Abstract
Background
The electrocardiographic criteria currently available for the diagnosis of left ventricular hypertrophy (LVH) are low in sensitivity. Thus, we compared the diagnostic performance of newly proposed electrocardiographic criteria to the existing criteria in a Chinese population.
Methods
A total of 235 consecutive hypertensive patients, hospitalized in our department between May 2017 and April 2018, were included. They were divided into two groups based on the gold standard echocardiogram: those with (n = 116) and without LVH (n = 119). The newly proposed ECG criteria were calculated by summating the amplitude of the deepest S wave (SD) in any single lead and the S‐wave amplitude of lead V4 (SV4). The area under the curve was calculated and compared against the sex‐specific Cornell limb lead and Sokolow–Lyon criteria.
Results
ECG analysis of the cohort showed that the newly proposed criteria had the highest sensitivity in diagnosing LVH (male: 65.5%; female: 81%), followed by the Cornell limb lead criteria (male: 55.2%; female: 56.9%). The specificities of both sets of criteria were higher than 70%, with no significant differences between them. Receiver operator curve analysis showed an optimal cutoff of ≥2.1 mV for females (AUC: 0.832; 95% CI: 0.757–0.906) and ≥2.6 mV for males (AUC: 0.772; 95% CI: 0.687–0.856).
Conclusion
The newly proposed SD + SV4 criteria provide an improved sensitivity for the ECG diagnosis of LVH compared to existing criteria, but its routine use will require further validation in larger populations.
Keywords: cornell voltage criteria, ECG criteria, left ventricular hypertrophy, peguero‐Lo presti criteria, sensitivity
1. INTRODUCTION
Left ventricular hypertrophy (LVH) is a pathological adaptation to underlying cardiovascular disease and a strong determinant for cardiovascular morbidity and mortality (Bombelli et al., 2009; Gosse et al., 2012; Schillaci, Battista, & Pucci, 2012; Verdecchia et al., 2001). In recent years, studies have reported the reversible nature of LVH, which has led to a reduction in adverse clinical outcomes (Verdecchia et al., 2003). As such, the early and correct diagnosis of LVH is of paramount importance. The electrocardiogram (ECG), being simple, economic, and convenient, is one of the most common tools for the screening and diagnosis of LVH. However, 37 electrocardiographic criteria have been endorsed by the American Heart Association to date, the abundance of which leads to confusion among diagnosing clinicians (Bacharova & Ugander, 2014; Hancock et al., 2009). Moreover, most of these criteria have high specificities but low sensitivities overall, for instance, the Cornell voltage criteria, with 90% specificity but only 20%‐40% sensitivity (Casale et al., 1985; Schillaci et al., 1994). Therefore, a new ECG standard with higher sensitivity and specificity must be explored with utmost urgency. Recently, Peguero et al. (2017) established novel ECG criteria that take the voltage amplitudes that occur within each lead into consideration. The authors found that the summation of the amplitude of the deepest S wave in any lead (SD) with the S wave in lead V4 (SV4) improves the sensitivity of the currently existing criteria, while maintaining an adequate specificity for the diagnosis of LVH. Our aim was to investigate the correlation of these novel ECG criteria in the diagnosis of LVH with hypertensive patients in a Chinese population.
2. METHODS
2.1. Study population
A total of 235 consecutive hypertensive patients who were hospitalized in our department between May 2017 and April 2018 were recruited sequentially for participation in the study. The patients were divided into two groups according to echocardiographic findings: those with LVH (n = 116) and those without LVH as controls (n = 119). Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg (Mancia et al., 2013), or if the patient was receiving any type of antihypertensive medication. Transthoracic echocardiography was used to diagnose LVH, defined as interventricular septum and left ventricular posterior wall thickness of over 11 mm each (Lang et al., 2016). Diabetes mellitus was defined as having a history of antidiabetic medication use or a fasting glucose level ≥126 mg/dl (Nang et al., 2009). Hypercholesterolemia was defined as total cholesterol >220 mg/dl, low‐density lipoprotein cholesterol >140 mg/dl, high‐density lipoprotein cholesterol <40 mg/dl, fasting triglycerides >150 mg/dl, or if the patient was being medically treated for the condition. A smoking habit was defined as smoking >2 pack‐years. The exclusion criteria were as follows: (a) atrial or ventricular arrhythmias, (b) complete left or right bundle branch block, (c) inability to obtain or unclear echocardiographic images, (d) a history of myocardial infarction, and (e) ventricular paced rhythm. All subjects provided written informed consent before enrollment in the study.
