Abstract
Background
The clinical relevance of extended monitoring of AF in the general population is unclear. The study evaluated the detection of AF using transtelephonic electrocardiography and the clinical relevance of additional AF findings, especially with regard to stroke risk and mortality.
Methods
The data of 1678 volunteers participating in the tele‐ECG‐subproject of the Study of Health in Pomerania was evaluated. Occurrence of AF as revealed by tele‐ECG and conventional ECG was evaluated. Associations with mortality, history of stroke, and other clinical parameters were analyzed.
Results
AF was detected in 21 subjects (1.3%) by conventional ECG (ECG‐AF) and in 43 (2.6%) by tele‐ECG. All individuals with AF revealed by conventional ECG were also diagnosed to have AF by tele‐ECG; 22 were diagnosed by tele‐ECG only (Tele‐AF).
During follow‐up (median: 6.3 years) 42/1635, 1/22, and 5/21 participants died in the no‐AF‐, tele‐AF‐, and ECG‐AF groups (p < .001). Whereas, in comparison to the no‐AF group, the risk of death was higher in the ECG‐AF group (HR 9.4; 3.7–23.8; p < .001), there was no significant increase in mortality in the tele‐AF group (HR 1.9; 0.26–14.0; p = .52).
Prevalence of stroke history was higher in the ECG‐AF group (19%; 5.5–42%) than with the no‐AF (1.9%; 1.3–2.7%; p = .001) and the tele‐AF groups (0%; 0–15%; p = .05).
Conclusions
Tele‐ECG identifies significantly more AF cases in a population‐based setting compared to conventional ECG. The impact of AF diagnosed only by extended monitoring differs from conventionally diagnosed AF. Additional studies are warranted, since this might have an impact on clinical management.
Keywords: atrial fibrillation, mortality, population based study, stroke
1. Introduction
Due to its prevalence and clinical consequence, atrial fibrillation (AF) is the most relevant clinical arrhythmia. AF nearly doubles mortality (Benjamin et al., 1998; Friberg, Hammar, Pettersson, & Rosenqvist, 2007) and significantly increases the risk of heart failure (Stewart, Hart, Hole, & McMurray, 2002) and stroke (Olesen et al., 2011; Wolf, Abbott, & Kannel, 1991). Although AF is clearly associated with impaired quality of life (Thrall, Lane, Carroll, & Lip, 2006), it can be completely asymptomatic (Patten et al., 2006; Roche et al., 2002), making exact estimation of AF prevalence complicated. Recent data on the prevalence in the general population vary between 0.4% and greater than 2.9% (Camm et al., 2012; Friberg & Bergfeldt, 2013; Fuster et al., 2011).
The fact that deleterious events such as ischemic stroke are associated with AF (Olesen et al., 2011; Stewart et al., 2002; Wolf et al., 1991) leads to the argument that intensified monitoring for AF could facilitate early intervention to reduce clinical events. Although it has clearly been shown that intensifying AF monitoring leads to a greater proportion of subjects diagnosed with AF (Charitos et al., 2012; Senatore et al., 2005), present estimations are based on extrapolation of patient cohorts or on sparse in‐office electrocardiogram (ECG) recordings. Furthermore, the impact of AF coincidentally detected outside a clinical context remains unclear. We therefore hypothesized that, in a population‐based setting, 4 weeks of tele‐ECG monitoring would lead to significantly higher AF detection compared to conventional ECG recording. We then investigated the clinical effect of these additional findings, especially with regard to stroke and mortality.
2. Methods
2.1. Study population
The present study is based on data from the Study of Health in Pomerania (SHIP), a population‐based investigation conducted in the northeastern part of Germany. Details on study design and recruitment have previously been published (Völzke et al., 2011).
In brief, sampling of adults aged 20–79 took place based on population registries that included a total population of 213,057. In order to provide an unbiased sample of the general population no other specific inclusion or exclusion criteria besides the age range the residence in the study region were applied. Of 6,265 eligible subjects, 4,308 participated in the baseline examination between 1997 and 2001 (SHIP‐0). The 5‐year follow‐up (SHIP‐1) was conducted between 2002 and 2006. Of the initial cohort, 130 were passive nonresponders due to migration, and 231 subjects had died between the two examinations. Of the remaining 3,949 eligible persons, 649 were active nonresponders, which resulted in successful follow‐up for 3,300 subjects (Haring et al., 2009). As part of SHIP‐1, a subgroup of 1,797 persons volunteered to take part in the tele‐electrocardiography project. Analysis of the data of 1678 participants ultimately took place, excluding 119 subjects with insufficient ECG or without tele‐ECGs (see below).
The study conforms to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the University of Greifswald.
