Abstract
Background
Patients with hypertrophic cardiomyopathy (HCM) are at a fourfold to sixfold higher risk of developing atrial fibrillation (AF) compared to the general population, though incidence rates among patients undergoing alcohol septal ablation (ASA) are not well characterized. The purpose of this study was to evaluate atrial fibrillation incidence following ASA.
Methods
We studied 132 consecutive HCM patients without comorbid AF that underwent 154 ASA procedures. The incidence of AF in follow‐up was assessed through chart abstraction including electrocardiography. Survival free of AF was estimated using Kaplan‐Meier methodology.
Results
Over a mean follow‐up of 3.6 ± 2.7 years (maximum 11.3 years), 10 (7.6%) patients developed new‐onset AF. Of those who developed AF, both resting and provoked left ventricular outflow tract (LVOT) gradients had improved significantly (difference –79.78 mm Hg, P ≤ 0.005). Severity of mitral regurgitation improved in 7 (70%) patients. Survival free of AF was estimated to be 99.1%, 93.7%, and 91.7% at 1, 3, and 5 years.
Conclusions
Despite relieving LVOT obstruction and improving mitral regurgitation severity via ASA, new‐onset AF remained a common complication of hypertrophic cardiomyopathy.
Keywords: alcohol septal ablation, hypertrophic cardiomyopathy, atrial fibrillation
Alcohol septal ablation (ASA) is an alternative to surgical myectomy for treatment of symptomatic hypertrophic cardiomyopathy (HCM) that provides long‐term resolution of left ventricular outflow tract (LVOT) obstruction, improves functional status, and prolongs longevity similar to that expected after surgical myectomy.1, 2 Nevertheless, patients with HCM are at a fourfold to sixfold higher risk of developing atrial fibrillation (AF), with a prevalence of 20% and an incidence of 3% per year.3, 4, 5, 6 AF confers increased risk of all‐cause mortality, including that due to heart failure and thromboembolic events and is associated with worsened exercise capacity and symptoms impacting quality of life.3, 4, 6 Despite multiple cohort studies of patients undergoing ASA for symptomatic HCM,1, 2 , 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 none has reported on the incidence of AF. We evaluated the incidence of AF following ASA for symptomatic HCM.
METHODS
We studied consecutive patients with symptomatic HCM without known comorbid AF prior to undergoing ASA from 2002 to 2011 at both the University of Colorado Hospital and the Denver Veterans Affairs Medical Center. Indications for ASA included persistent symptoms (New York Heart Association [NYHA] class II–IV) despite optimal medical therapy, significant resting or provoked LVOT gradients, and coronary anatomy amenable for proximal septal ablation. No patients had had prior surgical myectomy. The Colorado Multiple Institutional Review Board approved this analysis.
Procedure and Measurements
Prior to undergoing the ASA procedure all patients had a baseline transthoracic echocardiogram that demonstrated a normal left ventricular ejection fraction (>55%), as measured by the biplane method of discs.28 Additional echocardiographic indices included left atrial (LA) linear dimension, interventricular septal wall thickness, resting and provokable LVOT gradients, assessment of systolic anterior motion (SAM) of the mitral leaflet, and grading of the severity of mitral regurgitation.
The septal ablation procedure was performed in standard fashion as previously described.9 Patients were evaluated postprocedure to assess functional status (NYHA class), clinical symptoms, medication regimen, and echocardiographic indices. Routine follow‐up was performed postprocedure and then annually or as clinically indicated.
Our primary outcome was the incidence of AF assessed through systematic chart review and inspection of 12‐lead electrocardiograms. Ambulatory telemetric monitoring was not performed routinely in asymptomatic individuals. Comprehensive two‐dimensional and Doppler echocardiography included evaluation of septal wall thickness, left ventricular ejection fraction, LA linear dimension, presence or absence of SAM, mitral regurgitation severity, as well as both resting and provoked LVOT gradients.
