Abstract
Background
The role of underlying mechanisms of yogic strategies which exert beneficiary effects on cardiac autonomic control is poorly understood. We have performed heart rate variability (HRV) analysis on subjects performing yogic methods and control subjects who mimic them through paced breathing and focused attention tasks using external cues.
Methods
Heart rate (HR) time series is generated from electrocardiogram measured from subjects of yogic group (YG); performing yogic practices (n = 15), paced breathing group (PBG); involved in breathing exercises cued at breathing rates (BR) from 3 to 15 cycles per minute (cpm) (n = 23), normal breathing group (NBG) under regular breathing (n = 15), and subjects performing three different cognitive tasks designated as focused attention group (FAG), (n = 24). HRV is analyzed using coherence plots, spectrograms, HRV parameters, and instantaneous frequency recurrence plots (IFRP).
Results
HRV is similar among YG and PBG (at BR <12 cpm) and significantly different for YG vs. NBG (p < 0.001) and PBG vs. NBG (p < 0.001). Regularity of breathing oscillations observed in HR is quantified using IFRP and is identical among FAG, PBG, and YG and significantly different for YG vs. NBG (p < 0.01), PBG vs. NBG (p < 0.01), and FAG vs. NBG (p < 0.05).
Conclusions
Low‐frequency breathing (BR <12 cpm) plays a primary role in eliciting physiologically significant changes in HRV. By identifying a similarity in breathing oscillations of HR of FAG, YG, and PBG, the results recognize the coexistence of attention and breathing strategies and postulate their joint role in sustaining autonomic benefits, while effects induced by breathing alone on HRV could be attained even intermittently.
Keywords: focused attention, HRV, paced breathing, yogic methods
1. INTRODUCTION
The two main constituents of the autonomic nervous system, namely the sympathetic and parasympathetic controls work in a manner opposite to each other and as a consequence of their net effect, a regulation is achieved on certain physiological parameters such as heart rate (HR). Heart rate variability (HRV) measures have been used as noninvasive assessments of cardiac autonomic tone by quantifying their beat‐to‐beat variations (Acharya, Joseph, Kannathal, Lim, & Suri, 2006; Shaffer & Ginsberg, 2017). Parasympathetic dominance and increase in the magnitude of breathing oscillations on heart rates termed as respiratory sinus arrhythmia (RSA) have been reported in subjects practicing relaxation techniques like yoga (Bernardi et al., 2001; Cysarz & Bussing, 2005; Cysarz et al., 2004; Peng et al., 1999) and biofeedback training methods (Lehrer, Vaschillo, & Vaschillo, 2000) when compared against control subjects under rest condition. The presence of RSA patterns in the heart rate time series is ensured to enhance pulmonary gas exchange and baro‐reflex sensitivity (Hayano & Yasuma, 2003; Hirsch & Bishop, 1981).Yoga is a holistic approach which includes physical exercises, meditation, and spiritual practices to provide a wholesome wellness for an individual. Yogic methods claim to control bodily functions like heart rate by controlling the mind (Saraswati, 2006). On the other hand, the biofeedback training comprises deliberate exercises on breathing using external cues to control the heart rate. A study (Cysarz & Bussing, 2005) has reported that when RSA (or the parasympathetic dominance) could be created at will by breathing exercises (like in biofeedback training), the physiological impacts exerted by yoga could also be attained intermittently even by inexperienced subjects without a need for a special long‐term training. The involvement of the process of breathing to control the heart rate (HR) in yoga is known and is also conspicuous through various breath synchronized actions or protocols existing in yogic traditions (Saraswati, 2006). However, the exact mode of control of breathing in yogic methods is not clear to classify them as a volitional or a behavioral control. Similarly, yogic methods also use focused attention as an approach to concentrate on bodily functions. Studies which exclusively highlight the underlying mechanisms which exert significant variations in HRV with experimental validations have been only sparingly discussed (Steffen, Austin, DeBarros, & Brown, 2017). This may in part be due to lack of comprehensive assessments existing in the literature to probe the underlying psycho‐physiological mechanisms involved in yogic methods, while the physiological benefits which one could achieve by practicing yoga have been widely documented (Bernardi et al., 2001; Bertisch, Hamner, Tan, & Taylor, 2013; Muralikrishnan, Balakrishnan, Balasubramanian, & Visnegarawla, 2012; Telles, Nagarathna, & Nagendra, 1995; Telles & Vani, 2002). The prime objective of this study was to understand yogic strategies in a better way by studying their individual roles on HRV of nonpractitioners who could mimic the performance of regular practitioners using external cues. By recreating and comparing the heart rate variations of yogic subjects against control subjects, our work arrives at a conclusion that although the process of breathing as a strategy plays a primary role in the control of heart rate, the coexistence of attention might help in sustaining the autonomic benefits by psycho‐physiologically training an individual.
2. METHODS
2.1. Study population
Subjects recruited for this study were healthy young adults (59 Male and 8 female) with a mean age of 23 ± 11 years. All the subjects gave written informed consent in taking part in this investigation. Four study groups were involved in this study namely yogic group (YG), normal breathing group (NBG), paced breathing group (PBG), and focused attention group (FAG). In all the groups, ECG was measured from subjects in a comfortably sitting position using an electroencephalogram (EEG) system which can also function as a general purpose bio‐potential recorder (Compumedics Ltd., Vic., Australia). Subjects recruited for YG were sitting over a mat kept on the floor in their characteristic posture meant for meditation. Discrete Ag‐AgCl electrodes were fixed to configure ECG in Lead‐1, V1, and V2 positions. ECG measurements in all the four groups were recorded for about 10 min, and the signals were digitized with an analog‐to‐digital converter with a resolution of 24 bits at a sampling rate of 1,000 Hz and a bandwidth setting of 0–100 Hz. One among the three ECG channels which was noise‐free and showing prominent R‐wave peak was taken for HRV analysis.