2.2. Study protocol
Patient demographics (sex, age, and medical history) were collected, and the standard 12‐lead ECG and echocardiography examinations were conducted during the same visit. Complete blood count (CBC) was taken on admission, using established clinical laboratory methods (Coulter BC‐5380/6800 Hematology Analyzer; Mindray, Shenzhen, China) for white blood cell count (WBC), hemoglobin (Hb), and red blood cell distribution width (RDW). Uric acid (UA), serum creatinine (Cr), and fasting blood glucose (FBG) were measured after overnight fasting (12 hr) by a TBA‐120 FR analyzer (Toshiba, Japan), using the turbidimetric method.
A transthoracic echocardiographic examination was performed in all patients using the Vivid‐7 system equipped with a 2.4 MHz transducer (GE Medical Systems, Milwaukee, WI, USA). Left atrial diameter (LAD), interventricular septal thickness (IVST), left ventricular posterior wall thickness (LVPWT), and left ventricular end‐diastolic diameter (LVEDD) were assessed. Left ventricular ejection fraction (LVEF) was determined from apical four‐chamber and two‐chamber views using Simpson's biplane formula. All echocardiographic data were analyzed by two investigators who were blinded to the clinical status of the study subjects.
2.3. ECG analysis
A single 12‐lead ECG (25 mm, s/10 mm, mV; Beijing Foton Electronic Medical Instrument Co. LTD FX‐7402, China), taken on admission, was selected for each patient. It was independently interpreted by two cardiologists (Q.S and L.M). Individual leads were analyzed by measuring the tallest R or R’ and the deepest S or QS complex in all the precordial and limb leads using the PR segment as baseline. In cases of voltage differences within the same lead, only the largest complex was selected. SD + SV4 criteria were defined as the summation of the amplitude of the deepest S wave in any lead (SD) with the S wave in lead V4 (SV4). The sex‐specific Cornell voltage criteria were computed as the amplitude of R in aVL plus the amplitude of S or QS complex in V 3 (RaVL + SV3) with a cutoff of >2.8 mV in men and >2.0 mV in women (Casale et al., 1985). The Sokolow–Lyon voltage criteria (Hancock et al., 2009) were obtained by adding the amplitude of the S wave in V1 and the amplitude of R in V5 or V6 ≥3.5 mV (SV1 + RV5 or RV6; Figure 1).
Figure 1.
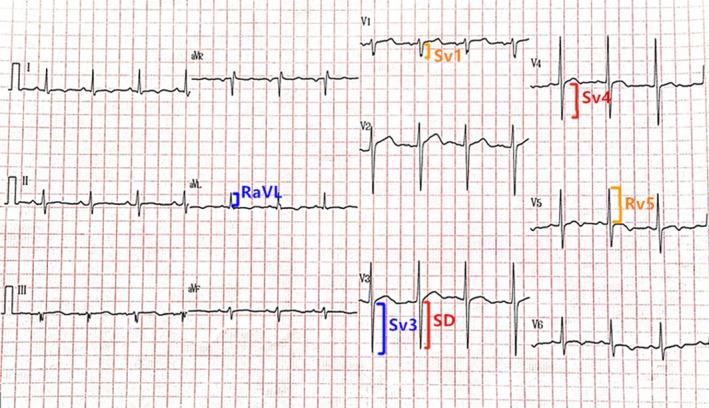
An example electrocardiogram of a 43‐year‐old man that meets criteria for left ventricular hypertrophy based on the newly proposed criteria (deepest S wave in any lead and S wave in V4 [SD + SV4]; 1.7 + 1.2 = 2.9 mV [male subjects ≥ 2.8 mV]). By contrast, LVH was not diagnosed using either the Cornell limb lead criteria (RaVL + SV3; 0.6 + 1.7 = 2.3 mV [male subjects > 2.8 mV]) or the Sokolow–Lyon criteria (SV1 + RV5; 0.4 + 1.3 = 1.7 mV [male subjects ≥ 3.5 mV])
2.4. Statistical analysis
The normality of the distribution of each continuous variable was tested by the Kolmogorov–Smirnov test. Categorical variables were reported as counts (percentage) and continuous variables as means ± SD or median (interquartile range). Statistical analysis was performed using the independent sample t test for continuous variables with normal distribution and Mann–Whitney U for non‐normally distributed data, while the chi‐square test was used to compare categorical variables. Receiver operating curves (ROC) were analyzed to assess the best cutoff values for ECG criteria (SD + SV4, RaVL + SV3) to discriminate LVH from hypertensive patients. Multivariable regression analyses were performed in order to investigate the factors that influence ECG morphology for the newly proposed SD + SV4 criteria in the study population. Only p values < 0.05 were regarded as statistically significant. All tests were two‐tailed, and analyses were performed using SPSS 17.0 Statistical Package Program for Windows (SPSS Inc., Chicago, IL, USA).
