Abstract
Background
The QT variability index (QTVI) is a noninvasive index of repolarization lability that has been applied to subjects with cardiovascular disease. QTVI provides a ratio of normalized QT variability to normalized heart rate variability, and therefore includes an assessment of autonomic nervous activity. However, measurement of QT time is particularly difficult in children, who exhibit physiologically high heart rates compared with adults. In this study, we developed a set of standard values of J‐point to Tpeak interval (JTp) for infants by age, and assessed the correlation of QTVI with the JTp variability index (JTpVI).
Methods
Subjects included 623 infants and children (0–7 years of age) without heart disease and 57 healthy university students. All subjects were divided into three groups by age. QTVI and JTpVI were calculated based on an electrocardiogram, and age‐specific standard values, a gender‐specific classification, and a standard growth curve were constructed.
Results
JTpVI markedly decreased in infancy and slowly decreased thereafter, reaching adult values by school age. There was also a strong correlation of JTpVI with QTVI (r = .856).
Conclusions
JTp can be used to evaluate the variability of the repolarization time in healthy infants, and may be useful for detection of early repolarization abnormalities.
Keywords: autonomic nervous system, JT peak interval, QT interval, variability index
1. Introduction
Body surface electrocardiography (ECG) can provide electrophysiological information on the myocardium, and is useful for evaluation of autonomic balance and arrhythmogenic substrates, and for prediction of patient prognosis. Heart rate variability is a measure of autonomic balance (Goldkorn et al., 2015; Akselrod et al., 1981) and the ventricular late potential (Simson, 1981; Malik et al., 1992), and was recently used to determine the delay and disturbance of myocardial depolarization based on the QRS complex, the heterogeneity of transmural dispersion of repolarization (Antzelevitch, Yan, & Shimizu, 1999; Smetana et al., 2011; Antzelevitch, 2005), and QT dispersion, for evaluation of repolarization based on the potential differences between myocardial regions (Napolitano, Priori, & Schwartz, 2000).
The QT variability index (QTVI) (Berger et al., 1997) is a measure of temporal instability, and is used to evaluate heart rate variability‐induced QT time variation by normalizing this variation using the mean and variance of heart rate. We previously reported standard childhood values of QTVI (Kusuki et al., 2011) and evaluated the lability of repolarization in pathological states using QTVI (Kuriki et al., 2011). However, evaluation using the QT time does not reflect the pure repolarization process as it contains the QRS time. Furthermore, it was often difficult to measure the end of the QT time, due to the overlap in the T‐wave endpoint and P wave of the next heartbeat in children, in whom baseline heart rate is physiologically high.
Several methods for analysis of the QT time using the subinterval between depolarization and repolarization have been reported. For example, the JT time is determined by excluding the QRS time (the depolarization time) from the QT time. JT time is then divided into the JTp time (from the end of the QRS complex [J‐point] to the T‐wave peak [Tpeak]) and the Tp‐e time (from Tpeak to the end of the T wave [Tend]). The Tp‐e time represents the late repolarization time, and arrhythmogenicity can be identified by analyzing the relationship between the Tp‐e and QT time (Gupta et al., 2008). Using Tpeak allows accurate measurement of the JTp time, which can be measured in waveforms of infants with a high heart rate. In addition, JTp time can be measured in waveforms with bundle branch block, as the QRS complex (a depolarization waveform) is excluded. Thus, the repolarization process can be evaluated, at least in part, in ECG waveforms that are difficult to evaluate using the QT time.
The electrophysiological significance of the early repolarization time is not fully understood. The autonomic nervous system is thought to have limited influence on the inward rectifier current (IK1), which determines this early repolarization phase, but can have marked effects on the inward calcium current, resulting in changes in the duration of the action potential (Wetzel & Klitzner, 1996; Lopatin & Nichols, 2001). Moreover, variation of the repolarization time, including the JT peak, among the ECG lead sites is predicted due to the potential gradient between the endocardium and the epicardium, and the spatial potential gradient between the apex and base of the heart (Malik & Batchvarov, 2000).
