Abstract
Background
So far, the specific appearance of QRS complex, ST‐segment, and T wave was observed in aortic stenosis (AS). S‐wave dynamic change in leads V1–V3 was not reported in AS.
Methods
In a single‐center, prospective study, we included a total number of 1.175 patients who underwent surgical aortic valve replacement (AVR). We conducted 3‐year gathering of patients with symptomatic and asymptomatic severe AS, and separated them by hemodynamic stability into groups A and B, through EFLV (of more or less than 50%), AVA (of more or less than 0.9 cm2), PG (between 55 and 75 mm Hg or over 75 mm Hg), and end‐diastolic LV dimension (of more or less than 56 mm). We evaluated the impact of S‐wave magnitude in right precordial leads before and after AVR in all patients. We followed S‐wave changes in electrocardiogram altogether with hemodynamic measurements derived from echocardiography.
Results
Analysis of echocardiographic parameters, measured in patients before surgery, did not show statistical significance between asymptomatic and symptomatic group. The statistical significance was observed in the change in S‐wave magnitude in the right precordial leads in both subsets of patients before AVR. We found statistically significant predictive value of S‐wave magnitude in leads V2–V3 for dependent variables PG and end‐diastolic LV dimension.
Conclusions
S‐wave changes in right precordial leads can predict increase in PG and critical narrowing of AVA, suggestive of timely referral for AVR.
Keywords: aortic valve area, asymptomatic aortic stenosis, electrocardiogram, mean pressure gradient
1. Introduction
Electrocardiographic appearance is often observed in search of predictors to identify patients with aortic stenosis (AS) at high risk of cardiac events that should be referred to aortic valve replacement (AVR). Precise assessment and adequate gradual follow up of ECG are imperative for those patients with cardiac discomforts (Drezner et al., 2013, 2013; Schillaci, Battista, & Pucci, 2012). ECG as a noninvasive and simple tool is the cheapest and the most widespread method that can be easily applied in the management of cardiac patients.
So far, several studies were conducted in the evaluation of AS severity. The newest one showed association of ST‐segment elevation in leads V1–V2 with different clinical outcomes in patients and therefore poorer prognosis and more frequent need for AVR in patients with severe aortic stenosis (Taniguchi et al., 2016). Another study emphasis diffuse ST‐segment depression and ST‐segment elevation as indicators of severe ischemic change also in connection with poor prognosis in aortic stenosis (Huang, Lee, Lin, Lu, & Lee, 2015). The well‐known ECG pattern in the evaluation of aortic stenosis and its risk assessment is QRS complex duration. Most of the studies with the research objectives based on ECG characteristic in aortic stenosis included QRS morphology as a main independent factor for severity prediction in aortic stenosis (Açıkgöz et al., 2015; Ağaç et al., 2014; Greve et al., 2012; Sebag et al., 2014; Sénéchal, 2014).
ECG recording is a great tool for adequate preoperative and/or postoperative monitoring of the patients with aortic stenosis. However, ECG could never replace hemodynamic parameters derived from echocardiography. Discovering new signs on ECG, regarding aortic stenosis, could save money on large number of dobutamine stress echocardiography examinations that aim to delineate asymptomatic subset of patients for surgical AVR.
The aim of this study was to evaluate S wave in right precordial leads as an independent ECG marker of aortic stenosis before the deterioration of cardiac function.
2. Methods
2.1. Study population
From 15 October 2010 to 15 October 2013 patients with severe AS who underwent surgical AVR at the Clinic for Cardiac Surgery, Clinical Centre of Serbia, were included in this prospective study. The total number of patients included in the study was 1,175. During the 3‐year follow up, the data needed for the study were collected from patient anamnesis and standard diagnostic procedures for preoperative assessment. Inclusion criteria were symptomatic patients with normal coronarography and positive ergometry testing as well as asymptomatic group with positive echocardiography criteria of aortic valve area (AVA) narrowing less than 1.5 cm2, mean pressure gradient (MG), more than 40 mm Hg, aortic peak velocities more than 4 m/s. Exclusion criteria were combined stenosis and aortic valve insufficiency and also underlying CAD or mitral valve disease. None of the studied patients had pacemaker, congenital heart disease, or any pulmonary disease. This prospective study was approved by the local Ethical Committee on Human Research at the Clinical Centre of Serbia.