3. RESULTS
A total of 116 hypertensive patients with LVH (50% male; mean age 65.7 years), and 119 sex‐ and age‐matched hypertensive patients without LVH (48.7% male; mean age 64.9 years) were included in our study. Both groups were comparable in terms of baseline characteristics, except for the higher incidences of stroke and renal insufficiency in the LVH group (p < 0.05; Table 1). Regarding the laboratory tests, the LVH group showed higher RDW, UA, and Cr levels, while WBC, Hb, and FBG levels did not differ significantly (Table 2). Echocardiographic analysis showed significantly higher LAD, LVEDD, LVPWT, and IVST, but similar LVEF (Table 2).
Table 1.
Baseline clinical and demographic properties of study population
|
Non‐LVH (n = 119) |
LVH (n = 116) |
p | |
|---|---|---|---|
| Age, years | 64.9 ± 9.8 | 65.7 ± 11.8 | 0.242 |
| Sex, male (n, %) | 58 (48.7) | 58 (50.0) | 0.847 |
| Diabetes mellitus (n, %) | 21 (17.6) | 50 (43.1) | 0.034 |
| Hypercholesterolemia (n, %) | 54 (45.4) | 53 (45.7) | 0.894 |
| Coronary heart disease (n, %) | 30 (25.2) | 34 (29.3) | 0.558 |
| Stroke (n, %) | 9 (7.6) | 23 (19.8) | 0.007 |
| Renal insufficiency (n, %) | 5 (4.2) | 19 (16.4) | 0.000 |
| Smoking habit (n, %) | 22 (18.5) | 27 (23.3) | 0.415 |
| Previous medication (n, %) | |||
| ACEI/ARB | 67 (56.3) | 79 (68.1) | 0.181 |
| Beta‐blocker | 24 (20.2) | 31 (26.7) | 0.151 |
| CCB | 65 (54.6) | 57 (49.1) | 0.435 |
| Diuretic | 12 (10.1) | 9 (7.8) | 0.649 |
ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blocking agents; CCB, calcium channel blockers; LVH, left ventricle hypertrophy.
Table 2.
Laboratory, electrocardiographic, and echocardiographic parameters of the study population
|
Non‐LVH (n = 119) |
LVH (n = 116) |
p | |
|---|---|---|---|
| Laboratory parameters | |||
| WBC Count (10*9/L) | 6.99 ± 1.41 | 6.90 ± 1.59 | 0.663 |
| Hb (g/L) | 138.46 ± 15.24 | 135.68 ± 19.40 | 0.227 |
| RDW (%) | 12.72 ± 0.46 | 13.28 ± 0.90 | 0.000 |
| UA (µmol/L) | 333.35 ± 79.00 | 365.44 ± 84.83 | 0.017 |
| Cr (µmol/L) | 67.64 ± 14.12 | 88.83 ± 36.47 | 0.000 |
| FBG (mmol/L) | 5.90 ± 1.60 | 5.88 ± 1.75 | 0.919 |
| Echocardiogram variables | |||
| LAD (mm) | 37.30 ± 4.77 | 41.67 ± 6.67 | 0.000 |
| LVEDD (mm) | 46.47 ± 4.10 | 48.04 ± 4.95 | 0.008 |
| LVPWT (mm) | 9.00 ± 0.85 | 12.25 ± 1.10 | 0.000 |
| IVST (mm) | 8.97 ± 0.93 | 12.45 ± 1.19 | 0.000 |
| LVEF (%) | 62.05 ± 5.53 | 61.36 ± 7.14 | 0.415 |
| Electrocardiographic parameters | |||
| Peguero‐Lo criteria (SD + SV4) | 1.47 ± 0.61 | 2.35 ± 0.95 | 0.000 |
| Cornell limb lead criteria (RavL + SV3) | 1.25 ± 0.51 | 1.68 ± 0.61 | 0.000 |
| Sokolow–Lyon criteria (SV1 + RV5) | 1.90 ± 0.71 | 2.09 ± 0.86 | 0.061 |
Cr: serum creatinine; FBG: fasting blood glucose; Hb: hemoglobin; IVST: interventricular septal thickness; LAD: left atrial diameter; LVEDD: left ventricular end‐diastolic diameter; LVEF: left ventricular ejection fraction; LVH: left ventricle hypertrophy; LVPWT: left ventricular posterior wall thickness; RDW: red blood cell distribution width; UA: uric acid; WBC: white blood cell.
SD + SV4: deepest S wave in any lead plus S wave in V4. RavL + SV3: the amplitude of R in aVL plus the amplitude of S or QS complex in V3. Sv1 + Rv5: the amplitude of S wave in V1 plus the amplitude of R wave in V5.
