Abstract
Background
Electrocardiograms (ECGs) are routinely obtained in patients with advanced congestive heart failure (CHF) before and after surgical implantation with a left‐ventricular assist device (LVAD). As the number of patients with CHF is increasing, it is necessary to characterize the changes present in the ECG of patients with LVADs.
Methods
ECGs of 43 patients pre‐ and postimplantation of a HeartMate II LVAD were compared to characterize the presence of an LVAD using the following six criteria (LVADS2): low limb‐lead voltage, ventricular pacing, artifact (electrical), duration of the QRS > 120 milliseconds, ST‐elevation in the lateral leads, and splintering of the QRS complex. Additionally, 50 ECGs of non‐LVAD patients coded as “lateral myocardial infarction (MI)” and 50 ECGs coded as “ventricular pacing” were chosen at random and scored. Odds ratios were calculated using Fisher's exact test. Logistic regression models were built to predict the presence of an LVAD in all patients.
Results
Univariate analysis of the pre‐ and post‐LVAD ECGs confirmed that all criteria except the “Duration of QRS > 120 milliseconds” characterized the ECG of a patient with an LVAD. Electrical artifact and low limb‐lead voltage yielded the greatest association with an LVAD‐ECG.
Conclusions
The ECG of a patient with end‐stage CHF significantly changes with LVAD implantation. The LVADS2 criteria provide a framework towards characterizing and establishing a new baseline of the ECG in a patient with a continuous‐flow LVAD.
Keywords: noninvasive techniques—electrocardiography, clinical, implantable devices—biventricular pacing/defibrillation
Left‐ventricular assist devices (LVADs) significantly improve survival and quality of life in patients with dilated, end‐stage congestive heart failure (CHF), by increasing cardiac output.1, 2, 3 The most commonly implanted LVADs are continuous‐flow, with a central electromagnetic axial rotor spinning from 8000 to 10000 rpm, driving blood from an inlet cannula at the LV apex to an outflow graft into the ascending aorta.4
The electrocardiogram (ECG) is a standard, noninvasive, and ubiquitous graphical recording of the cardiac cycle obtained on nearly every patient admitted with cardiac disease, and examined frequently in outpatient clinics. Most ECGs in patients with CHF are abnormal, with ischemic or nonspecific changes of the ST‐T segments and the QRS complex aberrations such as conduction defects and ventricular hypertrophy.5 Pacemakers are implanted in approximately 8% of patients with CHF and bradycardia, and randomized‐controlled trials support the use of biventricular pacing (BiV) in patients with a low ejection fraction and a prolonged QRS duration.6, 7 Reproducible patterns on recorded ECGs from implanted technological devices, such as single, dual‐lead, or cardiac‐resynchronization pacemakers, have led to establishing defining characteristics pertaining to a respective device.8, 9
The ECG interpretation of implanted LVAD remains largely uncharacterized. We propose a retrospective study to identify prominent attributes of an ECG that are highly associated with patients who have an implanted, continuous‐flow LVAD.
METHODS
Patient Population
The MUSE ECG archival system of Barnes‐Jewish Hospital in St. Louis, MO was queried for 12‐lead ECGs run at a speed of 25 mm/second on the horizontal axis and 10 mm/mV on the vertical axis, prior to and after HeartMateII (continuous‐flow) LVAD implantation from 43 patients from 2007 to 2010. The “preimplantation” ECGs were obtained within 24 hours prior to LVAD implantation, while the patient was an inpatient. Postimplantation ECGs were obtained at an average of 347 ± 278 days, with a range from 8 days to 1065 days. This variability is due to availability on the electronic medical record. We did not longitudinally examine ECGs for each patient. Two additional queries were performed for ECGs from 100 unique individuals: 50 coded as “lateral myocardial infarction” and 50 ECGs coded as “ventricular pacing.” All identifying information from the ECG and computerized interpretations of the tracings were removed. This retrospective study to examine patient ECGs only was approved by the Institutional Review Board of the Washington University School of Medicine (protocol #201102233).