2.2. Electrocardiography
Standard 12‐lead ECG with a paper speed of 25 mm/s was performed in the supine position at patient inclusion before AVR, and repeated at each follow up after AVR. S‐wave magnitude was assessed manually in leads V1–V3 as shown in Figure 1.
Figure 1.
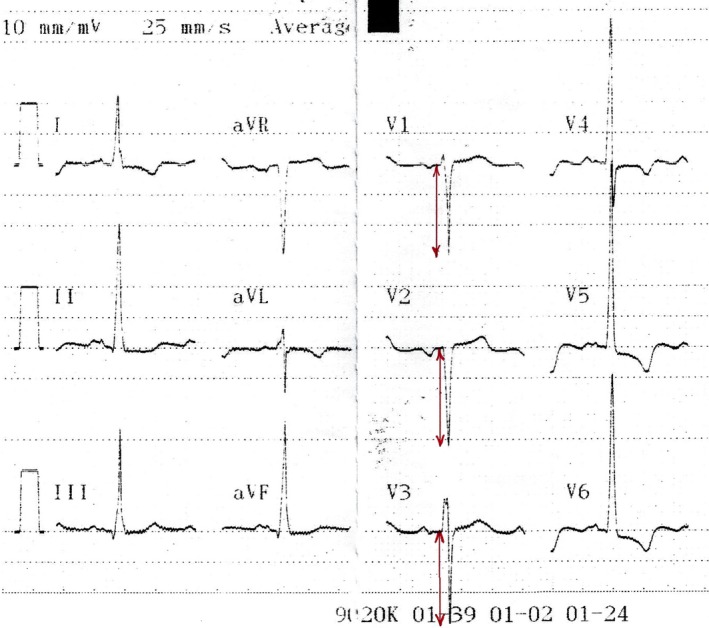
Typical ECG appearance in AS. Arrows depict S‐wave magnitude measurement in leads V1–V3 in AS
2.3. Echocardiography
All patients underwent a comprehensive Doppler echocardiographic study using commercially available ultrasound systems. Peak aortic velocity (V max) was recorded using continuous‐wave Doppler in several acoustic windows (apical 5 chamber, right parasternal, suprasternal, and epigastric views). The highest velocity was used to calculate aortic time velocity integral and subsequently derived parameters of peak pressure gradient (PG), MG, and V max. Furthermore, AVA was calculated using the continuity equation. LV dimensions in end systole and end diastole were measured and EFLV was calculated using Simpson's biplane method. All symptomatic patients were subdivided into categories A and B according to the hemodynamic state. Category A were patients who were compensated with EFLV > 50%, AVA > 0.9 cm2, PG > 55 and <75 mm Hg, and LV diastolic dimension <56 mm. Category B were patients who developed intermittent decompensating on exertion, with EFLV < 50% AVA < 0.9 cm2, PG > 75 mm Hg, and LV diastolic dimension >56 mm.
2.4. Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as percentages. The Shapiro–Wilk test was used to verify whether the data obtained on analysis of variance approximated a normal distribution. If this test failed, nonparametric Mann–Whitney U test was used. Wilcoxon's test was used to evaluate pre‐ and post‐AVR values of S‐wave magnitude. To evaluate the correlation of the studied ECG parameters, the Spearman's method was used. To identify independent predictors related to S‐wave depth, linear regression model was used. Statistically significant parameters in an univariate analysis were included in the multivariate linear regression model to determine the independent effect of S‐wave magnitude on several variables (PG, MG, EFLV, end‐diastolic LV dimension). According to the used test, p value <.05 and/or p‐value <.01 was accepted as statistically significant. The SPSS 21 statistical package was used for calculations.
3. Results
The study population consisted of 1,175 patients, 748 (64%) were men and 427 (36%) were women. The age range in men was from 46 to 77 years, whereas in women it was from 43 to 74 years. All patients with AVR survived in a follow up of 12 months.