Electrocardiographic analysis revealed higher voltage values in the LVH group compared to the non‐LVH group using both the newly proposed (SD + SV4; 2.35 ± 0.95 vs. 1.47 ± 0.61, p < 0.0001) and Cornell limb lead criteria (RavL + SV3; 1.68 ± 0.61 vs. 1.25 ± 0.51, p < 0.0001). By contrast, the Sokolow–Lyon criteria (SV1 + RV5) found no significant difference between the groups (2.09 ± 0.86 vs. 1.90 ± 0.71, p = 0.06). Subsequently, sensitivity and specificity values were compared between the different sets of criteria. The newly proposed criteria showed the best sensitivity (male: 65.5%; female: 81.0%), followed by the Cornell limb lead criteria (male: 55.2%; female: 56.9%), and finally the Sokolow–Lyon criteria (male: 63.8%; female: 51.7%). The specificity of the newly proposed criteria (male: 74.1%; female: 77%) and the specificity of the Cornell limb lead criteria (male: 75.9%; female: 94.0%) were both higher than that of the Sokolow–Lyon criteria (male: 56.9%; female: 59.0%).
Receiver operating characteristic curves were then constructed. This permitted optimal cutoff values to be determined according to the maximum Youden index (the sum of the sensitivity and specificity). With regards to the novel criteria, values of ≥2.6 mV for male (AUC: 0.772; 95% CI: 0.687–0.856) and ≥2.1 mV for female subjects (AUC: 0.832; 95% CI: 0.757–0.906) were considered positive for LVH (Figures 2 and 3). Our data suggest that the Cornell limb lead criteria predicted LVH at lower values (males: 2.04 mV; females: 1.71 mV) than in the guidelines (2.8, 2.0 mV, respectively). We also observed the same phenomenon in the standard of the Sokolow–Lyon criteria (males: 2.05 mV; females: 1.95 mV). This may suggest different cutoff values for Chinese ethnicity.
Figure 2.
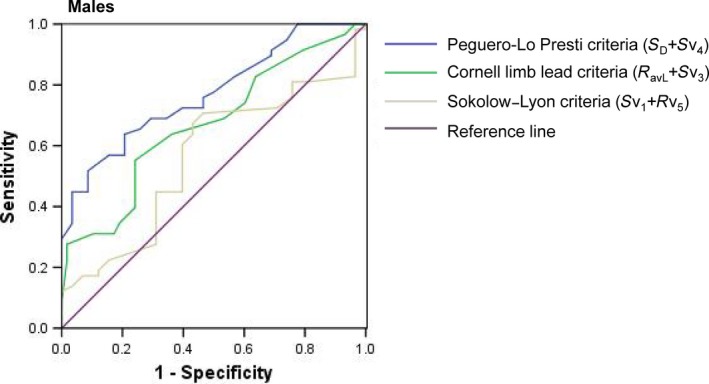
Receiver operating curve of the three criteria for the diagnosis of left ventricular hypertrophy in the study population for males
Figure 3.
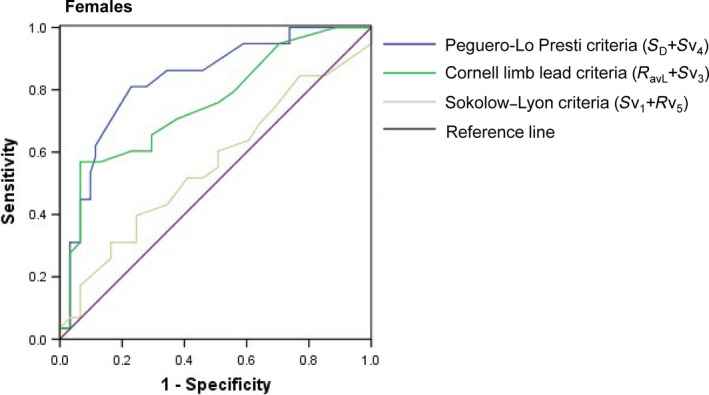
Receiver operating curve of the three criteria for the diagnosis of left ventricular hypertrophy in the study population for females
Multivariable regression analyses were performed in order to investigate the factors that influence ECG morphology for the newly proposed SD + SV4 criteria in the study population. A few of factors that were variably distributed in the population were selected for the regression analysis. We found that that age and LVEF were independently associated with ECG morphology for the newly proposed SD + SV4 criteria in the study population for males (OR 1.041, 95% CI 1.004–1.077; OR 0.908, 95% CI 0.841–0.981; p < 0.05, respectively; Table 3), and only LVEF was independently associated with ECG morphology for the newly proposed SD + SV4 criteria in the study population for females (OR 0.901, 95% CI 0.813–0.998; p < 0.05; Table 4). In addition, chest lead voltage is, of course, heavily dependent on the location of the electrode. We showed a figure where we vary the position of V4 electrode for a patient and record the consequent changes in S‐wave voltage (Figure 4), so the accurate placement of electrode is the primary factor.
Table 3.