A group of regular practitioners of yoga involved in performing specific yogic practice were recruited for the YG (n = 5). ECG was measured from these subjects while they were performing Ajapa japa meditation. Ajapa japa deals with internal repetition of melodically structured verses or hymn called mantras syllable‐by‐syllable and paying attention to its meaning (Gonda, 1963) for a total period of about 10 min initiated by an experienced yoga master. Apart from the subjects who were already recruited for YG (n = 5), R‐R interval data sets of subjects (n = 10) following Kundalini and Chi meditation sects were downloaded from the Physionet database (Goldberger et al., 2000) containing the original work of Peng et al. (1999) were pooled to YG. Normal breathing group (NBG) consisted of subjects (n = 15) under rest condition and breathing normally at their own rates. PBG encompassed volunteers (n = 23) performing paced breathing exercises guided by an external arrangement. They were instructed to breathe at specific paced rates using an external visual cue derived from a Arduino Uno microcontroller‐based programmable kit (SparkFun Electronics, Niwot, CO, USA) to control the glowing of two LEDs of two different colors (to give a signal to the subject for inhalation and exhalation) in an alternate fashion with a user adjustable delay for a total duration of ~10 min. This procedure helped to record and analyze the R‐R interval data of subjects, whose breathing was deliberately paced at pacing rates of 3–6 cycles per minute (cpm) (n = 7), 7–10 cpm (n = 8) and 12–15 cpm (n = 8) in different experimental runs. FAG included subjects (n = 24) who were involved in cognitive tasks with a stimulus presentation computer (Compumedics Ltd.) which formed the part of the EEG system. Three tasks (Todd, 2005) were given to three different subgroups of subjects under three experimental sessions (n = 8 in each). The first set of subjects was given 1‐back paradigm task in which they were presented an image sequence with geometric shapes arranged on the four sides of the screen. If the current image matched (with reference to shape of the objects and their spatial locations) with the previously shown image, the subjects were asked to elicit a response by pressing a response pad. In the second set of attention tasks, the subjects were given an odd ball paradigm experiment, in which they were presented a low‐frequency auditory tone which was interrupted infrequently by a high‐frequency tone. Whenever this deviant tone occurred, the subjects were asked to deliver a response. Subjects in the third subgroup were asked to visually track and merge moving colored dots on the screen (which were under the control of subjects by moving the mouse) inside randomly moving circles of the same color. Tasks in FAG were designed to keep the subjects attentively engaged in different ways (i.e., visual/auditory/ motor tracking). The subgroups of FAG with three different tasks were referred as FAG‐1, FAG‐2, and FAG‐3, respectively. Subjects in FAG were not given any instruction about breathing and were unaware of any yogic method. Subjects of each group were new, and they did not take part in other study groups.
2.2. Signal processing and analysis
DC offset and baseline drift which corrupted the ECG traces were eliminated, and the R‐wave peaks were identified on a beat‐by‐beat basis using an automated algorithm (Sengottuvel et al., 2012). The R‐R interval series was generated from the time differences of the identified R‐peak instants. The R‐R interval series was interpolated with cubic spline technique and uniformly resampled with a higher sampling rate of 4 Hz. A second‐order Butterworth high‐pass filter with a cutoff frequency of 0.01 Hz was used to filter the R‐R time series following a procedure discussed in the literature for HRV analysis (Carvalho et al., 2003). Processed R‐R interval of the four study groups were analyzed based on different approaches, namely coherence, spectrograms, classical HRV parameters, and instantaneous frequency recurrence plots. Student's t test was performed to reject the null hypothesis that the differences in HRV parameters of YG (n = 15), PBG‐3 cpm (n = 7), PBG‐7 cpm (n = 8), PBG‐12 cpm (n = 8), FAG‐1 (n = 8), FAG‐2 (n = 8), and FAG‐3 (n = 8) were not statistically different when compared against NBG (n = 15). HRV was also analyzed by pooling the sub groups of PBG and those of FAG to independently compare them with NBG. The values were expressed as mean ± standard deviation. Statistical level of significance was fixed at a p value <0.05. All the statistical tests were two‐tailed and unmatched. All the pre‐processing procedures and analysis methods mentioned in the present work were implemented using MATLAB (The Mathworks Inc., Natick, MA, USA) using standard routines and some custom written codes.
2.3. Coherence analysis
Coherence analysis was performed between the R‐R interval time series, and the instantaneous magnitudes of R waves of the measured ECG signals which are taken as estimates of respiratory patterns, namely the ECG derived respiration (EDR) (Lipsitz et al., 1995). Coherence analysis identified the frequency at which the two signals exhibited maximum correlation by computing magnitude square coherence using the following equation.
P XY refers to cross power spectral densities of R‐R and EDR signals, while P XX and P YY refer to their individual power spectral densities. Coherence analysis for the YG had been calculated only for the subjects who were exclusively recruited for this study (n = 5), as EDR could not be generated from the R‐R interval data of yogic subjects which were downloaded from the database.
2.4. Spectrogram
Spectrograms of R‐R time series were generated from short time Fourier transforms taken using a Hamming window of length 128 sample points moved over the entire length of time series with 50% overlap. The power spectral densities (PSD) of each overlapping segments were expressed in dB/Hz. PSD plotted as a function of time and frequency with their intensities indicated in color gave useful information on the spectral components present in R‐R interval time series during the period of measurement.