The largest number of patients, 729 (62%) had normal left ventricular dimensions in diastole. The increased size of the left ventricle during diastole was found in 446 patients (38%) of the total number. Regarding the systole, the normal dimensions were found in 776 patients (66%), whereas the increased dimensions in systole were found in 399 (34%) of 1,175 observed patients.
Table 1 shows comparative aspect of electrocardiographic appearance of asymptomatic and symptomatic patients before and after AVR. In this regard, ECG changes related to arrhythmias mostly disappeared after AVR and also inverted T waves were back to normal. However, cardiac axis deviation and left fascicular hemiblock persisted.
Table 1.
The tendency of changes in the electrocardiographic signs of aortic stenosis after surgery in asymptomatic and symptomatic patients
| Aortic stenosis | Inverted T wave | Left‐axis deviation | ST horizontal depression | Left branch hemiblock | Arrhythmia |
|---|---|---|---|---|---|
| PREOP asymptomatic | 5% (N = 8) | 3% (N = 5) | 2% (N = 3) | 3% (N = 5) | 1% (N = 2) |
| PREOP symptomatic | 63% (N = 644) | 33% (N = 337) | 25% (N = 255) | 43% (N = 439) | 30% (N = 307) |
| POSTOP asymptomatic | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | 1% (N = 2) | 0.7% (N = 1) |
| POSTOP symptomatic | 12% (N = 122) | 15% (N = 153) | 7% (N = 72) | 22% (N = 225) | 3% (N = 31) |
The obtained results of standard echocardiographic parameters (Table 2) showed no significant differences between asymptomatic and symptomatic patients preoperatively in terms of PG, MG, and AVA, which have been so far considered as the most important parameters in assessment of the appropriate time for surgical intervention. By using U test, statistically significant difference (p < .001) was found between preoperative asymptomatic and symptomatic groups in end‐systolic and end‐diastolic measurements.
Table 2.
Values of the most important echocardiographic parameters of aortic stenosis
| Aortic stenosis | EF LV % | End‐diastolic dimension LV | End‐systolic dimension LV | AVA | MG | PG |
|---|---|---|---|---|---|---|
| PREOP asymptomatic | 60 ± 5 | 53 ± 7 | 37 ± 5 | 1.0 ± 0.2 | 44 ± 13 | 77 ± 7 |
| PREOP symptomatic | 55 ± 10 | 58 ± 2* | 39 ± 4* | 1.0 ± 0.4 | 50 ± 14 | 100 ± 4 |
| POSTOP asymptomatic | 55 ± 5 | 51 ± 3 | 32 ± 3 | av | av | av |
| POSTOP symptomatic | 50 ± 10 | 56 ± 3 | 35 ± 4 | av | av | av |
EF, ejection fraction; LV, left ventricle; AVA, aortic valve area; av, artificial valve; MG, mean pressure gradient; PG, peak pressure gradient; * p‐value ˂.05.
Table 3 shows changes in S‐wave magnitude in right precordial leads before and after AVR in asymptomatic and symptomatic AS. In the group of asymptomatic AS, slight progression of S‐wave magnitude was documented after AVR (55%). Contrary, in the group of symptomatic aortic stenosis S‐wave magnitude diminished after AVR in a majority of patients (70%).
Table 3.
Changes in the values of S‐wave depth in the right precordial leads in relation to the symptomatic and asymptomatic subcategory pre‐ and postoperatively
| Aortic stenosis | S1 preop | S2 preop | S3 preop | S1 postop | S2 postop | S3 postop |
|---|---|---|---|---|---|---|
| Asymptomatic | 6 ± 4 mma | 10 ± 6 mma | 12 ± 1 mma | 13 ± 7 mm | 15 ± 5 | 12 ± 0,5 mm |
| Symptomatic | 15 ± 10 mm | 26 ± 7 mm | 26 ± 7 mm | 12 ± 7 mm | 22 ± 3 mm | 19 ± 8 mm |
p‐Value ˂ .05.