Multivariate logistic regression analysis on influencing ECG morphology for the newly proposed SD + SV4 criteria in the study population for males
| B | SE | Wald | p | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| LAD | 0.090 | 0.052 | 3.057 | 0.080 | 1.095 | 0.989 | −1.211 |
| LVEDD | 0.023 | 0.067 | 0.124 | 0.725 | 1.024 | 0.898 | −1.167 |
| LVEF | −0.096 | 0.039 | 5.994 | 0.014 | 0.908 | 0.841 | −0.981 |
| age | 0.042 | 0.019 | 4.607 | 0.032 | 1.041 | 1.004 | −1.077 |
| Hypercholesterolemia | −0.306 | 0.524 | 0.341 | 0.559 | 0.736 | 0.263 | −2.058 |
| Cr | 0.001 | 0.007 | 0.018 | 0.892 | 1.001 | 0.987 | −1.015 |
| K | 0.075 | 0.454 | 0.027 | 0.869 | 1.078 | 0.442 | −2.626 |
Cr: creatinine; LAD: left atrial diameter; LVEDD: left ventricular end‐diastolic diameter; LVEF: left ventricular ejection fraction.
Table 4.
Multivariate logistic regression analysis on influencing ECG morphology for the newly proposed SD + SV4 criteria in the study population for females
| B | SE | Wald | p | OR | 95% CI | ||
|---|---|---|---|---|---|---|---|
| LAD | 0.062 | 0.052 | 1.451 | 0.228 | 1.064 | 0.962 | −1.178 |
| LVEDD | 0.163 | 0.086 | 3.585 | 0.058 | 1.177 | 0.994 | −1.394 |
| LVEF | −0.105 | 0.052 | 4.013 | 0.045 | 0.901 | 0.813 | −0.998 |
| age | −0.013 | 0.029 | 0.198 | 0.656 | 0.987 | 0.934 | −1.044 |
| Hypercholesterolemia | −0.293 | 0.585 | 0.251 | 0.616 | 0.746 | 0.237 | −2.347 |
| Cr | 0.020 | 0.016 | 1.563 | 0.211 | 1.020 | 0.989 | −1.053 |
| K | −1.233 | 0.655 | 3.545 | 0.060 | 0.291 | 0.081 | −1.052 |
Cr: creatinine; LAD: left atrial diameter; LVEDD: left ventricular end‐diastolic diameter; LVEF: left ventricular ejection fraction.
Figure 4.
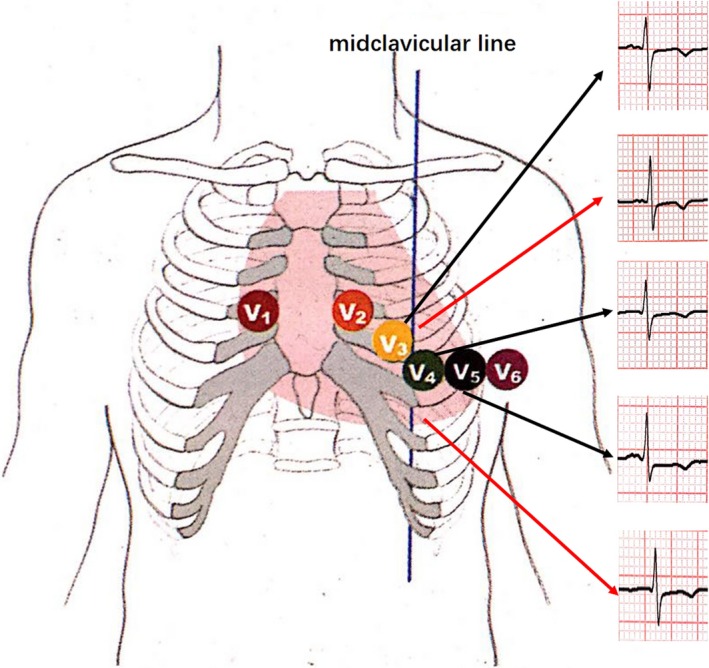
An example picture of the consequent changes in S‐wave voltage according to different position of V4 electrode for a patient
4. DISCUSSION
LVH is an independent risk factor associated with a high risk of adverse outcomes (Agabiti‐Rosei & Muiesan, 2002; Vakili, Okin, & Devereux, 2001). Currently, LVH can be diagnosed using the standard 12‐lead ECG, as well as imaging methods such as echocardiography and cardiac magnetic resonance (CMR; Alfakih, Reid, Hall, & Sivananthan, 2006). Out of these three modalities, the ECG is most frequently used due to its convenient, economic, and user‐friendly nature (Oseni et al., 2017; Vassiliou et al., 2014). LVH assessed by electrocardiography has been shown to be a good marker of subclinical cardiac damage and a strong predictor of adverse cardiovascular events (Brinkley et al., 2018). The amplitude of the electrical signals depends not only on myocardial cell numbers (Tse, Wong, Tse, & Yeo, 2016), but also on the active and passive electrical characteristics of these cells. These in turn are modified by influencing factors such as the difference between the surface electrodes and the myocardial tissue, electrodeposition, conduction abnormalities, myocardial fibrosis, emphysema, and other factors (Bacharova & Ugander, 2014; Casale, Devereux, Alonso, Campo, & Kligfield, 1987; Tse & Yeo, 2015).