Statistical Analysis
Incident AF‐free survival was evaluated by censoring patients at the date of newly diagnosed AF or last known follow‐up. Survival estimates were determined with traditional Kaplan‐Meier estimation.29 For a small subset of patients who underwent additional ASA procedures, our time to event analysis is based on the first procedure. Continuous variables are presented as mean ± standard deviation. Longitudinal changes of continuous variables were assessed using either a paired or unpaired 2‐tailed Student's t test. Mann‐Whitney U testing was used to assess both categorical and longitudinal changes of rank order variables. For all assessments, a P value ≤ 0.05 was deemed statistically significant. Statistical analysis was performed using R: A Language and Environment for Statistical Computing, version 3.1.3 (Vienna, Austria).29, 30
RESULTS
Of the 145 consecutive patients with HCM, 13 were excluded because of preexisting AF. The remaining 132 patients with HCM and without comorbid AF underwent 154 ASA procedures. Immediately following the procedure, resting and provoked LVOT gradients decreased to ≤30 mm Hg in 118 (91%) patients (of 130 with complete data). Prior to the procedure, all patients had symptoms attributable to LVOT obstruction with a mean NYHA class of 2.9 ± 0.4, which on follow‐up improved significantly to 1.3 ± 0.5 (change = –1.6, P < 0.001). We previously described the complications and longitudinal echocardiographic indices that persisted after the ASA procedure 17, 31, but we reanalyzed these same parameters for this subset of patients who did not have AF at baseline.
The mean follow‐up time to last known clinical assessment was 3.6 ± 2.7 years (maximum 11.3 years) and was available for 130 (98%) patients. Of the 129 patients with echocardiographic follow‐up, 125 (98%) had preserved (>55%) left ventricular ejection fraction. Serial echocardiography revealed persistent improvement in LVOT obstruction gradients and no significant change in LA linear dimension (41 ± 7 mm vs 42 ± 7 mm, P = 0.13) in the overall study cohort.
During this period 10 (7.6%) patients developed AF. Characteristics of the study population at baseline for those who remained free of AF and those who later developed AF are outlined in Table 1. Patients who went on to have AF were older (64.8 ± 16.9 years vs 56.4 ± 15.3 years, respectively) but had the same NYHA functional class (2.9) at baseline. LVEF remained preserved in all but one patient who developed AF. Eight patients experienced paroxysmal AF, while two patients developed persistent AF. Three of the eight patients with paroxysmal AF (38%) were treated with antiarrhythmic drugs. No patients underwent pulmonary vein isolation or left atrial catheter ablation, though one individual required atrioventricular node ablation. The mean CHA2DS2Vasc score among patients with incident AF was 2.3 ± 1.2. Despite therapeutic anticoagulation in all patients, a thromboembolic stroke occurred in one during follow‐up.
Table 1.
Characteristics of Patient Population at Baseline (n = 132)
| Survival Free of Atrial | Incident Atrial | ||||
|---|---|---|---|---|---|
| Fibrillation (n = 122) | Fibrillation (n = 10) | ||||
| Mean (n) | SD (%) | Mean (n) | SD (%) | P value | |
| Age (years) | 56.4 | ±15.3 | 64.8 | ±16.9 | 0.158 |
| Women | 60 | 49% | 5 | 50% | 0.963 |
| Prior stroke | 7 | 6% | 1 | 10% | 0.686 |
| Hypertension | 58 | 48% | 4 | 40% | 0.666 |
| Diabetes mellitus | 9 | 7% | 1 | 10% | 0.804 |
| Coronary artery disease | 50 | 42% | 5 | 50% | 0.654 |
| Chronic kidney disease | 5 | 4% | 2 | 20% | 0.267 |
| Family history of sudden cardiac death | 13 | 11% | 2 | 20% | 0.168 |
| Syncope | 36 | 30% | 2 | 20% | 0.510 |
| NYHA functional class | 2.9 | ± 0.4 | 2.9 | ± 0.3 | – |
| Medications | |||||
| Beta‐blocker | 89 | 75% | 8 | 80% | 0.716 |
| Calcium channel blocker | 57 | 48% | 3 | 30% | 0.286 |
| Angiotensin converting enzyme inhibitor | 19 | 16% | 1 | 10% | 0.583 |
| Disopyramide | 9 | 8% | 1 | 10% | 0.818 |
| Amiodarone | 1 | 1% | 1 | 10% | 0.385 |
Values are expressed as either mean ± standard deviation (SD) or number (n) and percentage (%). NYHA = New York Heart Association; – = not enough observations for comparison.