Shapiro–Wilk test did not show normal distribution of our results in all aspects. Mann–Whitney U test showed statistically significant difference (p < .001) for the comparison of S‐wave magnitude in leads V1–V3 in preoperative asymptomatic versus symptomatic group. Nonparametric correlation was calculated by Spearman test and p < .01 was noticed for S2, S3 regarding low and high EFLV, and normal or increased end‐diastolic LV dimension subgroups.
In regression analysis, S‐wave magnitude in right precordial leads significantly predicted PG and end‐diastolic LV dimensions (p < .001). Beta weight of S‐wave magnitude in V3 lead in predicting PG was 0.185 with adjusted R squared of .034. Also, beta weight of S‐wave magnitude in V2 lead in predicting PG was 0.14 with adjusted R squared of .02.
Furthermore, S‐wave magnitude in right precordial leads in regression analysis showed significant negative predictive value for end‐diastolic LV dimensions (p < .001). The results showed beta weight of −0.146 and adjusted R squared of .021 for independent variable S‐wave magnitude in V2 lead for predicting end‐diastolic LV dimensions, whereas beta weight of −0.199 and adjusted R squared of .04 were calculated for independent variable S‐wave magnitude in V2 lead for predicting end‐diastolic LV dimensions.
Regression analysis showed no predictive values of S‐wave magnitude in right precordial leads for dependent variables EFLV and MG, with adjusted R squared of 0 and p < .001.
Figure 2a and b shows regression plot of dependent variable end‐diastolic LV dimension with independent variable S‐wave magnitude in lead V2 and V3, showing negative correlation.
Figure 2.
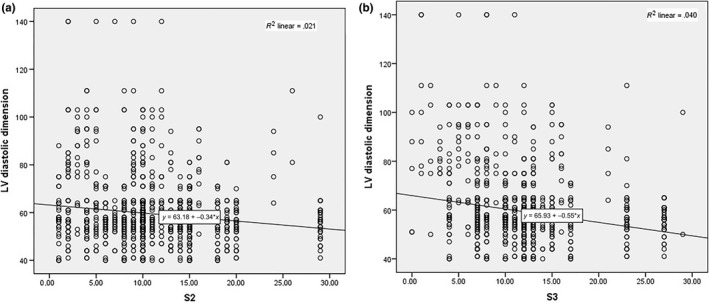
Data interpolation of S wave in V2‐3 prediction potential for LVd. Regression plot of dependent variable end‐diastolic LV dimension (LVd) with independent variable S wave in lead V2 (a) and lead V3 (b)
Figure 3a and b shows regression plot of dependent variable PG with independent variable S‐wave magnitude in lead V2 and V3, depicting positive correlation.
Figure 3.
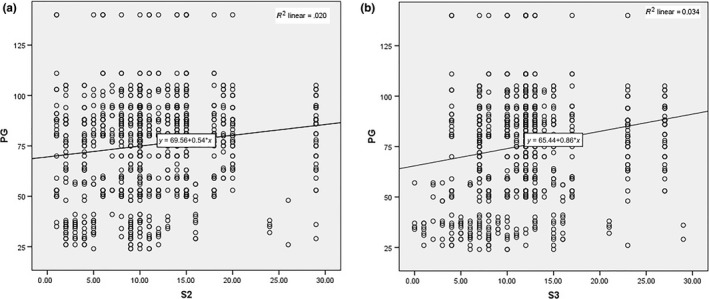
Data interpolation of S wave in V2‐3 prediction potential for PG. Regression plot of dependent variable PG with independent variable S wave in lead V2 (a) and lead V3 (b)
4. Discussion
To the best of our knowledge, this study is the first to explore S‐wave magnitude in right precordial leads preoperatively and postoperatively (12 months after surgery) in a large population of severe AS.
There have been many attempts in finding reliable ECG markers in AS. The SEAS study showed that QRS duration and morphology in asymptomatic patients with AS are independently associated with a poor prognosis, particularly the risk of sudden cardiac death (Greve et al., 2012). Another study demonstrated a higher frequency of fragmented QRS in patients with severe AS compared to controls (Ağaç et al., 2014).