2.5. Classical HRV parameters
Short‐term HRV analysis was performed using heart rate variability analysis software (Kubios HRV toolbox version 2.1, University of Kuopio, Finland) (Tarvainen, Niskanen, Lipponen, Ranta‐aho, & Karjalainen, 2014) for the R‐R interval data. Time domain parameters included mean R‐R interval, standard deviation of normal‐to‐normal R‐R interval (SDNN), square root of the mean of the squared differences of successive normal‐to‐normal R‐R intervals (RMSSD), normal‐to‐normal R‐R intervals differing by 50 ms (NN50), percentage of NN50 (pNN50), and a geometric index calculated from the width of R‐R interval histogram, namely triangular interpolation of normal–normal R‐R intervals (TINN). Frequency domain parameters included in this analysis were normalized low‐ and high‐frequency powers (LF nu and HF nu, respectively) estimated from their auto‐regressive spectrum in the frequency regimes of 0.05–0.15 Hz (LF) and 0.15–0.4 Hz (HF). Detailed descriptions of each of these parameters are given elsewhere (Acharya et al., 2006; Shaffer & Ginsberg, 2017). Nonlinear parameters of HRV were calculated from the HR Poincare recurrence plot (Brennan, Palaniswami, & Kamen, 2001) by plotting the current R‐R interval against the preceding R‐R interval (delay of one beat) which identified the oscillatory variations in HRV. By fitting an ellipse on the line of identity of the Poincare plot (a 45 degree diagonal line with slope = 1), short‐ and long‐term fluctuations around the mean were calculated as standard deviations 1 and 2, that is, SD1 and SD2 of the HR series. The two measures represented variations along the horizontal and vertical axes of the fitted ellipse. As opposed to their conventional notations in HRV literature (Shaffer & Ginsberg, 2017), they were denoted as TSD1 and TSD2, respectively, in the present work, to differentiate them from similar plots generated for instantaneous frequency series of R‐R intervals. A heart rate time series for which the variations are governed by parasympathetic control, TSD1 and TSD2 are expected to be higher indicating higher HRV (Shaffer & Ginsberg, 2017).
2.6. Instantaneous frequency recurrence plots (IFRP)
Instantaneous frequencies (IF) were calculated from the R‐R interval time series using Hilbert transform by following a procedure discussed in the literature to analyze nonstationary signals (Battista, Knapp, McGee, & Goebel, 2007). The computed instantaneous frequency series was expressed as recurrence graphs similar to those generated for R‐R intervals to quantify the deviations occurring in instantaneous frequencies of HR series. HR variations which exhibited a sustained RSA pattern throughout the measurement was thus expected to show minimum spread (in Hertz) in its IFRP (from respective mean breathing frequencies) characterized by low values for both short‐ and long‐term fluctuations (FSD1 and FSD2) about its mean.
3. RESULTS
3.1. Coherence plots
Figure 1 shows the coherence analysis performed for a subject in YG and a subject in normal breathing group. Prominent oscillations displaying a higher variability in R‐R intervals are observed in YG in the time period of meditation as shown in Figure 1a. The Figure 1b shows the corresponding EDR signals representing the breathing pattern measured from R peaks of the subject. As evidenced from the coherence plot shown in Figure 1c, the two signals exhibit a maximum correlation at a frequency around 0.07 Hz which corresponds to a breathing rate of ~4 cpm, hence ascribing these oscillations to breathing. Similar analysis performed for a control subject from NBG shown in Figure 1d–f reveals the maximum correlation between EDR and R‐R occurring at a frequency of 0.27 Hz (16 cpm). Values calculated for the maximum coherence (C xy) and the frequencies at which the coherence was measured for all the subjects in the study groups have been tabulated in Table 1. As seen in Figure 1 and Table 1, C xy of subjects engaged in yogic meditation occur at lower breathing frequencies around 0.1 Hz (6 cpm) as opposed to the conventional breathing regime of 0.2–0.4 Hz (12–24 cpm) seen in the normal breathing control group. The mean breathing frequencies for the YG, PBG (at paced rate 3 and 7 cpm) are significantly different when compared to NBG (p < 0.01). The frequency of maximum coherence for both FAG and NBG occurred around 0.35 Hz. The measured magnitude square coherence for all the groups vary between 0.5 and 0.7, and their values do not show significant differences across the groups. It is obvious that correlation exists between R‐R interval and EDR in PBG. Table 1 particularly endorses the inter‐relationship between respiration and heart rate for YG and FAG and ensures that the prominent oscillations observed in HR are unambiguously RSA patterns.
Figure 1.
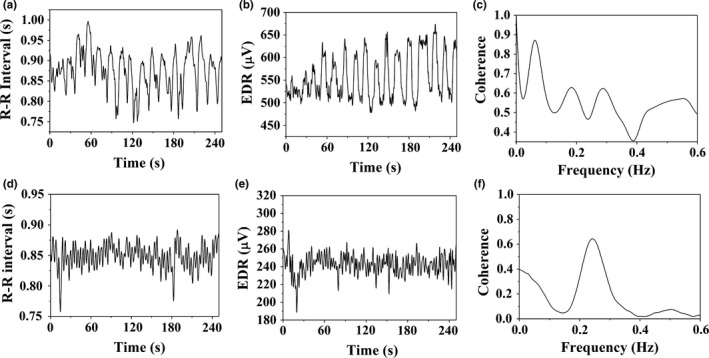
(a) A portion of the R‐R interval time series of a yogic subject (b) the corresponding EDR signal extracted from the ECG‐R‐wave magnitudes (c) coherence measured between the signals in (a) and (b). Similar plots generated from a control subject are shown in (d)–(f)
Table 1.
