mean QRS and T vectors and defined abnormal spatial QRS‐T angle as >116° for females and >130° for male subjects. In the second study (Tereshchenko et al., 2016), the investigators used unfiltered averaged xyz orthogonal ECG signal derived from standard x, y, and z orthogonally placed leads for the measurement of spatial QRS‐T angle that was calculated as the angle between spatial mean QRS vector and spatial peak T vector and a cut off of 75° was determined based on receiver operating characteristic curves. The difference in cut‐off values can be explained by the different methods of calculation of the spatial QRS‐T angle (Hnatkova et al., 2017). Our results confirm the predictive value of a cut off of approximately 100° (total cosine R to T –0.21 = 102.12°) derived from 5618 adults in general population with a predefined specificity of 85% (Porthan et al., 2013).
In this study, only LF/HF was significantly associated with mortality and major cardiac event amongst the calculated spectral HRV parameters but we did not observe statistically significant differences in LF and HF. In a previous Japanese study of 333 HD patients that used 24 hr ambulatory ECG monitoring all spectral HRV parameters predicted mortality and cardiac death (Oikawa et al., 2009). In another study of 120 Japanese HD patients, only LF/HF amongst all spectral HRV parameters predicted death but not cardiovascular mortality (Fukuta, 2003). Finally, in another Japanese study involving 281 HD patients with 24‐hr Holter ECG all conventional HRV parameters were different between dead and alive subjects (Suzuki et al., 2012). The differences in the predictive value of the HRV parameters we report may be explained by the small number of patients included in our study that may not have been sufficient to show differences in LF and HF in absolute values.
In our study, patients who received a kidney transplant (20) had lower QRS‐T angle by TCRT and higher LF/HF compared to patients who died (16) indicating that transplanted patients had less aberrant ECG risk profiles. Our survival analysis was performed with censoring at time of transplantation thus removing from the survival data systematically fitter and younger patients who are likely to have better survival prospects compared with the remaining population. This approach can underestimate the survival differences in the total population, especially if the transplant rate is high, and should be taken into consideration when interpreting the survival analysis.
5. LIMITATIONS
The main limitation is the small number of participants. This study was designed to assess reproducibility of selected ECG descriptors and was not powered to detect mortality differences. Secondly, although we aimed at assessing cardiac autonomic regulation from short term recordings, we did not include autonomic postural provocations that are likely to improve the predictive value of spectral HRV parameters (Wellens et al., 2014). Finally we did not include echocardiographic assessment and measurement of arterial stiffness.
6. CLINICAL IMPLICATIONS
QRS‐T angle is a promising descriptor of uraemic cardiomyopathy that can be calculated from a standard 12 lead ECG that is routinely collected in maintenance dialysis patients. Different groups have used different methods for its calculation and expectedly report different cut‐off values. There is a need to standardize the measurement of QRS‐T angle in order to increase our knowledge about its characteristics in large populations of HD patients, define normal and abnormal values and characterize longitudinal pattern changes that may be associated with increased cardiac risk. QRS‐T angle has the potential to be included as a cardiac risk marker in clinical practice to guide to support the clinical evaluation and management of cardiovascular disease in HD patients.
HRV appears to measure cardiac autonomic dysregulation which is particularly relevant in HD patients. However, HRV measurements require meticulous environmental and methodological standardization and substantial postprocessing analysis to yield reliable results that so far pose challenges in incorporating HRV measurement particularly from long‐term (e.g., 24 hr) ECG recordings in clinical practice. Future studies should focus on short term HRV measurements with standardized provocations around the dialysis treatment aiming at simplifying the process of signal analysis and optimizing the measurements.
7. CONCLUSION
QRS‐T angle calculated as TCRT and HRV measurement from short term recordings may have a value in risk stratification in HD patients and should be prospectively assessed. Standardized autonomic provocations are likely to improve the predictive value of short term spectral HRV parameters. Future collaborative work in digital ECG databases may be useful to expand our knowledge on QRS‐T angle and its calculation for risk stratification purposes in HD population.
ACKNOWLEDGEMENTS
Supported in part by the British Heart Foundation New Horizons Grant NH/16/2/32499.
Poulikakos D, Hnatkova K, Banerjee D, Malik M. Association of QRS‐T angle and heart rate variability with major cardiac events and mortality in hemodialysis patients. Ann Noninvasive Electrocardiol. 2018;23:e12570 10.1111/anec.12570
REFERENCES
- Acar, B. , Yi, G. , Hnatkova, K. , & Malik, M. (1999). Spatial, temporal and wavefront direction characteristics of 12‐lead T‐wave morphology. Medical & Biological Engineering & Computing, 37(5), 574–584. 10.1007/bf02513351 [DOI] [PubMed] [Google Scholar]
- De Bie, M. K. , Koopman, M. G. , Gaasbeek, A. , Dekker, F. W. , Maan, A. C. , Swenne, C. A. , … Jukema, J. W. (2012). Incremental prognostic value of an abnormal baseline spatial QRS‐T angle in chronic dialysis patients. EP Europace, 15(2), 290–296. 10.1093/europace/eus306 [DOI] [PubMed] [Google Scholar]
- Electrophysiology, T. F. (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation, 93(5), 1043–1065. 10.1161/01.cir.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Fukuta, H. (2003). Prognostic value of heart rate variability in patients with end‐stage renal disease on chronic haemodialysis. Nephrology Dialysis Transplantation, 18(2), 318–325. 10.1093/ndt/18.2.318 [DOI] [PubMed] [Google Scholar]
- Getemed, CardioDay® (2006). CardioDay®, holter ECG analysis software, user manual (REF 90270‐US 0505S1‐LAB‐Rev‐C‐GA‐CardioDay‐2‐0‐ENG_US.doc 12/16/2006). Getemed Medizin‐ und Informationstechnik AG.