In the present study, we developed a set of standard values of the early repolarization time (JTp) for infants by age, and assessed the correlation of clinical QTVI with JTpVI, a variability index of the early repolarization time.
2. Methods
The subjects were 646 neonates and children who had been determined to have no organic heart disease by electrocardiograph and cardiac ultrasonography and no systemic inflammatory disease by laboratory examinations, in Fujita Health University Hospital between April 2008 and November 2015. For the present analysis, eight patients were excluded because stable ECG recordings were not possible due to vigorous motion. An additional 15 patients were excluded because they showed extremely high heart rates (HRs > 180 bpm) with overlapping of T‐wave terminal portion and P wave. Finally, enrolled 623 patients had sufficient records of electrocardiography, and the T‐wave endpoint could be measured automatically. Fifty‐seven university students were also examined as healthy controls. Written informed consent was obtained from the subjects or their parents. The study was approved by the Epidemiological Clinical Study Ethics Committee of our university.
ECGs (single CM5 lead) were recorded using a polygraph device (MP‐150; Biopac Systems Inc., Goleta, CA, USA) during echocardiography performed between 4:00 and 6:00 p.m., prior to dinner. Infants of 3 months to 1 year of age were premedicated with Tricloryl syrup (0.7 ml/kg) for sedation. Records of 60 heartbeats with a stable baseline were subjected to automatic analysis of the interbeat (RR) interval using analytical software (Acqknowledge v3.9; Biopac Systems Inc.). First‐derivative and absolute‐value processing were applied to the ECG waveforms. The starting point and endpoint (J‐point) of the QRS complex and Tend were determined, and the RR interval, the QT time for the same heartbeat, and the JTp time were measured. The QTc interval was calculated by using Fridericia's formula. The mean and variance of each time parameter of the analyzed heartbeat were also calculated.
Subsequently, QTVI, JTVI, and JTpVI were calculated using Berger's formula, as follows: QTVI = Log10 (QTv/QTm2)/(HRv/HRm2), JTVI = Log10 (JTv/JTm2)/(HRv/HRm2), JTpVI = Log10 (JTpv/JTpm2)/(HRv/HRm2), where the numerator (QTVN, normalized QT variability; JTVI, normalized JT variability; JTpVI, normalized JTp variability) contains the variance of intervals (QTv, JTv, JTpv) divided by the square of the mean interval (QTm, JTm, JTpm). The denominator (HRVN, normalized HR variability) contains the variance of HR (HRv) divided by the squared mean HR (HRm). The logarithm is taken for statistical reasons to ensure a normal distribution of the otherwise skewed variability indexes distribution.
Subjects were divided into two groups to assess age‐related changes in values obtained from the ECG waveforms, as follows: group I, age < 1 year (n = 309, boys:girls = 180:129); group II, 1–7 years (n = 314, 172:142); and group III, healthy university students (mean age: 21.0 ± 1.2 years; n = 57, 30:27).
Statistical analysis was performed using JMP software (SAS Institute Inc., Cary, NC, USA). The correlation of QTVI with JTpVI was investigated using linear regression analysis. Comparisons of each age group with university students were performed by Dunnett's test, the Wilcoxon signed rank test was used for pairwise comparison in each age‐specific group, with the significance level at p < .05.
3. Results
3.1. Comparison of electrocardiography parameters
ECG parameters in each age group (groups I and II) were compared with those for the university students (group III) (Table 1). The mean heart rate decreased with age, reflecting physiological changes (Table 1). By contrast, the QRS time increased with age, and was significantly higher in group III compared with groups I and II. The corrected QT time was significantly higher in groups I and II compared with group III, while the corrected JT time was higher in groups I and II compared with group III. In groups I–II, the measured JTp time was significantly lower, and in groups I and II, the corrected JTp time was higher, compared with group III.
Table 1.