Coherence analysis
| Coherence | YG (n = 5) | NBG (n = 15) | PBG | FAG | ||||
|---|---|---|---|---|---|---|---|---|
| 3 cpm (n = 7) | 7 cpm (n = 8) | 12 cpm (n = 8) | FAG‐1 (n = 8) | FAG‐2 (n = 8) | FAG‐3 (n = 8) | |||
| Max Freq (Hz) | 0.1 ± 0.06a | 0.35 ± 0.26 | 0.09 ± 0.02a | 0.12 ± 0.06a | 0.29 ± 0.12 | 0.3 ± 0.27 | 0.37 ± 0.34 | 0.36 ± 0.3 |
| C xy | 0.62 ± 0.3 | 0.7 ± 0.47 | 0.7 ± 0.4 | 0.63 ± 0.4 | 0.6 ± 0.3 | 0.65 ± 0.39 | 0.46 ± 0.4 | 0.5 ± 0.4 |
p < 0.05—statistical level of significance when compared against normal breathing group (NBG).
3.2. Spectrogram plots
Figure 2 shows spectrogram plots of some of the representative cases from each group, namely YG, NBG, PBG‐3 cpm, and FAG‐1, respectively, indicated in figure from (a) to (d). It is evident from the plots that RSA components are seen aligned at their respective breathing frequencies with higher intensities (red color patches) in YG, PBG, and FAG. The breathing frequencies for YG and PBG (PBG‐3 cpm) occur around 0.08 Hz (~5 cpm), and further their magnitudes and the way of alignment of RSA are similar among the two groups. The RSA occurred around a frequency of 0.3 Hz (18 cpm) for FAG and magnitude wise it is weak as compared to that seen in YG and PBG‐3 cpm. Nevertheless, the alignment of breathing frequencies seen in FAG is appreciable. In contrast to the three study groups, the spectrogram of NBG shows unaligned and sporadic RSA patterns.
Figure 2.
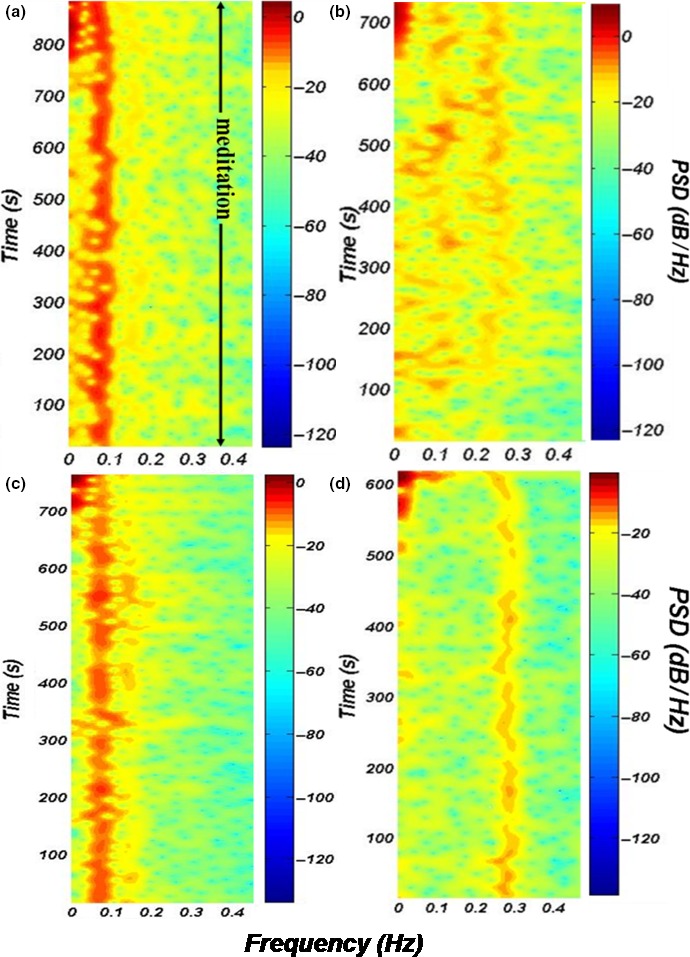
Representative spectrograms of R‐R interval series of a subject from (a) YG (b) NBG (c) PBG‐3 cpm and (d) FAG‐1. The color bars signify the intensities of the spectral power in all the spectrograms
3.3. HRV parameters
Box plots of various HRV parameters and maximum PSD values of spectrograms across groups and subgroups of this study are compared in Figure 3. YG, PBG‐3 cpm, PBG‐7 cpm, PBG‐12 cpm, FAG‐1, FAG‐2, FAG‐3, and NBG are sequentially indicated in the plots with alphabets from A‐H. HRV parameters which show significant differences when compared against NBG alone are shown in the figure. The plots display the overall variation in each subgroup with the whiskers representing the range and the boxes denoting the median of distribution of the parameters in each group. The parameters of each group are compared against those measured on NBG. The star marks (one–four) in the box plots refer to the statistical levels of significance calculated by comparing each one of them with NBG. The number of star marks represents the levels of statistical significance with p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively.
Figure 3.