Regarding the outcome of ASA among those who later developed AF, both resting and provoked LVOT gradients had improved significantly (P ≤ 0.005) and the severity of mitral regurgitation had improved in 7 (70%) of these patients (Figs. 1A and B). LA linear dimension appeared to improve in the group of patients with AF, but this was not significantly different from those without AF (difference –6.6 mm, P = 0.09) as shown in Figure 1C. Survival free of AF was estimated to be 99.1%, 93.7%, and 91.7% at 1, 3, and 5 years (Fig. 2).
Figure 1.
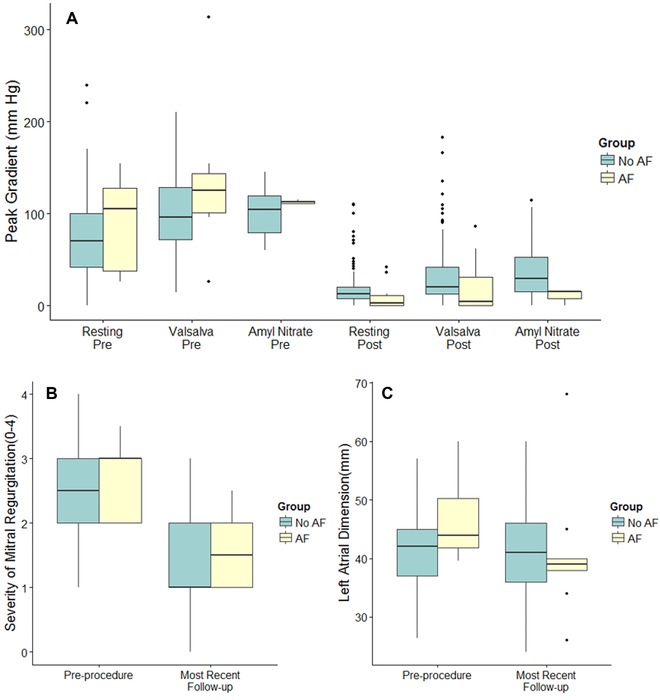
(A) Peak left ventricular outflow tract gradients at baseline and postprocedure in hypertrophic cardiomyopathy patients with and without incident atrial fibrillation (yellow and green fill respectively). Graphical depictions of inter‐quartile range (IQR, 25th–75th percentiles; box), median (horizontal line), and outliers (>1.5*IQR; points). Between groups comparisons were not statistically significant with the exception of with amyl nitrate (0.047 and 0.014 pre = and post = respectively). (B) Severity of Mitral regurgitation on a semi‐quantitative scale of 0–4 (none = 0, trace = 1, mild = 2, moderate = 3, and severe = 4) at baseline and at most recent follow‐up in hypertrophic cardiomyopathy patients with and without incident atrial fibrillation (yellow and green fill respectively). Graphical depictions of interquartile range (IQR, 25th–75th percentiles; box), median (horizontal line), and outliers (>1.5*IQR; points). Whiskers extend to 1.5 times the IQR. Between groups comparisons were not statistically significant. (C) Left atrial linear dimension at baseline and at most recent follow‐up in HCM patients with and without incident atrial fibrillation (yellow and green fill, respectively). Graphical depictions of interquartile range (IQR, 25th to 75th percentiles), median (horizontal line), and outliers (>1.5*IQR; points). Whiskers extend to 1.5 times the IQR. Between groups comparisons were not statistically significant.
Figure 2.
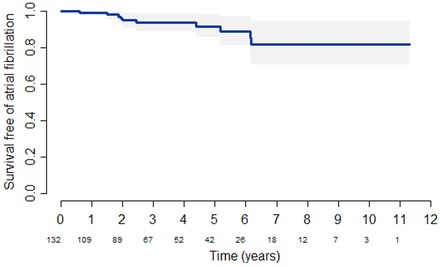
Incidence of atrial fibrillation after alcohol septal ablation. Bands represent 95% confidence interval of survival estimates. Number of patients at risk appears below x‐axis.