ECG characteristics
| I (n = 309) | II (n = 314) | III (n = 57) | |
|---|---|---|---|
| HR (bpm) | |||
| Mean ± SD | 155.3 ± 25.3a | 100.7 ± 15.8a | 68.3 ± 8.7 |
| Median | 159.7 | 99.3 | 67.9 |
| (2nd–98th) | 101.8–198.1 | 73.1–140.0 | 48.8–86.3 |
| RR (ms) | |||
| Mean ± SD | 397.7 ± 73.6a | 612.5 ± 95.2a | 900.1 ± 121.3 |
| Median | 375.3 | 606.7 | 899.7 |
| (2nd–98th) | 305.0–589.7 | 429.0–824.8 | 700.4–1236.6 |
| QRS (ms) | |||
| Mean ± SD | 67.8 ± 8.7a | 85.5 ± 9.2a | 104.4 ± 8.8 |
| Median | 65.8 | 85.9 | 103.0 |
| (2nd–98th) | 56.1–91.5 | 66.4–102.4 | 83.0–130.6 |
| QT (ms) | |||
| Mean ± SD | 258.1 ± 28.4a | 323.4 ± 28.5a | 380.9 ± 26.4 |
| Median | 252.9 | 323.1 | 381.2 |
| (2nd–98th) | 212.8–321.7 | 267.5–382.0 | 325.7–449.0 |
| QTc (ms) | |||
| Mean ± SD | 410.5 ± 20.4a | 414.5 ± 17.5a | 403.0 ± 24.0 |
| Median | 411.4 | 414.8 | 406.9 |
| (2nd–98th) | 368.4–450.4 | 378.2–447.4 | 350.2–441.0 |
| JT (ms) | |||
| Mean ± SD | 190.4 ± 23.6a | 237.8 ± 26.3a | 276.6 ± 27.1 |
| Median | 187.7 | 239.3 | 279.7 |
| (2nd–98th) | 149.6–242.8 | 182.4–294.6 | 218.2–345.6 |
| JTc (ms) | |||
| Mean ± SD | 302.7 ± 21.1a | 304.4 ± 19.2a | 292.5 ± 25.4 |
| Median | 305.4 | 304.6 | 295.5 |
| (2nd–98th) | 254.7–341.2 | 262.6–337.2 | 236.8–335.3 |
| JTp (ms) | |||
| Mean ± SD | 133.6 ± 21.3a | 169.3 ± 24.2a | 193.9 ± 26.9 |
| Median | 130.1 | 170.8 | 193.4 |
| (2nd–98th) | 96.4–179.7 | 117.8–217.2 | 134.8–261.0 |
| JTpc (ms) | |||
| Mean ± SD | 212.2 ± 22.6a | 216.4 ± 21.1a | 204.8 ± 24.8 |
| Median | 213.0 | 216.3 | 207.5 |
| (2nd–98th) | 164.9–257.5 | 170.5–254.8 | 148.4–254.7 |
Each value is expressed as the mean ± SD.
p < .05 (vs. III) with Dunnett's test.
3.2. Variability indexes
QTVI, JTVI, and JTpVI in each age group (groups I–II) were compared with those for university students (group III) (Table 2). All the variation rates were significantly different in groups I compared with group III, while there were no differences between group II and group III. Normalized variabilities (QTVN, JTVN, JTpVN, HRVN) were significantly different in groups I compared with group III, while there were no differences between group II and group III.
Table 2.