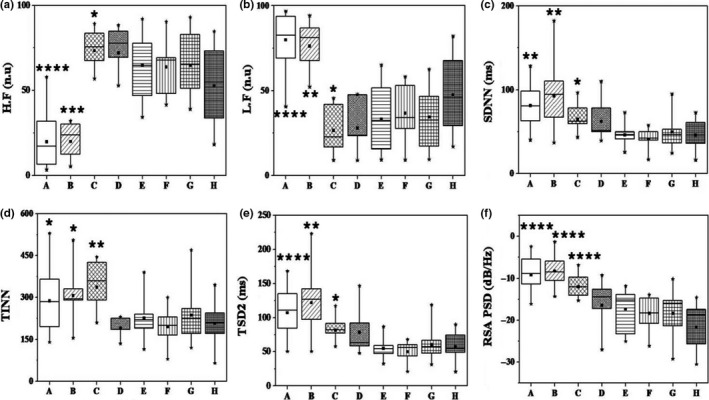
Comparison of HRV parameters and PSD of RSA in yogic group, paced breathing group at 3, 7, and 12 cpm, focused attention group with three tasks and normal breathing control group; indicated, respectively, from A, B, C, D, E, F, G, and H. Statistical levels of significance calculated by comparing each study group against the control group are indicated as star marks. Star marks from one to four represent p < 0.05, p < 0.01, p < 0.001, and p < 0.0001
Normalized HF power (HF nu) is significantly low for YG and PBG‐3 cpm, while for PBG‐7 cpm it is high as compared to NBG. Exactly reciprocal variations are observed for normalized LF power (LF nu) for YG and PBG. SDNN, TINN, and TSD2 are significantly high for YG, PBG‐3 cpm and PBG‐7 cpm as compared to NBG. Magnitudes of PSD at RSA frequencies are also high for YG, PBG‐3 cpm and PBG‐7 cpm and are remarkably different as compared to those of NBG (p < 0.0001). HRV parameters of PBG‐12 cpm and the FAG do not show significant differences as compared to NBG. YG and PBG‐3 cpm stand distinct by exhibiting identical variations as opposed to the rest of the groups. Pooling PBG (breathing rate <12 cpm), that is, PBG‐3 cpm (n = 7) and PBG‐7 cpm (n = 8) exhibit significant difference against NBG (n = 15) with p < 0.001 (graph not shown).
3.4. Instantaneous frequency analysis
Table 2 tabulates the short‐ and long‐term variations of IFRP plots, namely the FSD1 and FSD2 calculated for all the subjects in the study groups. It could be observed from Table 2 that the long‐term variations (FSD2) are significantly lower (represented in milli Hertz) for YG, PBG‐3 cpm, PBG‐ 7 cpm, PBG‐12 cpm, FAG‐1, and FAG‐2 when compared to those computed for NBG. The short‐term variations (FSD1) do not show significant differences among the study groups. By pooling the data of their respective subgroups, PBG and FAG are independently compared against NBG to improve the statistics (not shown). It is found that FSD2 is significantly lower in PBG (n = 23) (p < 0.01) and FAG (n = 24) (p < 0.05) when independently compared with NBG (n = 15).
Table 2.
Instantaneous frequency analysis
| IF parameters | YG (n = 15) | NBG (n = 15) | PBG | FAG | ||||
|---|---|---|---|---|---|---|---|---|
| 3 cpm (n = 7) | 7 cpm (n = 8) | 12 cpm (n = 8) | FAG‐1 (n = 8) | FAG‐2 (n = 8) | FAG‐3 (n = 8) | |||
| FSD1 (mHz) | 23 ± 20 | 24 ± 8 | 14 ± 4 | 19 ± 4 | 22 ± 8 | 23 ± 16 | 20 ± 4 | 22 ± 12 |
| FSD2 (mHz) | 87 ± 36** | 143 ± 46 | 88 ± 13** | 102 ± 13** | 120 ± 23* | 103 ± 19* | 128 ± 32* | 132 ± 49 |
**p < 0.01, *p < 0.05—statistical level of significance when compared against normal breathing group (NBG).
4. DISCUSSION
4.1. Breathing and HRV
The involvement of the process of breathing in yogic methods has been well recognized by researchers (Bertisch et al., 2013; Cysarz & Bussing, 2005; Peng et al., 1999; Telles & Vani, 2002). The present investigation revisits this aspect with a more comprehensive analysis with the help of a detailed experimental validation and reaffirms the fact; breathing is a fundamental strategy in eliciting physiologically significant changes in HRV. The ubiquitous nature of breathing is felt throughout the analysis for all the groups with the help of coherence plots, spectrograms, HRV parameters, and IFRP. Observing the similitude of the variations in heart rates, the study unequivocally demonstrates that low‐frequency breathing less than 12 cpm and the internal mechanisms associated with yogic methods are related. PBG less than 12 cpm effectively recreates the HRV of YG, characterized by highly intense RSA patterns in heart rate time series which make them uniquely different from those of NBG. Significant differences are observed in HRV parameters only up to a limit on the breathing rate (<12 cpm) as shown in Figure 3. It is to be noted from Figure 3f, unlike PBG‐3 cpm, paced breathing at rates greater than 12 cpm exhibit relatively weak RSA and insignificant variations in HRV parameters similar to those observed for NBG. A general inference is the explicit association of the magnitude of RSA with HRV parameters. It is obvious that low‐frequency breathing has caused the LF power to increase in YG, PBG than NBG. The increase in HF power for PBG‐7 may be attributed to the slight overlap of their breathing rates with the conventional HF regime (0.15–0.4 Hz) reported in HRV literature. Statistically significant increase in TINN, SDNN, and TSD2 of YG, PBG‐3 cpm, and PBG‐7 cpm as compared to NBG mark a higher variability in heart rate that could be expected for a healthy heart (Shaffer & Ginsberg, 2017). The sensitivity of these parameters to respiration (or parasympathetic dominance) has been discussed in a few reports (Fortrat, Yamamoto, & Hughson, 1997; Radhakrishna, Dutt, & Yeragani, 2000), and the present study reaffirms their correlation with breathing. In a physiological perspective, it is well known that the increase in RSA would exert a favorable impact on pulmonary gas exchange by keeping tidal volume or the breathing frequency constant (Cysarz & Bussing, 2005; Hayano & Yasuma, 2003; Hirsch & Bishop, 1981; Steffen et al., 