2.2. Clinical characterization
Information on sociodemographic characteristics, medication use, and medical history was collected by trained and certificated medical staff during a computer‐assisted standardized interview. All participants underwent an extensive standardized medical examination. Waist‐to‐hip ratio was calculated from the relevant circumferences measured to the nearest 0.5 cm. Waist circumference was measured midway between the lower rib margin and the iliac crest in the horizontal plane in subjects standing comfortably with weight distributed evenly on both feet. Hip circumference was measured horizontally at the level of the greatest lateral extension of the hips or buttocks. BMI, expressed in units of kg/m², was calculated as body mass in kg divided by the square of body height in m. After an initial 5‐min rest period, systolic and diastolic blood pressures were measured three times on the right arm of seated subjects using a digital blood pressure monitor (HEM‐705CP, Omron Corporation, Tokyo, Japan). Each reading was followed by further rest periods of 3 min. The cuff size was matched to the circumference of the participant's arm. Pulse pressure was then calculated as systolic blood pressure minus diastolic blood pressure. We used the mean of the second and third measurements for the present analyses.
Two‐dimensional and M‐mode echocardiography was performed by certified physicians using a Vingmed CFM 800A system (GE Medical Systems, Waukesha, WI, USA). All data and measurements were stored digitally. We applied the leading‐edge technique to measure cardiac dimensions: left atrial (LA) diameter, interventricular septum thickness (IVS), posterior wall thickness (LVPW), left ventricular end‐diastolic (LVDD) diameter, and left ventricular end‐systolic (LVDS) diameter (Reichek & Devereux, 1981). Left ventricular mass (LVM) was calculated using the formula according to Devereux (Devereux et al., 1986): LVM = 0.80 × [1.04 × (LVDD + IVS + LVPW)3 − LVDD3)] + 0.60 and indexed to height 2.7 (LVMI). Assessment of intrareader, intraobserver, interreader, and interobserver variability for all echocardiographic measurements revealed Spearman correlation coefficients of >0.85 and differences in mean (±2 SD) of 5% (Dörr et al., 2005).
A 12‐lead resting ECG of 10 sec duration was recorded and stored digitally with an ACTA electrocardiograph (ESAOTE, Florence, Italy) at a sampling frequency of 500 Hz. All ECGs were analyzed centrally using the modular ECG analysis system (MEANS). The MEANS program has been evaluated and validated extensively (de Bruyne et al., 1997). Bazett's formula (QTc = QT/√RR interval) was used to correct the QT interval for heart rate (Bazett, 1920).
Information on vital status was collected from population registries at annual intervals, from time of enrollment into the study through December 15, 2013. Subjects were censored at either death or failure to follow‐up. The number of months between baseline examination and censoring was used as follow‐up length.
2.3. Tele‐electrocardiography
The methodology of tele‐ECG monitoring in SHIP has previously been described (Alte et al., 2006). In brief, the participants were handed out an ECG card (Sensor Mobile 100, Telemedizinische Systeme, Chemnitz, Germany) for 4 weeks and were instructed to record two ECGs of 30 sec duration daily. They were asked to record additional ECGs in case of symptoms of arrhythmia, dizziness, or chest pain. All ECGs were sent via telephone, and the ECG transmissions (n = 89,220) were visually interpreted in terms of quality and presence of arrhythmias, including AF. Interpretation was by one of 8 physicians who had previously been trained and certified. Extensive validation of the findings took place. All ECG transmissions (n = 2,397) from participants with at least one AF event in a tele‐ECG transmission, or in a 12‐lead resting ECG recording, were reassessed by one of four different certified physicians, all of whom were specialists in ECG rhythm interpretation. In case of result mismatch (n = 405), the final diagnosis was consensually established by 2 rhythm specialists.
To ensure sufficient informative value of the tele‐ECG monitoring, participants in whom fewer than 20 tele‐ECG transmissions could be assessed were excluded from the analysis (n = 108). An additional 11 subjects were excluded for missing conventional baseline 12‐lead resting ECGs, resulting in a final study population of 1,678 participants for analysis.
2.4. Statistical methods
Continuous variables are given as mean (standard deviation). Proportions are reported with the 95% Clopper‐Pearson confidence interval. Comparison of the diagnostic yield of conventional versus tele‐ECG was performed using McNemar's test. For comparison of continuous variables, we applied nonparametric tests (Kruskal–Wallis, Mann–Whitney). Categorical data were compared using the chi‐square test.
Mortality was investigated using Kaplan–Meier analysis with the log‐rank test. Multivariable analyses and hazard‐ratio calculations were performed using Cox regression. All analyses were additionally weighted for loss to follow‐up from SHIP‐0 to SHIP‐1 (tele‐electrocardiography) (Hogan, Roy, & Korkontzelou, 2004). The assumption for using dropout weights is that the missing mechanism is at random, meaning that missing data can be explained by the available data. The inverse probability weights were calculated with a logistic regression model for participation in the tele‐electrocardiography substudy (1 if the person participated in the tele‐ECG substudy; 0 if not). We worked with baseline variables as independent variables (age, sex, smoking status, physical activity, education, equivalent income, alcohol consumption, HDL cholesterol, diabetes status, HbA1c, systolic and diastolic blood pressure, and body mass index). From this regression model we calculated the probability to participate in the tele‐electrocardiography substudy and took the inverse of this as dropout weight. A p < .05 was considered as statistically significant. All analyses were carried out with SPSS Statistics 23 (IBM, Armonk, NY, USA).