Comparison of variability indexes
| I (n = 309) | II (n = 314) | III (n = 57) | |
|---|---|---|---|
| QTVI | |||
| Mean ± SD | −0.41 ± 0.58a | −1.20 ± 0.48 | −1.35 ± 0.45 |
| Median | −0.39 | −1.30 | −1.29 |
| (2nd–98th) | −1.49 to 0.81 | −1.89 to 0.02 | −2.36 to −0.61 |
| JTVI | |||
| Mean ± SD | −0.16 ± 0.56a | −0.94 ± 0.47 | −1.06 ± 0.42 |
| Median | −0.16 | −1.02 | −0.95 |
| (2nd–98th) | −1.21 to 1.06 | −1.62 to 0.24 | −2.09 to −0.34 |
| JTpVI | |||
| Mean ± SD | 0.07 ± 0.64a | 0.80 ± 0.56 | −0.89 ± 0.45 |
| Median | 0.04 | −0.91 | −0.83 |
| (2nd–98th) | −1.15 to 1.36 | −1.65 to 0.65 | −1.85 to 0.03 |
| QTVN × 1000 | |||
| Mean ± SD | 0.46 ± 0.35a | 0.21 ± 0.19 | 0.21 ± 0.21 |
| Median | 0.38 | 0.14 | 0.13 |
| (2nd–98th) | 0.03 to 1.38 | 0.03 to 0.65 | 0.02 to 1.05 |
| JTVN × 1000 | |||
| Mean ± SD | 0.80 ± 0.61a | 0.36 ± 0.32 | 0.38 ± 0.34 |
| Median | 0.68 | 0.28 | 0.29 |
| (2nd–98th) | 0.07 to 2.37 | 0.07 to 1.20 | 0.04 to 1.72 |
| JTpVN × 1000 | |||
| Mean ± SD | 1.56 ± 1.58a | 0.57 ± 0.89 | 0.52 ± 0.39 |
| Median | 1.14 | 0.34 | 0.44 |
| (2nd–98th) | 0.11 to 5.93 | 0.10 to 2.51 | 0.08 to 1.82 |
| HRVN × 1000 | |||
| Mean ± SD | 1.46 ± 1.64a | 3.61 ± 3.48 | 3.97 ± 3.23 |
| Median | 0.86 | 2.63 | 3.17 |
| (2nd–98th) | 0.08 to 6.42 | 0.17 to 13.14 | 0.59 to 16.40 |
Each value is expressed as the mean ± SD.
p < .05 (vs. III) with Dunnett's test.
3.3. Gender differences
There were no gender differences in JTpVI between any age groups (Table 3).
Table 3.
Gender differences in JTpVI in each group
| I (n = 309) | II (n = 314) | III (n = 57) | |
|---|---|---|---|
| M/F | 180/129 | 172/142 | 30/27 |
| Age (month) | |||
| Median | 1 | 48 | 264 |
| (2nd–98th) | 0–11 | 12–91 | 233–289 |
| Male | 0.05 ± 0.65 | −0.84 ± 0.58 | −0.95 ± 0.56 |
| Female | 0.11 ± 0.64 | −0.76 ± 0.54 | −0.82 ± 0.26 |
| p‐value | .44 | .09 | .30 |
Each value is expressed as the mean ± SD. There is no significance with the Wilcoxon signed‐rank test.
3.4. Changes in JTpVI after birth
A scatter plot of the relationship between JTpVI and age in months is shown in Figure 1. The JTpVI value markedly decreased from the neonatal period to infancy, moderately decreased thereafter, and then stabilized by 3 years of age.
Figure 1.
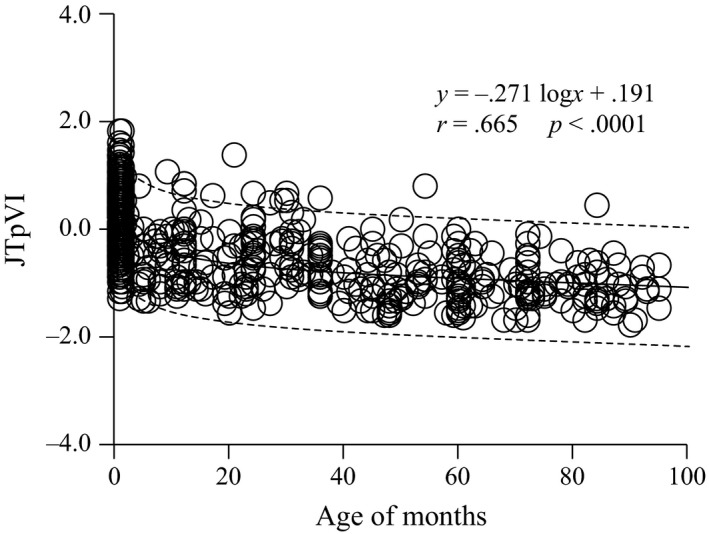
Relationship between JTpVI and age in months. The JTpVI value decreased rapidly until 3 months after birth, decreased slowly during infancy, and then stabilized by elementary school age
3.5. Comparing QTVI to other ECG‐based variability indexes
Correlation analysis showed a strong correlation (r = .856, p < .0001) of QTVI with JTpVI in children (Figure 2) and a significant relationship between JTVI and JTpVI (r = .897, p < .0001).