DISCUSSION
AF contributes to the exacerbation of symptoms and the development of heart failure in patients with HCM.5 It has been shown that increased LA afterload in HCM corresponds well with LV diastolic dysfunction32 and that septal reduction therapy improves the severity of mitral regurgitation.7, 11, 17, 18, 20, 21, 25, 33 While septal reduction therapy including ASA is not primarily performed to reduce subsequent AF burden in HCM, the favorable anatomic correction leading to reduced mitral regurgitation and decreased LA afterload—as demonstrated in our study—might reasonably be expected to lead to a corresponding decrease in the AF. Table S1 summarizes previous literature documenting that most patients with HCM have enlarged LA linear dimension at baseline or prior to septal reduction therapy and confirms our findings that while there is improvement in the severity of MR, LA linear dimension is not substantially reduced.
We found that despite favorable alterations in echocardiographic structure—reduced septal hypertrophy—and function—reduced LVOT gradients and mitral regurgitation—as well as improved patient symptoms, the incidence of AF in this cohort of patients with HCM after undergoing ASA mirrored that reported in studies of HCM populations that did not undergo ASA. In a meta‐analysis of 22 cohort studies (6413 patients) investigating AF in HCM as either a primary or secondary outcome, Guttmann et al. found the overall AF incidence to be 3.08% per year; however, very few patients had undergone ASA (n: 27; 0.42%).4 To our knowledge, ours is the first HCM cohort study to report the incidence of AF after ASA and demonstrates remarkable consistency in the incidence of AF when compared with previous cohorts of more general HCM populations.
Several groups have reported clinical and echocardiographic covariates associated with AF incidence in the HCM population. In their study of a general HCM cohort, Losi et al. found LA dysfunction, defined by LA fractional shortening ≤ 16% and LA linear dimension ≥45 mm were strongly associated with the development of AF. Similarly, Olivotto et al. found that increased LA linear dimension was strongly associated with subsequent AF.3, 5 This was corroborated in the largest single‐center study of HCM where multiple echocardiographic parameters were significantly correlated with the prevalence of AF, including higher LA volume index, LV posterior wall thickness, medial E/e’ ratio, and shorter mitral E‐wave deceleration time, a marker of restrictive physiology.6 Surprisingly, this same study also found that patients with obstructive physiology defined by a rest LVOT peak gradient of >30 mm Hg or a provoked LVOT peak gradient of >50 mm Hg were less likely to develop AF.6 More recently, LA percent ejection fraction has been shown to reliably identify patients with HCM at risk for development of AF.34 Yet others found that the LA wall in HCM patients with AF was not thicker than that of matched patients without structural heart disease, suggesting that overt atrial fibrosis and hypertrophy do not contribute to the incidence of AF.35
We surmise that the symptoms that prompt consideration for performing ASA may indicate that the condition has progressed sufficiently to irreversibly alter the structure and function of the LA. Alternatively, the underlying cardiomyopathy might also impact the atria independent of its influence on LV diastolic dysfunction and mitral regurgitation. In fact the coexistence of ventricular and atrial myopathy can be as high as 36–45%, but, as currently measured, this has not yet been shown to have a direct association with AF.5
We acknowledge a number of limitations to the current analysis. This was a retrospective, nonrandomized, cohort study without a contemporaneous control group. All assessments were performed on a clinical basis rather than stipulated by protocol. Although the timing of assessments was not identical for all patients, this reflects real world experience with generalizability to the broader HCM population. Because routine surveillance ambulatory telemetry monitoring was not performed, incidence rates may be underestimated due to lower ascertainment of asymptomatic paroxysmal AF. In addition, atrial linear dimensions were utilized in the current study, though increasingly left atrial volumes indexed for body surface area are used in practice and research settings.28 Only 1 patient in our study developed AF complicated by an embolic stroke. A larger cohort of patients would be needed to assess whether stroke risk is modulated by ASA in HCM patients with AF. Finally, our sample size of incident AF patients precludes the use of multiple regression analysis to assess for covariates that reliably predict arrhythmia incidence.
We conclude that after relieving LVOT obstruction and improving mitral regurgitation severity via ASA, incident AF remained a common complication of hypertrophic cardiomyopathy. Significant decreases in LA size were not observed in our cohort. Atrial remodeling, fibrosis, or factors other than effective LA afterload may contribute to the high incidence of AF in this HCM population. Further studies should be done to compare the incidence of AF in cohorts of patients who do not all undergo septal reduction therapy such as ASA.
Supporting information
Table S1. Alcohol septal ablation for hypertrophic cardiomyopathy cohort studies.