ECG Criteria Tabulation
It had been routinely observed by the authors that an electrical artifact was most consistently present in ECGs post‐LVAD surgery. Comparison of a handful of test ECGs from pre‐ to post‐evaluation as part of clinical care were garnered and several ECG changes were empirically noted and defined on the 12‐lead ECG. From this test cohort, not included in our statistical samples, we formulated the following criteria: low limb‐lead voltage defined as a QRS amplitude in each of the standard limb leads <5 mm; ventricular pacing with ventricular pacing spikes present in 50% of QRS beats; the presence of an electrical artifact in all 12 of the ECG leads; a duration of the QRS complex > 120 milliseconds; ST‐elevation in the lateral leads; and splintering of the QRS complex in lateral or distal precordial leads (LVADS2). Splintering is defined as multiple QRS deflections, typically at the peak of the complex, leading to what has been referred to as M or W complexes.10 Each LVADS2 criterion was scored as present or absent by a cardiologist blinded to the clinical context of the ECGS: pre‐LVAD, post‐LVAD, lateral MI without LVAD, and ventricular pacing without an LVAD.
Statistical Analysis
All tests for significance were conducted at the 5% type I error level (i.e., α = 0.05). Analysis was conducted with SAS v9.3 (SAS Institute, Cary, NC, USA). The pre‐ and post‐LVAD comparisons were conducted with McNemar's test. Post‐LVAD findings were combined with ventricular‐pacing and lateral MI patients. Frequency counts and comparisons between post‐LVAD and ventricular‐pacing ECGs were conducted with Fisher's exact test. Odds ratios and 95% confidence intervals were calculated. Similar analyses were conducted for post‐LVAD versus lateral MI and for post‐LVAD versus ventricular‐pacing or lateral MI patients.
A logistic regression model was built to predict LVAD among all patients. Model fit was described by the generalized R2, and accuracy was reported by the area under the receiver operating characteristics (ROC) curve. Multiple models were considered.
RESULTS
Demographics of the LVAD Population
We identified 43 patients at random who had undergone continuous‐flow LVAD implantation of a Heart Mate II device between June 2007 and April 2010 at our institution (Table 1). The average age of our patients was approximately 54 ± 12.6 years, predominantly male (77%), and Caucasian (79%). Approximately 30% had diabetes mellitus, and the etiology of heart failure was nonischemic cardiomyopathy in half of our patients.
Table 1.
LVAD Patient Characteristics
| Variable | Overall (N = 43) |
|---|---|
| Age | 54.4 ± 12.6 |
| Male, No. (%) | 33(77%) |
| Race, No. (%) | |
| White | 34(79%) |
| African American | 9(21%) |
| Diabetes, No. (%) | 13(30%) |
| NICM, No. (%) | 22(51%) |
Value is mean ± SD unless otherwise indicated.
LVAD = left‐ventricular assist device; NICM = nonischemic cardiomyopathy.
ECG Changes from Pre‐ to Post‐LVAD Implantation
The ECGs from 43 patients pre‐ and post‐LVAD implantation were evaluated. From one patient, a pre‐ and post‐LVAD 12‐lead ECG is shown in the Figure 1. In Figure 2, we highlight the LVADS2 criteria. A comparison of the ECGs between men (n = 33) versus women (n = 10) and ischemic (n = 21) versus nonischemic (n = 22) cardiomyopathy yielded no significant differences in pre‐ or postpatterns. We observed that low‐limb lead voltage, the presence of an electrical artifact in all 12 leads, and a splintering of the QRS complex are each significantly increased in post‐LVAD ECGs (Table 2). An ICD was present in 37 patients (n = 43) prior to their LVAD implantation, although the occurrence of pacing by ECG was also significantly increased post‐LVAD. One patient received an ICD in the interval time from surgery to the post‐ECG. Interestingly, an increase in the amplitude of the electrical artifact was noted preceding and into the QRS complex, particularly in the lateral leads of I, aVL, and the precordial lead V1. Although not 100% present, it appears to be a nearly pathognomonic quality of the post‐LVAD ECG. A QRS‐complex duration > 120 milliseconds, was seen in exactly 67% of the pre‐ and post‐LVAD ECGs. There was also an insignificant increase in the occurrence of ST‐segment elevation post‐LVAD implantation from 28% to 44%, P = 0.11.
Figure 1.