Figure 2.
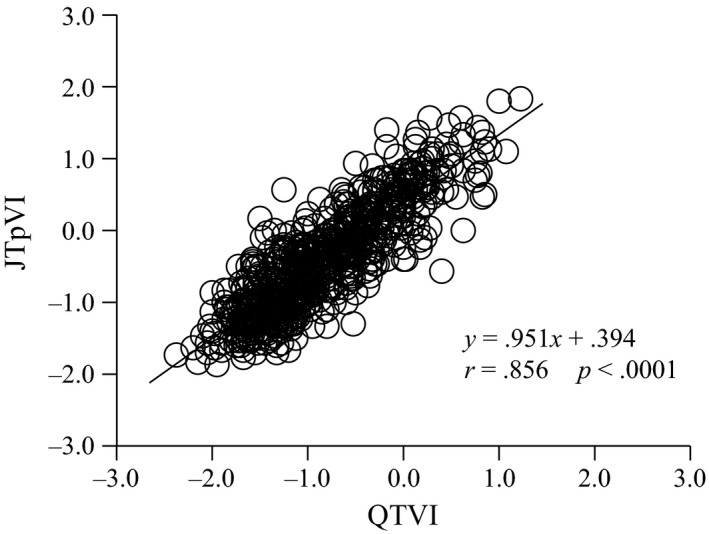
Relationship between QTVI and JTpVI
4. Discussion
In the present study, we used body surface electrocardiography to measure the early repolarization time, JTp, during childhood. The main finding was that the variability index (JTpVI) of the JTp time relative to the RR interval markedly decreased after birth, and then stabilized at 4–7 years of age. Furthermore, JTpVI was highly correlated with QTVI, which is used clinically to predict instability of myocardial repolarization.
Measurement of QT time is required for determining QTVI. However, as QT time includes the QRS complex time, false QT prolongation indicating QT extension is observed in cases with bundle branch block. Alternative methods have been developed to exclude the QRS complex when evaluating these waveforms, such as using the subinterval JT time from the J‐point, which represents the QRS complex endpoint (QRS end) to the T‐wave end. In a study of approximately 2,900 patients with bundle branch block, Zhou et al. (1992) reported that the JT time shortened with increasing QRS time. Furthermore, Berul, Hill, & Geggel (1997) measured the QRS, QT, and JT times in pediatric patients with long QT syndrome or right bundle branch block, and found QT prolongation in both groups, but JT prolongation only in patients with long QT syndrome. Thus, long QT syndrome and right bundle branch block can be differentiated by extraction and comparison of the JT time (Berul et al., 1997).
The ages of the subjects reported by Berul et al. (1997) ranged from 1 week to 18 years old, covering the neonatal period to school age and adolescence, suggesting that evaluation of repolarization time based on the JT time is useful in pediatric subjects. However, physiological heart rate is higher during infancy compared with in adulthood. Thus, the T‐wave end and P wave can overlap, which prevents accurate measurement of the QT time. To overcome this problem, we used the time from Q, termed the early repolarization time, to the T‐wave peak (QTp) in the repolarization process, or the time from the J‐point to the T‐wave peak (JTp). Although these are not complete substitutions for the QT time, we found a significant relationship between QTVI and JTpVI, indices of instability of myocardial repolarization.