3. Results
3.1. Diagnosis of AF by conventional ECG and tele‐ECG
A median of 54 (28–65) tele‐ECG transmissions was available per participant. Among the 1,678 subjects included in this study, AF was detected in 21 individuals (1.3%; 0.8–1.9%) by conventional ECG recording (ECG AF group) and in 43 individuals (2.6%; 1.9–3.4%) using tele‐ECG monitoring. All individuals with AF in the conventional ECG recording were also diagnosed as having AF by tele‐ECG. The diagnostic yield of AF by tele‐ECG monitoring is therefore significantly higher than by conventional ECG recording in a population‐based study (McNemar's test, p < .001). The participants presenting in sinus rhythm for conventional ECG in whom AF was detected by tele‐ECG are defined as the tele‐AF group. The group of participants in whom AF was detected neither by tele‐ECG nor by conventional ECG is further referred to as the no‐AF group (Table 1).
Table 1.
Diagnosis of AF with conventional ECG and tele ECG
Table 1 delineates the amount of AF diagnoses by conventional ECG or by tele‐ECG. Thus, the participants can be allocated to the following groups:
(no‐AF group: AF detected neither by single ECG nor by tele‐ECG.
(tele‐AF group): AF detected by tele‐ECG only.
(ECG‐AF group): AF detected by single in‐office ECG.
3.2. Clinical characteristics
Individuals with AF were older and more obese, including an unfavorable waist to hip ratio (Table 2). Male sex was more frequent among the participants with AF. With regard to these parameters, there was no significant difference between the tele‐AF group and the ECG‐AF group. Subjects with AF demonstrated a significantly higher pulse pressure and a nonsignificant trend toward higher systolic blood pressure values than subjects without AF.
Table 2.
Clinical characteristics
| No‐AF | Tele‐AF | ECG‐AF | p‐Value AF (tele‐AF + ECG‐AF) versus no‐AF | p‐Value tele‐AF versus no‐AF | p‐Value ECG‐AF versus no‐AF | p‐Value tele‐AF versus ECG‐AF | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), years | 51 (13) | 64 (14) | 69 (6) | <.001 | <.001 | <.001 | .30 |
| Male, (%, 95% CI) | 770 (47%; 45–50%) | 15 (68%; 45–86%) | 13 (62%; 38–82%) | .02 | .06 | .19 | .76 |
| Height, mean (SD), cm | 170 (9) | 170 (9) | 170 (9) | .81 | .75 | .98 | .96 |
| Weight mean (SD), kg | 80 (16) | 89 (18) | 86 (14) | .006 | .04 | .06 | .73 |
| BMI, mean (SD), m/kg2 | 27.9 (4.8) | 30.7 (6.9) | 30.0 (4.3) | .003 | .04 | .03 | .942 |
| WHR, mean (SD) | 0.87 (0.08) | 0.91 (0.08) | 0.90 (0.08) | .007 | .05 | .06 | .88 |
| BSA, mean (SD), m2 | 1.94 (0.23) | 2.04 (0.23) | 2.01 (0.20) | .013 | .05 | .13 | .72 |
| Blood pressure, mean (SD), mm Hg | |||||||
| Systolic | 130 (18) | 137 (19) | 134 (18) | .06 | .07 | .35 | .49 |
| Diastolic | 82 (10) | 78 (10) | 80 (11) | .04 | .05 | .34 | .54 |
| Mean arterial pressure | 98 (12) | 98 (11) | 98 (11) | .99 | .91 | .89 | .89 |
| Pulse pressure | 48 (13) | 60 (17) | 54 (17) | <.001 | .001 | .09 | .33 |
| History of stroke, (%, 95% CI) | 31 (1.9%; 1.3–2.7%) | 0 (0%; 0–15%) | 4 (19%; 5.5–42%) | .01 | .99 | .001 | .05 |
| CHADS2‐score, mean (SD) | 1.5 (0.7) | 2.4 (1.0) | 2.4 (1.0) | <.001 | <.001 | <.001 | .91 |
| Oral anticoagulation, (%, 95% CI) | 8 (0.5%; 0.2–1.0%) | 3 (13.6%; 2.9–34.9%) | 5 (23.8%; 8.2–47.1%) | <.001 | <.001 | <.001 | .322 |
BMI, body mass index; WHR, waist to hip ratio; BSA, body surface area.
Furthermore, participants with AF were more likely to have a history of stroke. Groupwise comparison showed this difference only between the no‐AF and the ECG‐AF group. No subject in the tele‐AF group had suffered a stroke in the past. The CHADS2 score was significantly higher in participants with AF than in the no‐AF group, with no difference between AF groups. Anticoagulation at baseline was more frequent in participants with AF than in the no‐AF group, with no difference between AF groups.
3.3. Echocardiographic and electrocardiographic characteristics
The LA diameter was significantly higher in the tele‐AF group than in the no‐AF group, with highest values in the ECG AF (Table 3). While no significant differences in left ventricular dimensions and fractional shortening (FS) were found, the LVMI was greater in both AF groups than in the no‐AF group, with no significant difference between AF groups (Table 3).
Table 3.