Ann Noninvasive Electrocardiol 2016;21(5):443–449
Disclosures: Krantz: General Electric, Abbott Vascular.
Funding Sources: None.
REFERENCES
- 1. Agarwal S, Tuzcu EM, Desai MY, et al. Updated meta‐analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;55(8):823–834. [DOI] [PubMed] [Google Scholar]
- 2. Fernandes VL, Nielsen C, Nagueh SF, et al. Follow‐up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the baylor and medical university of south carolina experience 1996 to 2007. JACC Cardiovasc Interv 2008;1(5):561–570. [DOI] [PubMed] [Google Scholar]
- 3. Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104(21):2517–2524. [DOI] [PubMed] [Google Scholar]
- 4. Guttmann OP, Rahman MS, O'Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: Systematic review. Heart 2014;100(6):465–472. [DOI] [PubMed] [Google Scholar]
- 5. Losi MA, Betocchi S, Aversa M, et al. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am J Cardiol 2004;94(7):895–900. [DOI] [PubMed] [Google Scholar]
- 6. Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: Prevalence, clinical correlations, and mortality in a large high‐risk population. J Am Heart Assoc 2014;3(3):e001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Airoldi F, Di Mario C, Catanoso A, et al. Progressive decrease of outflow gradient and septum thickness after percutaneous alcoholization of the interventricular septum in hypertrophic obstructive cardiomyopathy. Ital Heart J 2000;1(3):200–206. [PubMed] [Google Scholar]
- 8. Boekstegers P, Steinbigler P, Molnar A, et al. Pressure‐guided nonsurgical myocardial reduction induced by small septal infarctions in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2001;38(3):846–853. [DOI] [PubMed] [Google Scholar]
- 9. Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. One‐year follow‐up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: Predictors of hemodynamic and clinical response. Clin Res Cardiol 2007;96(12):864–873. [DOI] [PubMed] [Google Scholar]
- 10. Firoozi S, Elliott PM, Sharma S, et al. Septal myotomy‐myectomy and transcoronary septal alcohol ablation in hypertrophic obstructive cardiomyopathy. A comparison of clinical, haemodynamic and exercise outcomes. Eur Heart J. 2002;23(20):1617–1624. [DOI] [PubMed] [Google Scholar]
- 11. Guo H, Wang P, Xing Y, et al. Delayed electrocardiographic changes after percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy. J Electrocardiol 2007;40(4):356.e1–356.e6. [DOI] [PubMed] [Google Scholar]
- 12. Jensen MK, Almaas VM, Jacobsson L, et al. Long‐term outcome of percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: A scandinavian multicenter study. Circ Cardiovasc Interv 2011;4(3):256–265. [DOI] [PubMed] [Google Scholar]
- 13. Kazmierczak J, Kornacewicz‐Jach Z, Kisly M, Gil R, Wojtarowicz A. Electrocardiographic changes after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Heart 1998;80(3):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JJ, Lee CW, Park SW, et al. Improvement in exercise capacity and exercise blood pressure response after transcoronary alcohol ablation therapy of septal hypertrophy in hypertrophic cardiomyopathy. Am J Cardiol 1999;83(8):1220–1223. [DOI] [PubMed] [Google Scholar]
- 15. Kuhn H, Lawrenz T, Lieder F, et al. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): A 10 year experience. Clin Res Cardiol 2008;97(4):234–243. [DOI] [PubMed] [Google Scholar]
- 16. Leonardi RA, Kransdorf EP, Simel DL, Wang A. Meta‐analyses of septal reduction therapies for obstructive hypertrophic cardiomyopathy: Comparative rates of overall mortality and sudden cardiac death after treatment. Circ Cardiovasc Interv 2010;3(2):97–104. [DOI] [PubMed] [Google Scholar]
- 17. Moss TJ, Krantz MJ, Zipse MM, et al. Left ventricular systolic function following alcohol septal ablation for symptomatic hypertrophic cardiomyopathy. Am J Cardiol 2014;113(8):1401–1404. [DOI] [PubMed] [Google Scholar]