Evaluation of arrhythmogenic substrates requires analysis of the time from Tpeak to Tend, which reflects “variation” of the duration of the action potential. This method is well established and is electrophysiologically valid (Antzelevitch et al., 1999; Smetana et al., 2011; Antzelevitch, 2005). However, patients with arrhythmogenicity characterized by short QT syndrome were recently reported (Bjerregaard & Gussak, 2005). Although there are no established diagnostic criteria for this syndrome, Gollob, Redpath, & Roberts (2011) proposed criteria for adults where shortening of the early repolarization time (JTp < 120 ms) is scored as one point. Short QT syndrome also develops in childhood, and separate diagnostic criteria may be needed. The utility of the measured or corrected value of the JTp time or variability index remains to be investigated in this context.
To our knowledge, there are no previous reports on JTpVI, and no large‐scale studies on the repolarization time. The characteristics of the changes in JTpVI after birth (Figure 1) are similar to the age‐related changes in QTVI reported by Kusuki et al. (2011) Furthermore, the age‐related gradual changes in JTpVI closely resemble the frequency characteristic ratio (LF/HF) of heart rate variability described by Massin & Von Bernuth (1997) There is a progressive change in autonomic nervous system balance with growth after birth, with increased vagus nerve activity to match the predominant sympathetic nerve activity. LF/HF is a measure of autonomic nervous balance calculated from variation of the RR interval, and autonomic nervous input into the sinus node provides the basis for heart rate variability (De Rogalski et al., 2007; Longin et al., 2006). Thus, JTpVI might reflect the maturation of autonomic nervous control of the heart, similar to LF/HF and QTVI in healthy children.
Clinically, arrhythmia‐associated cardiac events can be predicted from a high QTVI in adults with an impaired myocardium. However, QTVI shows diurnal variation, and its general use should be assessed in a randomized prospective study (Dobson, Kim, & Haigney, 2013). In the present study, we used healthy university students as controls to match the control groups used in previous studies (Dobson et al., 2013; Atiga et al., 1998; Myredal, Karlsson, & Johansson, 2008). We previously reported that variation of QTVI was dependent on development after birth in children (Kusuki et al., 2011). Furthermore, in a clinical study of children with systemic inflammation due to Kawasaki disease (studies were performed in the febrile and recovery stages), a high QTVI was observed in the febrile stage, but no patients developed arrhythmia, suggesting that fever increased sympathetic tension (Kuriki et al., 2011). These studies suggest that JTpVI and QTVI reflect autonomic nervous system balance in subjects with no myocardial impairment. JTpVI combines measure of both JTp interval and heart rate variability, and these factors can be evaluated independently to assess the relative contributions of electrical instability and autonomic nervous activity.
4.1. Limitations
This study had several limitations. First, the T‐wave peak can vary among the 12‐lead ECG sites, and the early repolarization time is not homogenous. To overcome this problem, we only analyzed the single CM5‐lead ECG. Second, as the subjects were children with no organic heart disease and the QRS time did not exceed 120 ms in any subject, the influence of bundle branch block on JTpVI was not investigated. Future studies comparing the standard JTpVI values in cases with bundle branch block cases are required. Third, we administered sedative to infants who were 3–12 months. It is possible that the activity of sympathetic nervous system was decreased by the sedative. Fourth, we did not perform a comparison of subjects in the present study with excluded subjects that showed a T‐wave end overlap in the ascending limb of the P wave.
5. Conclusion
This study demonstrated that QTVI, which reflects instability of myocardial repolarization, and JTpVI determined by subinterval analysis, were positively correlated in healthy children, suggesting that JTpVI can be used to evaluate repolarization lability of the myocardium. Furthermore, JTpVI changed with age, reflecting fluctuations in the cardiac cycle and autonomic nervous input into repolarization (i.e., physiological development of the autonomic nervous system). Future studies are required to investigate the utility of JTpVI in subjects with organic heart pathology, with the goal of using this parameter for detection of early repolarization abnormalities.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This study was supported in part by the Japan Society for the Promotion of Science (KAKENHI) (#26350944). We are grateful to Dr. Tsuneaki Sadanaga, Seigatou Hospital, for his helpful suggestions and thank Mr. Hirofumi Kusuki for his technical assistance.