Echocardiographic characteristics
| No‐AF | Tele‐AF | ECG‐AF | p‐Value AF (Tele‐AF + ECG‐AF) versus no‐AF | p‐Value Tele‐AF versus no‐AF | p‐Value ECG‐AF versus no AF | p‐Value Tele‐AF versus ECG‐AF | |
|---|---|---|---|---|---|---|---|
| LA, mean (SD), mm | 34 (6) | 40 (7) | 45 (6) | <.001 | <.001 | <.001 | .02 |
| LVEDD, mean (SD), mm | 49 (5) | 49 (4) | 51 (4) | .17 | .76 | .12 | .41 |
| FS, mean (SD), % | 37 (8) | 37 (7) | 37 (10) | .85 | .69 | .91 | .82 |
| LVMI, mean (SD), g/m2.7 | 45 (16) | 52 (15) | 51 (10) | .001 | .03 | .009 | .63 |
LA, left atrial diameter; LVEDD, left ventricular end‐diastolic diameter; FS, fractional shortening; LVMI, left ventricular mass index.
There were significant differences in ECG parameters between individuals in the ECG‐AF group and in the two other groups (Table 4). In particular, heart rate in the tele‐AF group was lower and P‐wave duration, as well as PR and QRS intervals, were longer than in the group without AF. QT interval was higher in the tele‐AF group, but lower in the ECG‐AF group in comparison to the no‐AF group.
Table 4.
Electrocardiographic characteristics
| No‐AF | Tele‐AF | ECG‐AF | p‐Value AF (Tele‐AF + ECG‐AF) versus no‐AF | p‐Value tele‐AF versus no‐AF | p‐Value ECG‐AF versus no AF | p‐Value tele‐AF versus ECG‐AF | |
|---|---|---|---|---|---|---|---|
| Heart rate, mean (SD), bpm | 64 (10) | 58 (8) | 75 (24) | .98 | .01 | .02 | .001 |
| P duration, mean (SD), msec | 116 (13) | 124 (16) | N/A | .02 | |||
| PQ interval, mean (SD), msec | 165 (26) | 184 (35) | N/A | .04 | |||
| QRS duration, mean (SD), msec | 101 (15) | 113 (29) | 109 (24) | .01 | .02 | .19 | .37 |
| QT interval, mean (SD), msec | 412 (29) | 425 (34) | 398 (41) | .80 | .05 | .13 | .03 |
| QTc interval, mean (SD), msec | 421 (23) | 415 (18) | 435 (35) | .48 | .28 | .04 | .04 |
3.4. Mortality
During the median follow‐up time of 6.3 years (5–95 percentile: 4.5–7.5, total person‐years: 10,086), 42/1,635 (2.6%), 1/22 (4.5%), and 5/21 (23.8%) participants died in the no‐AF, tele‐AF, and ECG‐AF groups respectively. The Kaplan–Meier survival curves are displayed in Figure 1, which show significant differences in mortality (log rank test p < .001). Whereas Cox regression analysis revealed no significant difference in mortality between the no‐AF and the tele‐AF subjects (HR 1.9 (95% CI 0.26–14.0, p = .52), there was a significant difference between the no‐AF and the ECG‐AF groups (HR 9.4 (95% CI 3.7–23.8, p < .001). After adjustment for baseline risk factors (age, sex, hypertension, and diabetes), we determined a similar effect of AF status on mortality (HR for tele‐AF 0.67 (0.09–5.0), HR for ECG‐AF 3.9 (1.5–9.9). The results remained virtually unchanged when inverse probability weighting was used to account for possible selection effects owing to non‐participation in the tele‐electrocardiography substudy (Figure 2).
Figure 1.
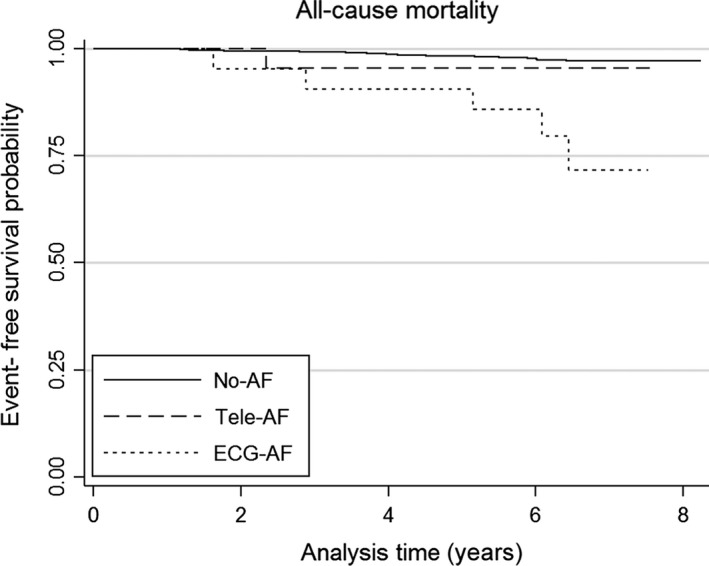
All‐cause mortality. Kaplan–Meier survival curves for all‐cause mortality in the no‐AF (solid line), tele‐AF (dashed line), and ECG‐AF groups (dotted line)
Figure 2.