A number of electrocardiographic criteria for LVH have been proposed, and the commonest used are the Sokolow–Lyon criteria (S‐wave depth in V1 + tallest R‐wave height in V5‐V6 >35 mm for both genders) and the Cornell limb lead criteria (S‐wave depth in V3 + R‐wave amplitude in aVL >28 mm in males and >20 mm in females; Okin, Hille, Kjeldsen, & Devereux, 2017). Although these criteria have high specificities, their sensitivities are low. A meta‐analysis has shown that LVH diagnosed electrocardiographically is a poor predictor of echocardiographic diagnosis of LVH (sensitivity of 10.5%–21% and specificity of 89%–99%; Pewsner et al., 2007). Similar results were obtained in a study that compared the diagnostic yield of LVH to CMR (Jain et al., 2010). Therefore, there is a clinical need to devise new ECG criteria with higher sensitivity values.
The traditional ECG criteria emphasize measurement of the R‐wave amplitude of the highest lead. Yet, the second part (S wave) can better reflect the main depolarization vector of the ventricular free wall. In the human heart, four depolarization vectors have been described. The first 30 ms consists of depolarization of the interventricular septum and the left ventricular endocardium (Ramanathan, Jia, Ghanem, Ryu, & Rudy, 2006). The third and fourth vectors represent depolarization of the left ventricular myocardium and epicardial free wall, which occur no earlier than 50 ms within the start of ventricular depolarization (Durrer et al., 1970). Thus, it is conceivable that changes in voltage that occur in patients with mild to moderate LVH are better represented by the latter part of the QRS complex, which corresponds to the S wave.
The measurement of maximum voltage in any lead, rather than a fixed lead, would significantly increase the sensitivity. Therefore, identifying these early changes may increase the sensitivity of the ECG. Peguero et al. (2017) showed that the SD is the best single lead with which to diagnose LVH, and furthermore, the diagnostic value of SD + SV4 is higher than that of SD alone. Our study also showed that SD + SV4 criteria had nominally the best sensitivity (for male: 65.5%; for female: 81%), followed by the Cornell voltage criteria (for male: 55.2%; for female: 56.9%). Therefore, application of these novel criteria in the diagnosis of LVH in lieu of traditional indicators results in higher sensitivity and specificity.
4.1. Limitations
Some limitations of this study should be considered. First, the study is from a single center with a relatively small sample size, for which the utility of the AUC statistical method may be limited (Cook, 2008, 2010 ). Secondly, interventricular septum and left ventricular posterior wall thickness were estimated by using two‐dimensional echocardiography, although better accuracy would be achieved using CMR (Bacharova & Ugander, 2014; Casale et al., 1987). Nonetheless, echocardiography is known to have good reproducibility for the diagnosis of LVH and remains the most frequently used method in clinical practice (Palmieri et al., 1999). Thirdly, subjects in this study were selected from a population that required echocardiography examinations. Therefore, our findings cannot be applied to the general and otherwise healthy population. Fourthly, we only compared the newly proposed criteria to the two commonest methods, the Cornell limb lead criteria, and Sokolow–Lyon criteria. We therefore cannot exclude the possibility that other criteria may perform with higher sensitivities than the latter two criteria studied here. Finally, the proposed criteria did not improve upon the limitations of previous criteria in diagnosing LVH in patients with right or left bundle branch block, ventricular paced rhythm, concomitant right ventricular hypertrophy, and other cardiomyopathies that might influence the accuracy of the SD + SV4 criteria, as these subgroups were excluded from the study and axis shits also influence the amplitude of S wave in V4. It needs large‐scale studies to verify the accuracy of the SD + SV4 criteria on axis shits or intraventricular conduction delay. Moreover, we found that the cutoff values for LVH used in this study were lower than in the guidelines, which may be explained by ethnic differences in optimal cutoffs. The impact of ethnicity on the sensitivity and specificity of the newly proposed criteria in diagnosing LVH was not addressed in this study; further exploration is required in this area.
5. CONCLUSIONS
The newly proposed SD + SV4 criteria provide an improved sensitivity for the ECG diagnosis of LVH compared to existing criteria, but its routine use will require further validation in larger populations. Early detection of LVH and interventions aimed at prevention and/or regression of LVH are to be encouraged. However, further validation on a larger population is warranted.
CONFLICTS OF INTEREST
None.