- Hnatkova, K. , Seegers, J. , Barthel, P. , Novotny, T. , Smetana, P. , Zabel, M. , … Malik, M. (2017). Clinical value of different QRS‐T angle expressions. EP Europace, Advance online publication. 10.1093/europace/eux246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa, K. , Ishihara, R. , Maeda, T. , Yamaguchi, K. , Koike, A. , Kawaguchi, H. , … Itoh, H. (2009). Prognostic value of heart rate variability in patients with renal failure on hemodialysis. International Journal of Cardiology, 131(3), 370–377. 10.1016/j.ijcard.2007.10.033 [DOI] [PubMed] [Google Scholar]
- Porthan, K. , Viitasalo, M. , Toivonen, L. , Havulinna, A. S. , Jula, A. , Tikkanen, J. T. , … Oikarinen, L. (2013). Predictive value of electrocardiographic T‐wave morphology parameters and T‐wave peak to T‐wave end interval for sudden cardiac death in the general population. Circulation: Arrhythmia and Electrophysiology, 6(4), 690–696. 10.1161/circep.113.000356 [DOI] [PubMed] [Google Scholar]
- Poulikakos, D. , Banerjee, D. , & Malik, M. (2013a). T wave morphology changes during hemodialysis. Journal of Electrocardiology, 46(6), 492–496. 10.1016/j.jelectrocard.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Poulikakos, D. , Banerjee, D. , & Malik, M. (2013b). Risk of sudden cardiac death in chronic kidney disease. Journal of Cardiovascular Electrophysiology, 25(2), 222–231. 10.1111/jce.12328 [DOI] [PubMed] [Google Scholar]
- Poulikakos, D. , Banerjee, D. , & Malik, M. (2015). Repolarisation descriptors and heart rate variability in hemodialysed patients. Physiological Research, 64, 487–493. [DOI] [PubMed] [Google Scholar]
- Poulikakos, D. , Malik, M. , & Banerjee, D. (2014). Sex‐dependent association between heart rate variability and pulse pressure in haemodialysis patients. Nephron Clinical Practice, 128(3–4), 361–366. 10.1159/000368436 [DOI] [PubMed] [Google Scholar]
- Sassi, R. , Cerutti, S. , Lombardi, F. , Malik, M. , Huikuri, H. V. , & Peng, C. (2015). Advances in heart rate variability signal analysis: Joint position statement by the e‐Cardiology ESC Working Group and the European Heart Rhythm Association co‐endorsed by the Asia Pacific Heart Rhythm Society. Europace, 17(9), 1341–1353. 10.1093/europace/euv015 [DOI] [PubMed] [Google Scholar]
- Suzuki, M. , Hiroshi, T. , Aoyama, T. , Tanaka, M. , Ishii, H. , Kisohara, M. , … Hayano, J. (2012). Nonlinear measures of heart rate variability and mortality risk in hemodialysis patients. Clinical Journal of the American Society of Nephrology, 7(9), 1454–1460. 10.2215/cjn.09430911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereshchenko, L. G. , Kim, E. D. , Oehler, A. , Meoni, L. A. , Ghafoori, E. , Rami, T. , … Parekh, R. S. (2016). Electrophysiologic Substrate and Risk of Mortality in Incident Hemodialysis. Journal of the American Society of Nephrology, 27(11), 3413–3420. 10.1681/asn.2015080916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USRDS (2017). Annual data report, US renal data system (mortality, chapter 5). Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; https://www.usrds.org/2017/view/v2_05.aspx [Google Scholar]
- Wellens, H. J. , Schwartz, P. J. , Lindemans, F. W. , Buxton, A. E. , Goldberger, J. J. , Hohnloser, S. H. , … Wilde, A. A. (2014). Risk stratification for sudden cardiac death: Current status and challenges for the future. European Heart Journal, 35(25), 1642–1651. 10.1093/eurheartj/ehu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel, M. , Acar, B. , Klingenheben, T. , Franz, M. R. , Hohnloser, S. H. , & Malik, M. (2000). Analysis of 12‐lead T‐wave morphology for risk stratification after myocardial infarction. Circulation, 102(11), 1252–1257. 10.1161/01.cir.102.11.1252 [DOI] [PubMed] [Google Scholar]