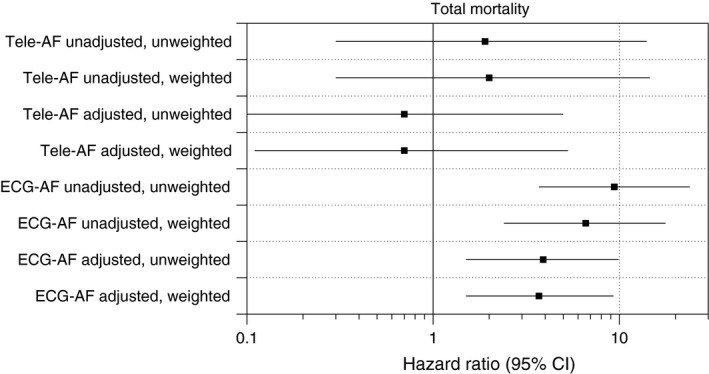
Hazard ratios for all‐cause mortality. Hazard ratios for all‐cause mortality are displayed dependent on AF class, adjustment for risk factors (age, gender, hypertension, diabetes) and weighting for probability of participation
4. Discussion
4.1. AF monitoring
In our study, a single 12‐lead ECG recording revealed an AF prevalence of 1.3%, which corresponds to the estimated ranges reported in current American and European guidelines of 0.4–1.0% (Fuster et al., 2006, 2011) and 1.5–2.0% (Camm et al., 2012). In the population‐based setting of our study, the use of tele‐ECG monitoring doubled the extent of AF diagnosis in comparison to diagnosis based on a conventional 12‐lead resting ECG.
Previous studies applied various extended ECG monitoring modalities to detect AF in patients with a history of AF (e.g., patients on antiarrhythmic therapy (Singh et al., 2007) or subjects following AF ablation (Senatore et al., 2005)), and those at great AF risk (e.g., patients with cryptogenic ischemic stroke (Kamel et al., 2013; Sinha et al., 2010)). In one study, the use of tele‐ECG monitoring consequently resulted in a higher detection rate of AF recurrences in patients after ablation therapy for AF than in subjects with conventional ECG and 24‐h Holter monitoring (Senatore et al., 2005). However, in another study, the use of similar ECG monitoring was unable to significantly improve the identification of AF patients in the setting of cryptogenic stroke (Kamel et al., 2013).
Data on AF in the general population is mainly derived from single conventional ECG recordings or anamnestic information (Miyasaka et al., 2006; Stewart, Hart, Hole, & McMurray, 2001). The ECG‐AF group in our study therefore corresponds to the AF cohorts in previous population‐based studies. Engdahl et al. used tele‐ECG monitoring in a population‐based setting, but only for individuals at greater risk for AF (age 75–76 and CHADS2‐score ≥ 2) (Engdahl, Andersson, Mirskaya, & Rosenqvist, 2013). Among 848 participants previously undiagnosed, silent AF was found in 10 (1%). In contrast with this study, our data are not based on subjects at great risk for AF, but on a sample from a population‐based study. This makes the observed high proportion of subjects with evidence for AF even more remarkable. Moreover, it must be taken into account that tele‐ECG monitoring lacks negative predictive value, as do all discontinuous ECG monitoring modalities, in contrast to monitoring based on implantable loop recorders (Charitos et al., 2012). Most likely, a considerable proportion of paroxysmal AF still remains undiagnosed in our study. Implantation of loop recorders, however, has until now been evidently unacceptable in a population‐based approach. With further miniaturization of implantable loop recorders, continuous AF monitoring may well become potentially feasible in population‐based research.
4.2. Clinical relevance
Common clinical classification distinguishes among paroxysmal, persistent, and permanent AF (Fuster et al., 2011). The modalities of AF diagnosis in our study do not allow for exact allocation according to such classification. Nevertheless, it is very likely that the ECG‐AF group comprises predominantly permanent or persistent forms of AF, whereas the tele‐AF group consists of paroxysmal AF or new‐onset AF.
The differences in baseline characteristics (such as age, sex, echocardiographic parameters, and CHADS2‐score) between participants with AF and the no‐AF group are consistent with the anticipated findings previously confirmed in numerous studies (Fuster et al., 2011; Lévy et al., 1999; Stewart et al., 2001). Interestingly, there are no significant differences with regard to these parameters between the ECG‐AF and the tele‐AF group, with the exception of LA diameter, which is larger in the first group. Although baseline characteristics, especially the CHADS2 score, are not different between the tele‐AF and the ECG‐AF group, there is a significant difference in the mortality risk and the association with history of stroke. The mortality and stroke prevalence greater in the ECG‐AF group than in the no‐AF group is consistent with previous studies (Benjamin et al., 1998; Friberg et al., 2007; Olesen et al., 2011; Stewart et al., 2002; Wolf et al., 1991). However, it is a very surprising finding that, in this regard, the tele‐AF group is similar to the no‐AF group. An earlier hypothesis has to be taken into account here: i.e., that AF per se increases mortality (Benjamin et al., 1998; Friberg et al., 2007; Stewart et al., 2002) and that there is a similar risk of stroke in the various clinical classifications of AF (paroxysmal, persistent, and permanent) (Friberg, Hammar, & Rosenqvist, 2010; Hohnloser et al., 2007). A possible explanation for this apparent discrepancy is that the findings in the studies cited were obtained in patients with an established diagnosis of AF, whereas the subjects in the tele‐AF group of our study were presumably in an earlier phase of their AF course prior to an overt AF diagnosis.