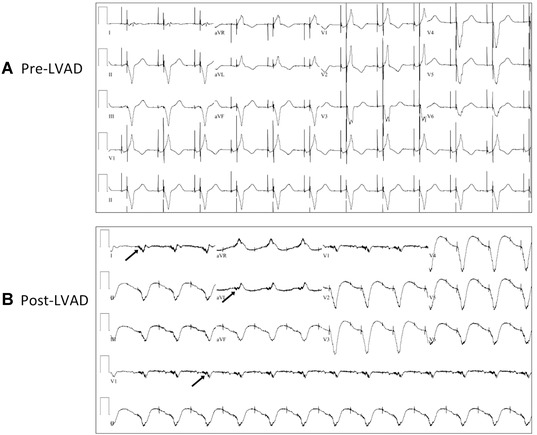
ECGs pre‐ and post‐LVAD in one patient. (A) An ECG obtained prior to LVAD placement. (B) The same patient with an ECG obtained approximately 2 months post‐LVAD implantation. Preceding the QRS complex in leads I, aVL, and V1, an increase in the amplitude of the electrical artifact can be noted, indicated by arrows.
Figure 2.
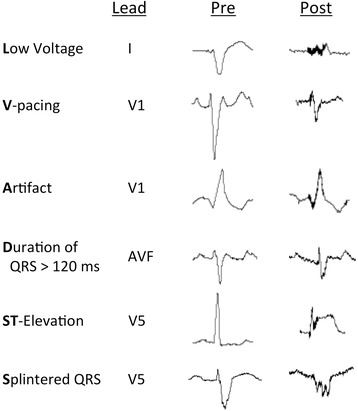
LVADS2 Criteria pre‐ and post‐HeartMate II LVAD implantation. Representative beats at the indicated leads of the same patient pre‐ and post‐LVAD implantation are shown to demonstrate each of the criteria.
Table 2.
Pre‐ versus Post‐LVAD Criteria Comparisons
| Variable | Pre‐LVAD (N = 43) | Post‐LVAD (N = 43) | P‐Value* | ||
|---|---|---|---|---|---|
| L, No. (%) | <.001 | ||||
| No | 28 | (65%) | 15 | (35%) | |
| Yes | 15 | (35%) | 28 | (65%) | |
| V, No. (%) | <0.001 | ||||
| No | 24 | (56%) | 9 | (21%) | |
| Yes | 19 | (44%) | 34 | (79%) | |
| A, No. (%) | <0.001 | ||||
| No | 40 | (93%) | 1 | (2%) | |
| Yes | 3 | (7%) | 42 | (98%) | |
| D, No. (%) | 1.00 | ||||
| No | 14 | (33%) | 14 | (33%) | |
| Yes | 29 | (67%) | 29 | (67%) | |
| S1, No. (%) | 0.11 | ||||
| No | 31 | (72%) | 24 | (56%) | |
| Yes | 12 | (28%) | 19 | (44%) | |
| S2, No. (%) | 0.012 | ||||
| No | 18 | (42%) | 7 | (16%) | |
| Yes | 25 | (58%) | 36 | (84%) | |
*Based on McNemar's test.
A = electrical artifact; D = duration of QRS complex > 120 ms; L = low limb‐lead voltage; LVAD = left‐ventricular assist device; S1 = ST elevation in ST segment of the lateral leads; S2 = splintering of the QRS complex; V = ventricular pacing.
Comparison of Post‐LVAD, Ventricular‐Pacing, and Lateral MI ECGs
ECGs from a random sample of 50 patients without LVADs and with ECGs coded as “ventricular‐pacing” (VPACE) or “lateral myocardial infarction” (Lat MI) had their identifying information and computerized interpretations removed and were then scored for the presence or absence of each of the LVADS2 criteria. We selected VPACE patients as one control because over 40% of our LVAD patients had ventricular pacing at baseline, which increased to 79% after LVAD insertion. LVAD implantation induces damage and scar formation secondary to myocardial removal and suturing around the LVAD cannula, which significantly led to QRS splintering in the lateral limb and precordial leads in our LVAD patients. Lateral myocardial infarctions were selected as another control cohort because we hypothesized that a greater percentage of those patients with lateral myocardial infarctions would have splintering of the QRS complex compared to those with ventricular pacing, providing a stringency to the S2 (splintering) criterion for our LVAD patients. Those scores of VPACE and LatMI patients were compared to the scores in the cohort of post‐LVAD ECGs, and Fisher's exact test was used for computing significance (Table 3). Corroborating odds ratios for LVADS2 are shown as Table 4. Criteria more represented in the LVAD than VPACE ECGs included: Low‐limb lead voltage, electrical artifact, and splintering of the QRS complex. ECGs labeled as Lat MI (n = 50) and combining the VPACE and Lat MI (n = 100), demonstrated a significantly greater prevalence of all LVADS2 criteria in the LVAD ECGs, with the exception of an increased QRS complex duration greater than 120 milliseconds.