Shao Q, Meng L, Tse G, et al. Newly proposed electrocardiographic criteria for the diagnosis of left ventricular hypertrophy in a Chinese population. Ann Noninvasive Electrocardiol. 2019;24:e12602 10.1111/anec.12602
The first two authors contributed equally to this study.
Contributor Information
Guangping Li, Email: liutongdoc@126.com.
Tong Liu, Email: tic_tjcardiol@126.com.
REFERENCES
- Agabiti‐Rosei, E. , & Muiesan, M. L. (2002). Left ventricular hypertrophy and heart failure in women. Journal of Hypertension, 20, S34–S38. [PubMed] [Google Scholar]
- Alfakih, K. , Reid, S. , Hall, A. , & Sivananthan, M. U. (2006). The assessment of left ventricular hypertrophy in hypertension. Journal of Hypertension, 24, 1223–1230. 10.1097/01.hjh.0000234097.47379.fd [DOI] [PubMed] [Google Scholar]
- Bacharova, L. , & Ugander, M. (2014). Left ventricular hypertrophy: The relationship between the electrocardiogram and cardiovascular magnetic resonance imaging. Annals of Noninvasive Electrocardiology, 19, 524–533. 10.1111/anec.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombelli, M. , Facchetti, R. , Carugo, S. , Madotto, F. , Arenare, F. , Quarti‐Trevano, F. , … Mancia, G. (2009). Left ventricular hypertrophy increases cardiovascular risk independently of in‐office and out‐of‐office blood pressure values. Journal of Hypertension, 27, 2458–2464. 10.1097/HJH.0b013e328330b845 [DOI] [PubMed] [Google Scholar]
- Brinkley, T. E. , Anderson, A. , Soliman, E. Z. , Bertoni, A. G. , Greenway, F. , Knowler, W. C. , … Espeland, M. A. (2018). Long‐Term Effects of an Intensive Lifestyle Intervention on Electrocardiographic Criteria for Left Ventricular Hypertrophy: The Look AHEAD Trial. American Journal of Hypertension, 31, 541–548. 10.1093/ajh/hpy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale, P. N. , Devereux, R. B. , Alonso, D. R. , Campo, E. , & Kligfield, P. (1987). Improved sex‐specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: Validation with autopsy findings. Circulation, 75, 565–572. 10.1161/01.CIR.75.3.565 [DOI] [PubMed] [Google Scholar]
- Casale, P. N. , Devereux, R. B. , Kligfield, P. , Eisenberg, R. R. , Miller, D. H. , Chaudhary, B. S. , & Phillips, M. C. (1985). Electrocardiographic detection of left ventricular hypertrophy: Development and prospective validation of improved criteria. Journal of the American College of Cardiology, 6, 572–580. 10.1016/S0735-1097(85)80115-7 [DOI] [PubMed] [Google Scholar]
- Cook, N. R. (2008). Statistical evaluation of prognostic versus diagnostic models: Beyond the ROC curve. Clinical Chemistry, 54, 17–23. 10.1373/clinchem.2007.096529 [DOI] [PubMed] [Google Scholar]
- Cook, N. R. (2010). Assessing the incremental role of novel and emerging risk factors. Current Cardiovascular Risk Reports, 4, 112–119. 10.1007/s12170-010-0084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrer, D. , van Dam, R. T. , Freud, G. E. , Janse, M. J. , Meijler, F. L. , & Arzbaecher, R. C. (1970). Total excitation of the isolated human heart. Circulation, 41, 899–912. 10.1161/01.CIR.41.6.899 [DOI] [PubMed] [Google Scholar]
- Gosse, P. , Jan, E. , Coulon, P. , Cremer, A. , Papaioannou, G. , & Yeim, S. (2012). ECG detection of left ventricular hypertrophy: The simpler, the better? Journal of Hypertension, 30, 990–996. 10.1097/HJH.0b013e3283524961 [DOI] [PubMed] [Google Scholar]
- Hancock, E. W. , Deal, B. J. , Mirvis, D. M. , Okin, P. , Kligfield, P. , Gettes, L. S. , … Wellens, H. (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology, 53, 992–1002. [DOI] [PubMed] [Google Scholar]
- Jain, A. , Tandri, H. , Dalal, D. , Chahal, H. , Soliman, E. Z. , Prineas, R. J. , … Bluemke, D. A. (2010). Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: The Multi‐Ethnic Study of Atherosclerosis (MESA). American Heart Journal, 159, 652–658. 10.1016/j.ahj.2009.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , … Voigt, J. U. (2016). Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of, Cardiovascular Imaging. European Heart Journal of Cardiovascular Imaging, 17, 412. [DOI] [PubMed] [Google Scholar]
- Mancia, G. , Fagard, R. , Narkiewicz, K. , Redon, J. , Zanchetti, A. , Böhm, M. , … Members, F. (2013). ESH/ESC Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal, 2013(34), 2159–2219. [DOI] [PubMed] [Google Scholar]