Our data are also evidently in contrast with the main finding of the ASSERT trial, in which subclinical atrial arrhythmias detected by implantable devices were associated with an increased risk of embolic stroke (Healey et al., 2012). However, post‐hoc analysis of the ASSERT data to examine the temporal relation of AF and systemic embolism (Brambatti et al., 2014) raises questions regarding their direct causal relationship. It seems rather possible that AF is a marker of atrial pathology, which for its part is partially responsible for embolic risk. Owing to inclusion criteria (e.g., illness requiring device therapy such as dual chamber pacemaker or ICD, age ≥65, and history of hypertension), the patients in ASSERT represent a population at higher risk and, as such, are not directly comparable to our study population. This constellation supports the hypothesis of an underlying “atriopathy” that is more advanced in studies showing an AF‐related risk for embolism and mortality, but that is less distinct in our study population.
In contrast to ASSERT, other studies in patients with implanted devices are in line with our findings, given that they suggest a threshold for the duration of AF per day (AF burden) for adverse implications (Boriani et al., 2014; Glotzer et al., 2009; Swiryn et al., 2016). Due to the methodology of our ECG monitoring, we cannot precisely determine AF burden; however, the tele‐AF group will naturally demonstrate a lesser AF burden than does the ECG‐AF group.
4.3. Limitations
Even though inverse probability weighting to account for possible selection effects showed no effect on the results, the voluntary participation to the tele‐ECG subproject might still have given rise to selection bias which cannot be fully accounted for by the measured data. The conventional ECG was performed once at baseline, which can be considered a limitation since detection of AF could probably be improved by repeated recordings. The proportion of anticoagulation at baseline is inadequate given the CHADS‐score and AF diagnosis. The noninterventional design of SHIP prohibited initiation of anticoagulation as part of the study. Although participants were informed of abnormal findings and were advised to contact their primary care physician the implementation of therapy could not be monitored within the study. The information on stroke rates is limited to the participants’ history at baseline. Since follow‐up is limited to mortality, no prospective diagnosis of stroke is available. Therefore, our data cannot address whether AF detection by extended monitoring can be utilized to identify patients at risk for stroke.
5. Conclusions
Extended monitoring for atrial fibrillation by means of transtelephonic ECG is detecting significantly more cases compared to conventional ECG recording. This might have implications for preventive therapy.
However, based on our data, incidental AF detection by extended monitoring was associated neither with increased stroke rates in the patients’ history at baseline nor with increased mortality during follow‐up. Due to the small number of incidental AF cases, our data must be interpreted with caution. Nevertheless, speculation is in order that the current methodology of risk stratification derived from patient cohorts may not apply to extended ECG monitoring of the general population. Therefore, it appears necessary to evaluate a larger cohort with incidental AF detected by extended monitoring in order to clarify which patient is in need of prophylactic therapy: e.g., anticoagulation.
Busch MC, Gross S, Alte D, et al. Impact of atrial fibrillation detected by extended monitoring – A population‐based cohort study. Ann Noninvasive Electrocardiol. 2017;22:e12453 10.1111/anec.12453
Funding information
The Study of Health in Pomerania is part of the Community Medicine Research Network of University Medicine Greifswald, which was funded by the German Federal Ministry for Education and Research; the Ministry for Education, Research and Cultural Affairs; and the Ministry for Social Affairs of the German state of Mecklenburg‐West Pomerania. The tele‐ECG subproject was funded by a research grant by Biotronik (Berlin, Germany).
No author has any relationship with industry or financial associations within the past 2 years that might pose a conflict of interest in connection with this article.