Takeuchi Y, Omeki Y, Horio K, et al. Relationship between QT and JT peak interval variability in prepubertal children.Ann Noninvasive Electrocardiol. 2017;22:e12444. 10.1111/anec.12444
Funding information
Supported in part by grants from the Japan Society for the Promotion of Science KAKENHI (#26350944).
References
- Akselrod, S. , Gordon, D. , Ubel, F. A. , Shannon, D. C. , Berger, A. C. , & Cohen, R. J. (1981). Power spectrum analysis of heart rate fluctuations: A quantitative probe of beat‐to‐beat cardiovascular control. Science, 213, 220–222. [DOI] [PubMed] [Google Scholar]
- Antzelevitch, C. (2005). Role of transmural dispersion of repolarization in the genesis of drug‐induced torsades de pointes. Heart Rhythm, 2, S9–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch, C. , Yan, G. X. , & Shimizu, W. (1999). Transmural dispersion of repolarization and arrhythmogenicity: The Brugada syndrome versus the long QT syndrome. Journal of Electrocardiology, 32, 158–165. [DOI] [PubMed] [Google Scholar]
- Atiga, W. L. , Calkins, H. , Lawrence, J. H. , Tomaselli, G. F. , Smith, J. M. , & Berger, R. D. (1998). Beat‐to‐beat repolarization lability identifies patients at risk for sudden cardiac death. Journal of Cardiovascular Electrophysiology, 9, 899–908. [DOI] [PubMed] [Google Scholar]
- Berger, R. D. , Kasper, E. K. , Baughman, K. L. , Marban, E. , Calkins, H. , & Tomaselli, G. F. (1997). Beat‐to‐beat QT interval variability: Novel evidence for repolarization lability in ischemic and non‐ischemic dilated cardiomyopathy. Circulation, 96, 1557–1565. [DOI] [PubMed] [Google Scholar]
- Berul, C. I. , Hill, S. L. , Geggel, R. L. , Hijazi, Z. M. , Marx, G. R. , Rhodes, J. , … Fulton, D. R. (1997). Electrocardiographic markers of late sudden death risk in postoperative tetralogy of Fallot children. Journal of Cardiovascular Electrophysiology, 8, 1349–1356. [DOI] [PubMed] [Google Scholar]
- Bjerregaard, P. , & Gussak, I. (2005). Short QT syndrome. Annals of Noninvasive Electrocardiology, 10, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rogalski Landrot, I. , Roche, F. , Pichot, V. , Teyssier, G. , Gaspoz, J. M. , Barthelemy, J. C. , & Patural, H. (2007). Autonomic nervous system activity in premature and full‐term infants from theoretical term to 7 years. Autonomic Neuroscience, 136(1–2), 105–109. [DOI] [PubMed] [Google Scholar]
- Dobson, C. P. , Kim, A. , & Haigney, M. (2013). QT variability index. Progress in Cardiovascular Diseases, 56(2), 186–194. [DOI] [PubMed] [Google Scholar]
- Goldkorn, R. , Naimushin, A. , Shlomo, N. , Dan, A. , Oieru, D. , Moalem, I. , … Goldenberg, I. (2015). Comparison of the usefulness of heart rate variability versus exercise stress testing for the detection of myocardial ischemia in patients without known coronary artery disease. American Journal of Cardiology, 115, 1518–1522. [DOI] [PubMed] [Google Scholar]
- Gollob, M. H. , Redpath, C. J. , & Roberts, J. D. (2011). The short QT syndrome: Proposed diagnostic criteria. Journal of the American College of Cardiology, 57, 802–812. [DOI] [PubMed] [Google Scholar]
- Gupta, P. , Patel, C. , Patel, H. , Narayanaswamy, S. , Malhotra, B. , Green, J. T. , & Yan, G. X. (2008). T(p‐e)/QT ratio as an index of arrhythmogenesis. Journal of Electrocardiology, 41, 567–574. [DOI] [PubMed] [Google Scholar]