2017; Tripathi, 2004). From the results obtained in this investigation, it is clear that these benefits which are imparted on yogic subjects are solely attributed to these mechanisms which are inherently to every yogic method. In PBG (also in biofeedback training methods), the respiratory frequency is deliberately kept constant by an external guiding reference creating an artificial physiological demand for the respiratory system to maintain ventilation. The RSA becomes intense and regular as long as the respiratory frequency is lower and a matching is facilitated to alveolar perfusion within each respiratory cycle (Cysarz & Bussing, 2005; Lehrer et al., 2000; Tripathi, 2004). It is possible to state that the yogic methods make use of these physiological gains by breathing at lower rates dictated by their internal breath control paradigm. Even though the exact respiratory parameter which is kept constant during yogic practices is not clear, it should be providing a time matched trigger as evidenced from the regularity of the R‐R interval quantified by IFRP. The trigger effectively achieves breath control which could for instance, be a rosary prayer (Bernardi et al., 2001), hexameter verse (Cysarz et al., 2004), Om mantra (Telles et al., 1995), breath synchronized physical actions (Cysarz & Bussing, 2005), etc. The yogic methods employed in the present study for YG are also conspicuous for their association with breathing. Whatsoever the mode of executing the low‐frequency breathing, the physiological effects have been observed to be similar for YG and PBG (<12 cpm) at least for the extent of cardiac autonomic control as evident from the results on PBG and YG which are in agreement with some reports (Cysarz & Bussing, 2005; Steffen et al., 2017).
4.2. Attention and HRV
The role of attention is felt in most of the yogic traditions similar to breathing (Saraswati, 2006). Cognitive attention tasks employed in this work are alternatives to the task of directed attention on a fixate practiced by yogic subjects who concentrate on one particular object or phenomenon for a prolonged period of time (Saraswati, 2006). It is clear from the results obtained from FAG, unlike low‐frequency breathing, attention as a strategy does not show any characteristic difference in HRV across the three subgroups on auditory, visual, and motor tracking tasks (FAG‐1, 2 and 3). However, it is worth noting that the alignment of RSA (occurring at breathing rates 0.25 Hz and above, i.e., 15 cpm and above) in heart rate series is markedly similar to those of the PBG and YG as opposed to NBG with sporadic patterns in their R‐R spectrograms (observed in Figure 2). For subjects in FAG where there are no specific instructions for breathing and are unaware of any yogic method, such a noticeable alignment in RSA has to be given its due recognition. Entrainment of spectral components of HRV for subjects involved in cognitive tasks has been recognized in an earlier study (Tripathi, Mukundan, & Mathew, 2003). However, the study has only highlighted the correlation existing between the modulation of HR and cognitive tasks and did not show any causational impacts.
One of the significant contributions of the present study is the quantification of the regularity of RSA with the help of IF recurrence plots, which, to our knowledge, has not been discussed so far in the literature. This has facilitated a comparison of entrainment of RSA components (with quantifiable spread in cardio‐respiratory interaction) across groups. A limited spread in instantaneous values of breathing frequencies quantified as FSD2 in Table 2 for YG, PBG and FAG (FAG‐1 and FAG‐2) marks a sustained synchronization in instantaneous breathing frequencies. Large deviations from the mean of the breathing frequencies might represent momentary phase slips in the synchrony between respiration and heart rate (Cysarz & Bussing, 2005). The demand for attention pertaining to the motor tasks of FAG‐3 might have caused an increase in cognitive load which is not in the scope of the present study. But it is worthwhile to note that such an increase in level of cognition causes reduction in cardio‐respiratory coupling (orderliness of RSA) which is consistent with a study which discusses the effect of arithmetic tasks on HRV (Cysarz & Bussing, 2005).
4.3. Relationship between attention and breathing
Paced breathing group is able to exhibit aligned RSA due to cueing by an external arrangement by focusing on the glowing of two LED lights. It is meaningful to assume that attention is confounded in this task as well, as evidenced from the aligned RSA seen in spectrograms of PBG which is qualitatively similar to those of FAG seen in Figure 2c,d. Paced breathing has been shown to increase low‐frequency alpha power in EEG, indicative of internal attention (Park & Park, 2012). Interestingly, the results obtained from FAG have demonstrated that tasks on attention could invoke paced breathing in control subjects and in turn, their coexistence. Quantifiable changes have been observed in HRV of subjects under cognitive tasks with changes in respiratory rates (Nagasawa & Hagiwara, 2016).