References
- Alte, D. , Völzke, H. , Robinson, D. M. , Kleine, V. , Grabe, H. J. , John, U. , & Felix, S. B. (2006). Tele‐electrocardiography in the epidemiological “Study of Health in Pomerania” (SHIP). Journal of Telemedicine and Telecare, 12(2), 103–107. [DOI] [PubMed] [Google Scholar]
- Bazett, H. C. (1920). An analysis of the time‐relations of electrocardiograms. Heart (British Cardiac Society), 7, 353–370. [Google Scholar]
- Benjamin, E. J. , Wolf, P. A. , D'Agostino, R. B. , Silbershatz, H. , Kannel, W. B. , & Levy, D. (1998). Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation, 98(10), 946–952. [DOI] [PubMed] [Google Scholar]
- Boriani, G. , Glotzer, T. V. , Santini, M. , West, T. M. , de Melis, M. , Sepsi, M. , … Singer, D. E. (2014). Device‐detected atrial fibrillation and risk for stroke: An analysis of >10 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). European Heart Journal, 35(8), 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambatti, M. , Connolly, S. J. , Gold, M. R. , Morillo, C. A. , Capucci, A. , Muto, C. , … Healey, J. S. (2014). Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation, 129(21), 2094–2099. [DOI] [PubMed] [Google Scholar]
- de Bruyne, M. C. , Kors, J. A. , Hoes, A. W. , Kruijssen, D. A. , Deckers, J. W. , Grosfeld, M. , … van Bemmel, J. H. (1997). Diagnostic interpretation of electrocardiograms in population‐based research: Computer program research physicians, or cardiologists? Journal of Clinical Epidemiology, 50(8), 947–952. [DOI] [PubMed] [Google Scholar]
- Camm, A. J. , Lip, G. Y. H. , De Caterina, R. , Savelieva, I. , Atar, D. , Hohnloser, S. H. , … Kirchhof, P. (2012). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European Heart Journal, 33(21), 2719–2747. [DOI] [PubMed] [Google Scholar]
- Charitos, E. I. , Stierle, U. , Ziegler, P. D. , Baldewig, M. , Robinson, D. R. , Sievers, H. H. , & Hanke, T. (2012). A comprehensive evaluation of rhythm monitoring strategies for the detection of atrial fibrillation recurrence: Insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation, 126(7), 806–814. [DOI] [PubMed] [Google Scholar]
- Devereux, R. B. , Alonso, D. R. , Lutas, E. M. , Gottlieb, G. J. , Campo, E. , Sachs, I. , & Reichek, N. (1986). Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. American Journal of Cardiology, 57(6), 450–458. [DOI] [PubMed] [Google Scholar]
- Dörr, M. , Wolff, B. , Robinson, D. M. , John, U. , Lüdemann, J. , Meng, W. , … Völzke, H. (2005). The association of thyroid function with cardiac mass and left ventricular hypertrophy. Journal of Clinical Endocrinology and Metabolism, 90(2), 673–677. [DOI] [PubMed] [Google Scholar]
- Engdahl, J. , Andersson, L. , Mirskaya, M. , & Rosenqvist, M. (2013). Stepwise screening of atrial fibrillation in a 75‐year‐old population: implications for stroke prevention. Circulation, 127(8), 930–937. [DOI] [PubMed] [Google Scholar]
- Friberg, L. , & Bergfeldt, L. (2013). Atrial fibrillation prevalence revisited. Journal of Internal Medicine, 274(5), 461–468. [DOI] [PubMed] [Google Scholar]
- Friberg, L. , Hammar, N. , Pettersson, H. , & Rosenqvist, M. (2007). Increased mortality in paroxysmal atrial fibrillation: Report from the Stockholm Cohort‐Study of Atrial Fibrillation (SCAF). European Heart Journal, 28(19), 2346–2353. [DOI] [PubMed] [Google Scholar]
- Friberg, L. , Hammar, N. , & Rosenqvist, M. (2010). Stroke in paroxysmal atrial fibrillation: Report from the Stockholm Cohort of Atrial Fibrillation. European Heart Journal, 31(8), 967–975. [DOI] [PubMed] [Google Scholar]
- Fuster, V. , Rydén, L. E. , Cannom, D. S. , Crijns, H. J. , Curtis, A. B. , Ellenbogen, K. A. , … Zamorano, J. L. (2006). ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: Full text: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace, 8(9), 651–745. [DOI] [PubMed] [Google Scholar]
- Fuster, V. , Rydén, L. E. , Cannom, D. S. , Crijns, H. J. , Curtis, A. B. , Ellenbogen, K. A. , … Yancy, C. W. (2011). 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation, 123(10), e269–367. [DOI] [PubMed] [Google Scholar]
- Glotzer, T. V. , Daoud, E. G. , Wyse, G. , Singer, D. E. , Ezekowitz, M. D. , Hilker, C. , … Ziegler, P. D. (2009). The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circulation: Arrhythmia and Electrophysiology, 2(5), 474–480. [DOI] [PubMed] [Google Scholar]
- Haring, R. , Alte, D. , Völzke, H. , Sauer, S. , Wallaschofski, H. , John, U. , & Schmidt, C. O. (2009). Extended recruitment efforts minimize attrition but not necessarily bias. Journal of Clinical Epidemiology, 62(3), 252–260. [DOI] [PubMed] [Google Scholar]
- Healey, J. S. , Connolly, S. J. , Gold, M. R. , Israel, C. W. , Van Gelder, I. C. , Capucci, A. , … Hohnloser, S. H. (2012). Subclinical atrial fibrillation and the risk of stroke. The New England Journal of Medicine, 366(2), 120–129. [DOI] [PubMed] [Google Scholar]