Table 3.
Criteria Comparison: LVAD versus V‐Pace, Lateral MI, V‐Pace, and Lateral MI
| V‐Pace | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LVAD | V‐Pace | Lat MI | &Lat MI | ||||||||
| Variable | (N = 43) | (N = 50) | P‐Value* | (N = 50) | P‐Value* | (N = 100) | P‐Value* | ||||
| L, No. (%) | <0.001 | <0.001 | <0.001 | ||||||||
| No | 15 | (35%) | 42 | (84%) | 36 | (72%) | 78 | (78%) | |||
| Yes | 28 | (65%) | 8 | (16%) | 14 | (28%) | 22 | (22%) | |||
| V, No. (%) | 0.06 | <0.001 | 0.008 | ||||||||
| No | 9 | (21%) | 3 | (6%) | 42 | (84%) | 45 | (45%) | |||
| Yes | 34 | (79%) | 47 | (94%) | 8 | (16%) | 55 | (55%) | |||
| A, No. (%) | <0.001 | <0.001 | <0.001 | ||||||||
| No | 1 | (2%) | 47 | (94%) | 48 | (96%) | 95 | (95%) | |||
| Yes | 42 | (98%) | 3 | (6%) | 2 | (4%) | 5 | (5%) | |||
| D, No. (%) | 0.022 | 0.21 | 0.69 | ||||||||
| No | 14 | (33%) | 6 | (12%) | 23 | (46%) | 29 | (29%) | |||
| Yes | 29 | (67%) | 44 | (88%) | 27 | (54%) | 71 | (71%) | |||
| S1, No. (%) | 0.13 | <0.001 | <0.001 | ||||||||
| No | 24 | (56%) | 36 | (72%) | 48 | (96%) | 84 | (84%) | |||
| Yes | 19 | (44%) | 14 | (28%) | 2 | (4%) | 16 | (16%) | |||
| S2, No. (%) | <0.001 | 0.037 | <0.001 | ||||||||
| No | 7 | (16%) | 31 | (62%) | 18 | (36%) | 49 | (49%) | |||
| Yes | 36 | (84%) | 19 | (38%) | 32 | (64%) | 51 | (51%) | |||
*Based on Fisher's exact test, compared to LVAD.
A = electrical artifact; D = duration of QRS complex > 120 ms; L = low limb‐lead voltage; Lat MI = ECGs coded as having lateral myocardial infarction; LVAD = left‐ventricular assist device; V = ventricular pacing; V‐Pace = ECGs coded as having ventricular pacing; ; S1 = ST elevation in ST segment of the lateral leads; S2 = splintering of the QRS complex.
Table 4.
Odds Ratios
| LVAD versus | LVAD versus | LVAD versus | ||||
|---|---|---|---|---|---|---|
| V‐Pace | Lat MI | V‐Pace & Lat MI | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| L | 9.8 | (3.35, 29.93) | 4.8 | (1.83, 12.73) | 6.62 | (2.82, 15.7) |
| V | 0.24 | (0.04, 1.08) | 19.83 | (6.23, 65.69) | 3.09 | (1.27, 8.06) |
| A | 658 | (59, 27078) | 1008 | (75, 41421) | 798 | (87, 32529) |
| D | 0.28 | (0.08, 0.91) | 1.76 | (0.7, 4.51) | 0.85 | (0.37, 2) |
| S1 | 2.04 | (0.79, 5.29) | 19 | (3.93, 175.93) | 4.16 | (1.72, 10.04) |
| S2 | 8.39 | (2.86, 26.34) | 2.89 | (0.98, 9.21) | 4.94 | (1.91, 14.28) |
A = electrical artifact; D = duration of QRS complex > 120 milliseconds; L = low limb‐lead voltage; Lat MI = ECGs coded as having lateral myocardial infarction; LVAD = left‐ventricular assist device; S1 = ST elevation in ST segment of the lateral leads; S2 = splintering of the QRS complex; V = ventricular pacing; V‐Pace = ECGs coded as having ventricular pacing.