The SEAS study also showed that LV hypertrophy and LV strain on ECG are independently predictive of poor prognosis in patients with asymptomatic AS (Greve et al., 2012). Left ventricular hypertrophy (LVH) by Romhilt and Estes criteria was found to be an independent predictor of early development of symptoms in AS, although the sensitivity of detecting LVH by ECG was found to be as low as 40% (Sathyamurthy & Jayanthib, 2016).
ST‐segment elevation in the precordial leads is often seen in patients with AS. It was shown that ST‐segment elevation in leads V1–V2 independently predicted poorer prognosis and more frequent need for AVR in patients with severe AS (Taniguchi et al., 2016).
Our study shows statistically significant difference between S‐wave magnitude in right precordial leads in asymptomatic versus symptomatic patients before AVR. We speculate the difference between groups is indicative of gradual increase in the left ventricular volume and pressure overload, as S waves tend to progress over time in AS. Also, by analyzing our population it was noticed that the majority of patients (65%) diminished S‐wave magnitude after AVR, whereas in a minority of them the same values persisted (10%) or even increased (25%). This finding is suggestive of the LV diastolic recovery after AVR that can be followed on ECG recordings.
Another finding in our study is positive predictive value of S‐wave magnitude in leads V2–V3 in the assessment of dependent variable PG, whereas we found no predictive values of the same independent variables in predicting MG and EFLV.
The obtained results show that there is no significant difference in echocardiographic measurements of AVA and MG observed in asymptomatic and symptomatic patients with aortic stenosis before AVR.
The last finding in our study is negative predictive value of S‐wave magnitude in leads V2 and V3 for the dependent variable end‐diastolic LV dimension.
We speculate that S‐wave changes in right precordial leads in AS are related to unraveling the two loops within double helicoid structure of LV that are due to pressure overload (Torrent‐Guasp et al., 2001).
The importance of these findings is to help determine the timely referral for AVR, as pressure generated in the LV increases in aortic stenosis over time and causes progressive changes on the ECG that can be measured before surgery. After the surgery, the changes that occurred preoperatively were due to blood flow obstruction and gradually reverse after AVR. It was noted in the follow up in the next year that, in the vast majority of operated symptomatic patients, ECG values return to normal in a timely manner.
It is difficult to determine when asymptomatic patients should be referred for surgery, as the PG measured over the stenotic orifice differs and thus the cut‐off values indicative for surgery cannot be determined. A study by Sylvestre Marechaux et al. evaluated the relationship between aortic valve area (AVA) and outcome in patients with severe asymptomatic aortic stenosis, where they also defined a specific threshold of AVA less than 0.6 cm2 identifying asymptomatic patients at very high risk of cardiac events based on their clinical outcome (Maréchaux et al., 2016).
In conclusion, our study demonstrated that S‐wave changes in right precordial leads can predict increase in PG and critical narrowing of AVA, suggestive of timely referral for echocardiographic testing for assessment of patients eligible for AVR or TAVI, especially in asymptomatic aortic stenosis. Further prospective and randomized studies are needed to confirm this study's results in a larger number of patients with severe AS.