It is well known that respiratory modalities such as tidal volume, respiratory rate, air flow pattern, recruitment profile of respiratory muscles, etc. get influenced either in an inhibitory or excitatory way by behavioral reflexes of central nervous system (CNS) (Shea, 1996). It is reasonable to hypothesize that the regularity in breathing frequency might represent an inhibitory effect exerted on the CNS by the act of vigilance of the subjects in being attentive to the experiment. By minimizing or subduing other behavioral influences the self sustained neural pacemaker at the brain stem might have competed to favor a regular respiratory rhythm (Shea, 1996). The susceptibility of the cardiac autonomic controls to external rhythms has been studied by some researchers (Charnock & Manenica, 1978; Steffen et al., 2017). Hence, by observing the aligned RSA patterns on FAG similar to YG, it is logical to conclude that the act of breath control in yogic methods is not volitional like in PBG but could be invoked by focused attention. Because attention as a strategy demands long‐term practice, yoga or meditation which is imbibed with internal guiding mechanisms (similar to tasks performed by FAG) might help in psycho‐physiologically train subjects to breathe at low pacing rates. Training in such pursuits might be the reason for achieving long‐term health benefits even under rest conditions as reported by some studies (Muralikrishnan et al., 2012; Telles & Vani, 2002), in contrary to short‐term intermittent benefits (Cysarz & Bussing, 2005) could be obtained at the time of the breathing exercises even by first‐time meditators or for that purpose by any individual.
5. CONCLUSION
A comprehensive analysis has been performed on the influence of two yogic strategies (paced breathing and focused attention) on the HRV of control subjects, and the results have been compared with those obtained on regular practitioners of yoga. The commonality and the characteristic differences existing across R‐R interval data recorded from subjects belonging to four distinct groups (and sub‐groups) have been extensively analyzed by keeping RSA as a key element of investigation using coherence plots, spectrograms, HRV parameters, and instantaneous frequency analysis. Consistent results have been obtained, which highlight the importance of controlled low‐frequency breathing, which is ubiquitous and fundamental in delivering physiologically significant variations in heart rates of yogic practitioners. In addition, the results on focused attention group, which exhibited RSA patterns similar to the paced breathing and yogic groups (but at slightly higher frequencies), have highlighted the contribution of the cognitive attributes like attention in achieving a sustained cardio‐respiratory synchronization. It has been observed that focused attention and low‐frequency breathing are co‐existing inherent strategies in yogic techniques which might play an important role in psycho‐physiologically adapting a nonpractitioner to get trained in yoga to derive long‐term physiological benefits.
5.1. Limitations of the study
Pooling of R‐R interval data of Kundalini and Chi groups was performed to improve the statistics for comparison with control subjects. However, the yogic sub‐group originally measured in this study also reflected significant differences when independently compared with control subjects.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest for this research study.
ACKNOWLEDGMENTS
The research reported in this manuscript was initiated and facilitated by Mr. V.V. Bhat, former Member‐Finance, Department of Atomic Energy, Government of India. It is a pleasure to thank our colleagues Mr. R. Baskaran, Dr. N.V. Chandra Shekar and Dr. G. Amarendra for their encouragement and support. The authors also thank all the volunteers who participated in the study.
Senthilnathan S, Patel R, Narayanan M, et al. An investigation on the influence of yogic methods on heart rate variability. Ann Noninvasive Electrocardiol. 2019;24:e12584 10.1111/anec.12584
REFERENCES
- Acharya, U. R. , Joseph, K. P. , Kannathal, N. , Lim, C. M. , & Suri, J. S. (2006). Heart rate variability: A review. Medical and Biological Engineering and Computing, 44, 1031–1051. 10.1007/s11517-006-0119-0 [DOI] [PubMed] [Google Scholar]
- Battista, B. M. , Knapp, C. , McGee, T. , & Goebel, V. (2007). Application of the empirical mode decomposition and Hilbert‐Huang transform on seismic reflection data. Geophysics, 72(2), H29–H37. 10.1190/1.2437700 [DOI] [Google Scholar]
- Bernardi, L. , Sleight, P. , Bandinelli, G. , Cencetti, S. , Fattorini, L. , Wdowczyc‐Szulc, J. , & Lagi, A. (2001). Effect of rosary prayer & yoga mantras on autonomic cardiovascular rhythms: Comparative study. British Medical Journal, 323(7327), 1446–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertisch, S. , Hamner, J. , Tan, C. , & Taylor, J. A. (2013). Comparison of cardiovascular responses to yoga and slow paced breathing. FASEB Journal, 27(903), 3 10.1096/fasebj.27.1_supplement.903.3 23012321 [DOI] [Google Scholar]
- Brennan, M. , Palaniswami, M. , & Kamen, P. (2001). Do existing measures of Poincare geometry reflect nonlinear features of heart rate variability? IEEE Transactions on Biomedical Engineering, 48(11), 1342–1347. 10.1109/10.959330 [DOI] [PubMed] [Google Scholar]
- Carvalho, J. L. H. , Rocha, A. F. , Santos, I. D. , Iltiki, C. , Junqueira Jr, L. F. , & Nascimento, F. A. O. (2003). Study on the optimal order for the auto‐regressive time‐frequency analysis of heart rate variability In Proc. 25th Annual International conference of the IEEE‐Engineering in Medicine and Biology Society. Cancun, Mexico September 17–21, 2003, 3 (pp. 2621–2624). 10.1109/iembs.2003.1280453 [DOI] [Google Scholar]