- Nang, E. E. , Khoo, C. M. , Tai, E. S. , Lim, S. C. , Tavintharan, S. , Wong, T. Y. , … Lee, J. (2009). Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease? The Singapore prospective study program. American Journal of Epidemiology, 169, 1454–1462. 10.1093/aje/kwp076 [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Hille, D. A. , Kjeldsen, S. E. , & Devereux, R. B. (2017). Combining ECG criteria for left ventricular hypertrophy improves risk prediction in patients with hypertension. Journal of the American Heart Association, 6, e007564 10.1161/JAHA.117.007564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseni, A. O. , Qureshi, W. T. , Almahmoud, M. F. , Bertoni, A. G. , Bluemke, D. A. , Hundley, W. G. , … Soliman, E. Z. (2017). Left ventricular hypertrophy by ECG versus cardiac MRI as a predictor for heart failure. Heart, 103, 49–54. 10.1136/heartjnl-2016-309516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri, V. , Dahlöf, B. , DeQuattro, V. , Sharpe, N. , Bella, J. N. , de Simone, G. , … Devereux, R. B. (1999). Reliability of echocardiographic assessment of left ventricular structure and function: The PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. Journal of the American College of Cardiology, 34, 1625–1632. 10.1016/S0735-1097(99)00396-4 [DOI] [PubMed] [Google Scholar]
- Peguero, J. G. , Lo Presti, S. , Perez, J. , Issa, O. , Brenes, J. C. , & Tolentino, A. (2017). Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. Journal of the American College of Cardiology, 69, 1694–1703. 10.1016/j.jacc.2017.01.037 [DOI] [PubMed] [Google Scholar]
- Pewsner, D. , Jüni, P. , Egger, M. , Battaglia, M. , Sundström, J. , & Bachmann, L. M. (2007). Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: Systematic review. BMJ, 335, 711 10.1136/bmj.39276.636354.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan, C. , Jia, P. , Ghanem, R. , Ryu, K. , & Rudy, Y. (2006). Activation and repolarization of the normal human heart under complete physiological conditions. Proceedings of the National Academy of Sciences of the United States of America, 103, 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillaci, G. , Battista, F. , & Pucci, G. (2012). A review of the role of electrocardiography in the diagnosis of left ventricular hypertrophy in hypertension. Journal of Electrocardiology, 45, 617–623. 10.1016/j.jelectrocard.2012.08.051 [DOI] [PubMed] [Google Scholar]
- Schillaci, G. , Verdecchia, P. , Borgioni, C. , Ciucci, A. , Guerrieri, M. , Zampi, I. , … Porcellati, C. (1994). Improved electrocardiographic diagnosis of left ventricular hypertrophy. American Journal of Cardiology, 74, 714–719. 10.1016/0002-9149(94)90316-6 [DOI] [PubMed] [Google Scholar]
- Tse, G. , Wong, S. T. , Tse, V. , & Yeo, J. M. (2016). Monophasic action potential recordings: Which is the recording electrode? Journal of Basic and Clinical Physiology and Pharmacology, 27, 457–462. 10.1515/jbcpp-2016-0007 [DOI] [PubMed] [Google Scholar]
- Tse, G. , & Yeo, J. M. (2015). Conduction abnormalities and ventricular arrhythmogenesis: The roles of sodium channels and gap junctions. IJC Heart Vasculature, 9, 75–82. 10.1016/j.ijcha.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakili, B. A. , Okin, P. M. , & Devereux, R. B. (2001). Prognostic implications of left ventricular hypertrophy. American Heart Journal, 141, 334–341. 10.1067/mhj.2001.113218 [DOI] [PubMed] [Google Scholar]
- Vassiliou, V. , Chin, C. , Perperoglou, A. , Tse, G. , Ali, A. , Raphael, C. , … Prasad, S. (2014). 93 Ejection fraction by cardiovascular magnetic resonance predicts adverse outcomes post aortic valve replacement. Heart, 100, A53–A54. 10.1136/heartjnl-2014-306118.93 [DOI] [Google Scholar]
- Verdecchia, P. , Angeli, F. , Borgioni, C. , Gattobigio, R. , de Simone, G. , Devereux, R. B. , & Porcellati, C. (2003). Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: A meta‐analysis. American Journal of Hypertension, 16, 895–899. 10.1016/S0895-7061(03)01018-5 [DOI] [PubMed] [Google Scholar]
- Verdecchia, P. , Porcellati, C. , Reboldi, G. , Gattobigio, R. , Borgioni, C. , Pearson, T. A. , & Ambrosio, G. (2001). Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation, 104, 2039–2044. 10.1161/hc4201.097944 [DOI] [PubMed] [Google Scholar]