- Kuriki, M. , Fujino, M. , Tanaka, K. , Horio, K. , Kusuki, H. , Hosoi, M. , … Hata, T. (2011). Ventricular repolarization lability in children with Kawasaki disease. Pediatric Cardiology, 32, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuki, H. , Kuriki, M. , Horio, K. , Hosoi, M. , Matsuura, H. , Fujino, M. , … Hata, T. (2011). Beat‐to‐beat QT interval variability in children: Normal and physiologic data. Journal of Electrocardiology, 44, 326–329. [DOI] [PubMed] [Google Scholar]
- Longin, E. , Gerstner, T. , Schaible, T. , Lenz, T. , & König, S. (2006). Maturation of the autonomic nervous system: Differences in heart rate variability in premature vs. term infants. Journal of Perinatal Medicine, 34(4), 303–308. [DOI] [PubMed] [Google Scholar]
- Lopatin, A. N. , & Nichols, C. G. (2001). Inward rectifiers in the heart: An update on I(K1). Journal of Molecular and Cellular Cardiology, 33, 625–638. [DOI] [PubMed] [Google Scholar]
- Malik, M. , & Batchvarov, V. N. (2000). Measurement, interpretation and clinical potential of QT dispersion. Journal of the American College of Cardiology, 36, 1749–1766. [DOI] [PubMed] [Google Scholar]
- Malik, M. , Odemuyiwa, O. , Poloniecki, J. , Kulakowski, P. , Farrell, T. , Staunton, A. , & Camm, A. J. (1992). Late potentials after acute myocardial infarction. Performance of different criteria for the prediction of arrhythmic complications. European Heart Journal, 13, 599–607. [DOI] [PubMed] [Google Scholar]
- Massin, M. , & Von Bernuth, G. (1997). Normal range of heart rate variability during infancy and childhood. Pediatric Cardiology, 18, 297–302. [DOI] [PubMed] [Google Scholar]
- Myredal, A. , Karlsson, A. K. , & Johansson, M. (2008). Elevated temporal lability of myocardial repolarization after coronary artery bypass grafting. Journal of Electrocardiology, 41, 698–702. [DOI] [PubMed] [Google Scholar]
- Napolitano, C. , Priori, S. G. , & Schwartz, P. J. (2000). Significance of QT dispersion in the long QT syndrome. Progress in Cardiovascular Diseases, 42, 345–350. [DOI] [PubMed] [Google Scholar]
- Simson, M. B. (1981). Use of signals in the terminal QRS complex to identify patients with ventricular tachycardia after myocardial infarction. Circulation, 64, 235–242. [DOI] [PubMed] [Google Scholar]
- Smetana, P. , Schmidt, A. , Zabel, M. , Hnatkova, K. , Franz, M. , Huber, K. , & Malik, M. (2011). Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: Peak to the end of the T wave interval and nondipolar repolarization components. Journal of Electrocardiology, 44, 301–308. [DOI] [PubMed] [Google Scholar]
- Wetzel, G. T. , & Klitzner, T. S. (1996). Developmental cardiac electrophysiology recent advances in cellular physiology. Cardiovascular Research, 31, E52–E60. [PubMed] [Google Scholar]
- Zhou, S. H. , Wong, S. , Rautaharju, P. M. , Karnik, N. , & Calhoun, H. P. (1992). Should the JT rather than the QT interval be used to detect prolongation of ventricular repolarization? An assessment in normal conduction and in ventricular conduction defects. Journal of Electrocardiology, 25, 131–136. [DOI] [PubMed] [Google Scholar]