- Charnock, D. M. , & Manenica, I. (1978). Spectral analysis of RR intervals under different work conditions. Ergonomics, 21, 103–108. [DOI] [PubMed] [Google Scholar]
- Cysarz, D. , & Bussing, A. (2005). Cardiorespiratory synchronisation during Zen meditation. European Journal of Applied Physiology, 95(1), 88–95. 10.1007/s00421-005-1379-3 [DOI] [PubMed] [Google Scholar]
- Cysarz, D. , von Bonin, D. , Lackner, H. , Heusser, P. , Moser, M. , & Bettermann, H. (2004). Oscillations of heart rate and respiration synchronize during poetry recitation. American Journal of Physiology – Heart and Circulatory Physiology, 287(2), H579–H587. 10.1152/ajpheart.01131.2003 [DOI] [PubMed] [Google Scholar]
- Fortrat, J. O. , Yamamoto, Y. , & Hughson, R. L. (1997). Respiratory influences on non‐linear dynamics of heart rate variability in humans. Biological Cybernetics, 77(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Goldberger, A. L. , Amaral, L. A. N. , Glass, L. , Hausdorff, J. M. , Ivanov, P. Ch. , Mark, R. G. , … Stanley, H. E. (2000). PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation, 101(23), E215–E220. [DOI] [PubMed] [Google Scholar]
- Gonda, J. (1963). The Indian mantra. Oriens, 16, 244–297. 10.2307/1580265 [DOI] [Google Scholar]
- Hayano, J. , & Yasuma, F. (2003). Hypothesis: Respiratory sinus arrhythmia is an intrinsic resting function of cardiopulmonary system. Cardiovascular Research, 58(1), 1–9. 10.1016/S0008-6363(02)00851-9 [DOI] [PubMed] [Google Scholar]
- Hirsch, J. A. , & Bishop, B. (1981). Respiratory sinus arrhythmia in humans: How breathing patterns modulates heart rate. American Journal of Physiology, 241, H620–H629. [DOI] [PubMed] [Google Scholar]
- Lehrer, P. M. , Vaschillo, E. , & Vaschillo, B. (2000). Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Applied Psychophysiology and Biofeedback, 25(3), 177–191. 10.1023/A:1009554825745 [DOI] [PubMed] [Google Scholar]
- Lipsitz, L. A. , Hashimoto, F. , Lubowsky, L. P. , Mietus, J. , Moody, G. B. , Appenzeller, O. , & Goldberger, A. L. (1995). Heart rate and respiratory rhythm dynamics on ascent to high altitude. British Heart Journal, 74(4), 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralikrishnan, K. , Balakrishnan, B. , Balasubramanian, K. , & Visnegarawla, F. (2012). Measurement of the effect of Isha yoga on cardiac autonomic nervous system using short‐term heart rate variability. Journal of Ayurveda and Integrative Medicine, 3(2), 91–96. 10.4103/0975-9476.96528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa, T. , & Hagiwara, H. (2016). Workload induces changes in hemodynamics, respiratory rate and heart rate variability In Proc. 16th IEEE conference on Bioinformatics and Bioengineering (BIBE) (pp. 176–181). Taichung, Taiwan, October 31‐November 2, 2016. 10.1109/bibe.2016.27 [DOI] [Google Scholar]
- Park, Y. J. , & Park, Y. B. (2012). Clinical utility of paced breathing as a concentration meditation practice. Complementary Therapies in Medicine, 20(6), 393–399. 10.1016/j.ctim.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Peng, C. K. , Mietus, J. E. , Liu, Y. , Khalsa, G. , Douglas, P. S. , Benson, H. , & Goldberger, A. L. (1999). Exaggerated heart rate oscillations during two meditation techniques. International Journal of Cardiology, 70(2), 101–107. [DOI] [PubMed] [Google Scholar]
- Radhakrishna, R. K. , Dutt, D. N. , & Yeragani, V. K. (2000). Nonlinear measures of heart rate time series: Influence of posture and controlled breathing. Autonomic Neuroscience, 83, 148–158. [DOI] [PubMed] [Google Scholar]
- Saraswati, S. (2006). A systematic course in the ancient tantric techniques of yoga and kriya (1st ed.). Munger, Bihar, India: Yoga Publications Trust. [Google Scholar]
- Sengottuvel, S. , Parasakthi, C. , Mariyappa, N. , Patel, R. , Gireesan, K. , Janawadkar, M. P. , … Muralidharan, T. R. (2012). Enhancing the reliability in the noninvasive measurement of the His bundle magnetic field. Annals of Noninvasive Electrocardiology, 17(3), 186–194. 10.1111/j.1542-474X.2012.00523.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer, F. , & Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea, S. A. (1996). Behavioural and arousal‐related influences on breathing in humans. Experimental Physiology, 81(1), 1–26. [DOI] [PubMed] [Google Scholar]
- Steffen, P. R. , Austin, T. , DeBarros, A. , & Brown, T. (2017). The impact of resonance frequency breathing on measures of heart rate variability, blood pressure and mood. Frontiers in Public Health, 5, 222 10.3389/fpubh.2017.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarvainen, M. P. , Niskanen, J. P. , Lipponen, J. A. , Ranta‐aho, P. O. , & Karjalainen, P. A. (2014). Kubios HRV‐heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220. 10.1016/j.cmpb.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Telles, S. , Nagarathna, R. , & Nagendra, H. R. (1995). Autonomic changes during “OM” meditation. Indian Journal of Physiology and Pharmacology, 39(4), 418–420. [PubMed] [Google Scholar]
- Telles, S. , & Vani, R. (2002). Increase in voluntary pulse rate reduction achieved following yoga training. International Journal of Stress Management, 9(3), 235–239. [Google Scholar]
- Todd, C. H. (Ed.) (2005). Event related potentials – A methods handbook. Cambridge, MA: MIT Press. [Google Scholar]
- Tripathi, K. K. (2004). Respiration and heart rate variability: A review with special reference to its application in aerospace medicine. Indian Journal of Aerospace Medicine, 48(1), 1–10. [Google Scholar]
- Tripathi, K. K. , Mukundan, C. R. , & Mathew, T. L. (2003). Attentional modulation of heart rate variability (HRV) during execution of PC based cognitive tasks. Indian Journal of Aerospace Medicine, 47(1), 64–75. [Google Scholar]