- Hogan, J. W. , Roy, J. , & Korkontzelou, C. (2004). Handling drop‐out in longitudinal studies. Statistics in Medicine, 23(9), 1455–1497. [DOI] [PubMed] [Google Scholar]
- Hohnloser, S. H. , Pajitnev, D. , Pogue, J. , Healey, J. S. , Pfeffer, M. A. , Yusuf, S. , & Connolly, S. J. (2007). Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: An ACTIVE W Substudy. Journal of the American College of Cardiology, 50(22), 2156–2161. [DOI] [PubMed] [Google Scholar]
- Kamel, H. , Navi, B. B. , Elijovich, L. , Josephson, S. A. , Yee, A. H. , Fung, G. , … Smith, W. S. (2013). Pilot randomized trial of outpatient cardiac monitoring after cryptogenic stroke. Stroke, 44(2), 528–530. [DOI] [PubMed] [Google Scholar]
- Lévy, S. , Maarek, M. , Coumel, P. , Guize, L. , Lekieffre, J. , Medvedowsky, J. L. , & Sebaoun, A. (1999). Characterization of different subsets of atrial fibrillation in general practice in France: The ALFA study. The College of French Cardiologists. Circulation, 99(23), 3028–3035. [DOI] [PubMed] [Google Scholar]
- Miyasaka, Y. , Barnes, M. E. , Gersh, B. J. , Cha, S. S. , Bailey, K. R. , Abhayaratna, W. P. , … Tsang, T. S. (2006). Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation, 114(2), 119–125. [DOI] [PubMed] [Google Scholar]
- Olesen, J. B. , Lip, G. Y. H. , Hansen, M. L. , Hansen, P. R. , Tolstrup, J. S. , Lindhardsen, J. , … Torp‐Pedersen, C. (2011). Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ, 342, d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten, M. , Maas, R. , Karim, A. , Müller, H.‐W. , Simonovsky, R. , & Meinertz, T. (2006). Event‐recorder monitoring in the diagnosis of atrial fibrillation in symptomatic patients: Subanalysis of the SOPAT trial. Journal of Cardiovascular Electrophysiology, 17(11), 1216–1220. [DOI] [PubMed] [Google Scholar]
- Reichek, N. , & Devereux, R. B. (1981). Left ventricular hypertrophy: Relationship of anatomic, echocardiographic and electrocardiographic findings. Circulation, 63(6), 1391–1398. [DOI] [PubMed] [Google Scholar]
- Roche, F. , Gaspoz, J.‐M. , Da Costa, A. , Isaaz, K. , Duverney, D. , Pichot, V. , … Barthelemy, J. C. (2002). Frequent and prolonged asymptomatic episodes of paroxysmal atrial fibrillation revealed by automatic long‐term event recorders in patients with a negative 24‐hour Holter. Pacing and Clinical Electrophysiology, 25(11), 1587–1593. [DOI] [PubMed] [Google Scholar]
- Senatore, G. , Stabile, G. , Bertaglia, E. , Donnici, G. , De Simone, A. , Zoppo, F. , … Fazzari, M. (2005). Role of transtelephonic electrocardiographic monitoring in detecting short‐term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. Journal of the American College of Cardiology, 45(6), 873–876. [DOI] [PubMed] [Google Scholar]
- Singh, B. N. , Connolly, S. J. , Crijns, H. J. G. M. , Roy, D. , Kowey, P. R. , Capucci, A. , … Hohnloser, S. H. (2007). Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. New England Journal of Medicine, 357(10), 987–999. [DOI] [PubMed] [Google Scholar]
- Sinha, A.‐M. , Diener, H.‐C. , Morillo, C. A. , Sanna, T. , Bernstein, R. A. , Di Lazzaro, V. , … Brachmann, J. (2010). Cryptogenic stroke and underlying atrial fibrillation (CRYSTAL AF): Design and rationale. American Heart Journal, 160(1), 36–41. [DOI] [PubMed] [Google Scholar]
- Stewart, S. , Hart, C. L. , Hole, D. J. , & McMurray, J. J. (2001). Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart (British Cardiac Society), 86(5), 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, S. , Hart, C. L. , Hole, D. J. , & McMurray, J. J. V. (2002). A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. American Journal of Medicine, 113(5), 359–364. [DOI] [PubMed] [Google Scholar]
- Swiryn, S. , Orlov, M. V. , Benditt, D. G. , DiMarco, J. P. , Lloyd‐Jones, D. M. , Karst, E. , … Waldo, A. L. (2016). Clinical implications of brief device‐detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the registry of atrial tachycardia and atrial fibrillation episodes. Circulation, 134(16), 1130–1140. [DOI] [PubMed] [Google Scholar]
- Thrall, G. , Lane, D. , Carroll, D. , & Lip, G. Y. H. (2006). Quality of life in patients with atrial fibrillation: A systematic review. American Journal of Medicine, 119(5), 448. e1–19. [DOI] [PubMed] [Google Scholar]
- Völzke, H. , Alte, D. , Schmidt, C. O. , Radke, D. , Lorbeer, R. , Friedrich, N. , … Hoffmann, W. (2011). Cohort profile: The study of health in Pomerania. International Journal of Epidemiology, 40(2), 294–307. [DOI] [PubMed] [Google Scholar]
- Wolf, P. A. , Abbott, R. D. , & Kannel, W. B. (1991). Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke, 22(8), 983–988. [DOI] [PubMed] [Google Scholar]