Vranic II. Electrocardiographic appearance of aortic stenosis before and after aortic valve replacement. Ann Noninvasive Electrocardiol. 2017;22:e12457 10.1111/anec.12457
References
- Açıkgöz, E. , Yaman, B. , Açıkgöz, S. K. , Topal, S. , Tavil, Y. , & Boyacı, N. B. (2015). Fragmented QRS can predict severity of aortic stenosis. Annals of Noninvasive Electrocardiology, 20(1), 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ağaç, M. T. , Korkmaz, L. , Bektas, H. , Acar, Z. , Erkan, H. , Kurt, I. H. , … Celik, S. (2014). Increased frequency of fragmented QRS in patients with severe aortic valve stenosis. Medical Principles and Practice, 23(1), 66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drezner, J. A. , Ackerman, M. J. , Anderson, J. , Ashley, E. , Asplund, C. A. , Baggish, A. L. , … Wilson, M. G. (2013). Electrocardiographic interpretation in athletes: The ‘Seattle criteria’. British Journal of Sports Medicine, 47(3), 122–124. [DOI] [PubMed] [Google Scholar]
- Drezner, J. A. , Ackerman, M. J. , Cannon, B. C. , Corrado, D. , Heidbuchel, H. , Prutkin, J. M. , … Wilson, M. G. (2013). Abnormal electrocardiographic findings in athletes: Recognising changes suggestive of primary electrical disease. British Journal of Sports Medicine, 47(3), 153–167. [DOI] [PubMed] [Google Scholar]
- Greve, A. M. , Boman, K. , Gohlke‐Baerwolf, C. , Kesäniemi, Y. A. , Nienaber, C. , Ray, S. , … Wachtell, K. (2012). Clinical implications of electrocardiographic left ventricular strain and hypertrophy in asymptomatic patients with aortic stenosis: The Simvastatin and Ezetimibe in Aortic Stenosis study. Circulation, 125(2), 346–353. [DOI] [PubMed] [Google Scholar]
- Greve, A. M. , Gerdts, E. , Boman, K. , Gohlke‐Baerwolf, C. , Rossebø, A. B. , Devereux, R. B. , … Wachtell, K. (2012). Impact of QRS duration and morphology on the risk of sudden cardiac death in asymptomatic patients with aortic stenosis: The SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study. Journal of the American College of Cardiology, 59(13), 1142–1149. [DOI] [PubMed] [Google Scholar]
- Huang, T. C. , Lee, M. K. , Lin, S. J. , Lu, Y. H. , & Lee, K. T. (2015). Diffuse ST‐segment depression with ST‐segment elevation in lead aVR in 12‐lead electrocardiography may indicate ischemic change of severe aortic stenosis. Acta Cardiologica Sinica, 31(5), 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchaux, S. , Ringle, A. , Rusinaru, D. , Debry, N. , Bohbot, Y. , & Tribouilloy, C. (2016). Prognostic value of aortic valve area by Doppler echocardiography in patients with severe asymptomatic aortic stenosis. Journal of the American Heart Association, 5(5), pii:e003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyamurthy, I. , & Jayanthib, K. (2016). Asymptomatic severe aortic stenosis with normal left ventricular function‐ A review. Indian Heart Journal, 68(4), 576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillaci, G. , Battista, F. , & Pucci, G. (2012). A review of the role of electrocardiography in the diagnosis of left ventricular hypertrophy in hypertension. Journal of Electrocardiology, 45(6), 617–623. [DOI] [PubMed] [Google Scholar]
- Sebag, F. A. , Lellouche, N. , Chaachoui, N. , Dubois‐Rande, J. L. , Gueret, P. , & Monin, J. L. (2014). Prevalence and clinical impact of QRS duration in patients with low‐flow/low‐gradient aortic stenosis due to left ventricular systolic dysfunction. European Journal of Heart Failure, 16(6), 639–647. [DOI] [PubMed] [Google Scholar]
- Sénéchal, M. (2014). What is the best therapeutic strategy in patients with low flow, low‐gradient aortic stenosis, and wide QRS? European Journal of Heart Failure, 16(6), 598–600. [DOI] [PubMed] [Google Scholar]
- Taniguchi, T. , Shiomi, H. , Kosuge, M. , Morimoto, T. , Nakatsuma, K. , Nishiga, M. , … Kimura, T. (2016). Prognostic significance of ST‐segment elevation in leads V1‐2 in patients with severe aortic stenosis. Circulation Journal, 80(2), 526–534. [DOI] [PubMed] [Google Scholar]
- Torrent‐Guasp, F. , Ballester, M. , Buckberg, G. D. , Carreras, F. , Flotats, A. , Carrió, I. , … Narula, J. (2001). Spatial orientation of the ventricular muscle band: Physiologic contribution and surgical implications. The Journal of Thoracic and Cardiovascular Surgery, 122(2), 389–392. [DOI] [PubMed] [Google Scholar]