- 18. Mutlak D, Gruberg L, Reisner S, Markiewicz W. Non‐surgical myocardial reduction in hypertrophic obstructive cardiomyopathy. Isr Med Assoc J 2002;4(2):86–90. [PubMed] [Google Scholar]
- 19. Osterne EC, Seixas TN, Paulo Filho W, Osterne EM, Gomes OM. Percutaneous transluminal septal alcoholization for the treatment of refractory hypertrophic obstructive cardiomyopathy: Initial experience in the federal district. Arq Bras Cardiol 2003;80(4):359–378. [DOI] [PubMed] [Google Scholar]
- 20. Qin JX, Shiota T, Lever HM, et al. Outcome of patients with hypertrophic obstructive cardiomyopathy after percutaneous transluminal septal myocardial ablation and septal myectomy surgery. J Am Coll Cardiol 2001;38(7):1994–2000. [DOI] [PubMed] [Google Scholar]
- 21. Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long‐term follow‐up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin Res Cardiol 2007;96(12):856–863. [DOI] [PubMed] [Google Scholar]
- 22. Sorajja P, Valeti U, Nishimura RA, et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation 2008;118(2):131–139. [DOI] [PubMed] [Google Scholar]
- 23. Streit S, Walpoth N, Windecker S, Meier B, Hess O. Is alcohol ablation of the septum associated with recurrent tachyarrhythmias? Swiss Med Wkly 2007;137(47‐48):660–668. [DOI] [PubMed] [Google Scholar]
- 24. Tsuchikane E, Takeda Y, Kobayashi T, et al. Percutaneous transluminal septal myocardial ablation for hypertrophic obstructive cardiomyopathy. Circ J 2003;67(9):763–767. [DOI] [PubMed] [Google Scholar]
- 25. van der Lee C, Scholzel B, Ten Berg JM, et al. Usefulness of clinical, echocardiographic, and procedural characteristics to predict outcome after percutaneous transluminal septal myocardial ablation. Am J Cardiol 2008;101(9):1315–1320. [DOI] [PubMed] [Google Scholar]
- 26. Veselka J, Duchonova R, Palenickova J, et al. Impact of ethanol dosing on the long‐term outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy: A single‐center prospective, and randomized study. Circ J 2006;70(12):1550–1552. [DOI] [PubMed] [Google Scholar]
- 27. Monakier D, Woo A, Puri T, et al. Usefulness of myocardial contrast echocardiographic quantification of risk area for predicting postprocedural complications in patients undergoing septal ethanol ablation for obstructive hypertrophic cardiomyopathy. Am J Cardiol 2004;94(12):1515–1522. [DOI] [PubMed] [Google Scholar]
- 28. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr 2015;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 29. Core Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2015;3.1.3. [Google Scholar]
- 30. Harrell FE Jr (2015). rms: Regression Modeling Strategies. R package version 4.3-0. Available at: http://CRAN.R-project.org/package=rms. Accessed February 16, 2015.
- 31. Schuller JL, Zipse MM, Krantz MJ, et al. Incidence and predictors of late complete heart block after alcohol septal ablation treatment of hypertrophic obstructive cardiomyopathy. J Interv Cardiol 2015;28(1):90–97. [DOI] [PubMed] [Google Scholar]
- 32. Briguori C, Betocchi S, Losi MA, et al. Noninvasive evaluation of left ventricular diastolic function in hypertrophic cardiomyopathy. Am J Cardiol 1998;81(2):180–187. [DOI] [PubMed] [Google Scholar]
- 33. Yu EH, Omran AS, Wigle ED, Williams WG, Siu SC, Rakowski H. Mitral regurgitation in hypertrophic obstructive cardiomyopathy: Relationship to obstruction and relief with myectomy. J Am Coll Cardiol 2000;36(7):2219–2225. [DOI] [PubMed] [Google Scholar]
- 34. Maron BJ, Haas TS, Maron MS, et al. Left atrial remodeling in hypertrophic cardiomyopathy and susceptibility markers for atrial fibrillation identified by cardiovascular magnetic resonance. Am J Cardiol 2014;113(8):1394–1400. [DOI] [PubMed] [Google Scholar]
- 35. Hayashi H, Hayashi M, Miyauchi Y, et al. Left atrial wall thickness and outcomes of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Interv Card Electrophysiol 2014;40(2):153–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Alcohol septal ablation for hypertrophic cardiomyopathy cohort studies.