Prediction Models Characterizing an LVAD‐ECG
Two logistic regression models were built to predict LVAD among all patients. One model utilized all six criteria (Model 1: LVADS2), and an additional model comprised of the presence of low limb‐lead voltage and electrical artifact (Model 2: L and A), as shown in Table 5). Both models displayed high accuracy with area under the ROC curve (AUC) values of 0.98 and 0.99, respectively. To obtain the probability of an ECG as belonging to a patient with an LVAD, the following equations derived from the models can be used:
Table 5.
LVAD‐ECG Prediction Models
| β | P‐Value | ||
|---|---|---|---|
| Model 1 | Intercept | −0.60 | 0.40 |
| Low limb‐lead voltage | 1.10 | 0.07 | |
| Ventricular pacing | −0.20 | 0.75 | |
| Artifact (electrical) in all leads | 3.56 | <0.001 | |
| Duration of QRS > 120 miiliseconds | −0.64 | 0.40 | |
| S1: ST segment elevation | 0.64 | 0.23 | |
| S2: Splintering of QRS complex | 0.27 | 0.66 | |
| Max‐rescaled generalized R2 = 0.89, AUC = 0.99 | |||
| Model 2 | Intercept | −1.05 | 0.06 |
| Low limb‐lead voltage | 1.17 | 0.038 | |
| Artifact (electrical) in all leads | 3.47 | <0.001 | |
| Max‐rescaled generalized R2 = 0.88, AUC = 0.98 | |||
DISCUSSION
Advanced heart failure therapies such as biventricular‐pacing and LVADs have improved the survival and quality of life for thousands of patients with end‐stage, dilated CHF.1, 11 With biventricular pacing, evaluation of the effect of the implanted electrical device on the ECG has proved to be a valuable tool in associating a patient's response to cardiac resynchronization therapy with clinical outcomes.12, 13 Currently, there are no identified descriptive or defining characteristics of the 12‐lead ECG associated with LVAD implantation.
In our study, we examined the pre‐ and post‐ECGs of 43 patients who underwent advanced heart failure therapy with an LVAD. The implantation of a metal conduit and impeller motor significantly changed the ECG, as demonstrated in four out of six of our suspect criteria: Low limb‐lead voltage, more ventricular pacing, electrical artifact presence in all leads, and a splintering of the QRS complex. Most patients had an ICD implanted prior to their LVADs, and one possible explanation for the significant increase in ventricular pacing would be the routine increase in program the pacing to a higher rate in the postoperative setting. It is unlikely that the atrioventricular node is damaged curing cannula insertion. Among comparison between post‐LVAD and patients with ventricular‐pacing or lateral myocardial infarction, the strongest criterion was presence of an electrical artifact in all leads, yielding the highest odds ratio and contributing the most towards the model probability of an LVAD‐ECG. We hypothesize that this artifact is most likely the result of the electromagnetically driven motor spinning at speeds between 8000 and 10,000 RPM.14
Low limb‐lead voltage is best described as a dampening of either the electrical voltage generation or transmission, with varied myocardial intrinsic or extrinsic causes such as an infiltrative cardiomyopathy and loss of myocardial contractile tissue, or pleural effusions and obesity.15 Prior ECG research in multiple myocardial infarction (MMI) models has shown a characteristic splintering of the QRS complex, proposed as secondary to a serrated edge of interdigitating normal myocardium and myocardial fibrosis.10 The loss of myocardium from the LV‐cannula displacement and positioning of the 10‐ounce motor within the thorax could explain, in part, a decrease in limb‐lead voltage, while the healing connection and suturing of the LV cannula into the apex might result in splintering of the QRS complex.
The presence or absence of an LVAD is obvious to patients and their treating physicians, however, the presence of atrial and ventricular arrhythmias can range from 30% to greater than 50% after implantation.16, 17 Every patient with an LVAD requires a new baseline ECG, with subsequent ECGs to be ordered as clinically indicated. In our study, we identify ECG criteria that collectively characterize an ECG of a patient with an LVAD, with the strongest odds ratios for the ubiquitous electrical artifact, followed by low limb‐lead voltage, and a splintering of the QRS complex. Future, ECG studies are indicated to determine the utility of our criteria and models in the post‐LVAD population towards LVAD optimization and outcomes.
Acknowledgments
The authors gratefully and respectfully acknowledge the indelible contributions from the late John P. Boineau, M.D. with his masterful ECG insights and discussions towards this project.
REFERENCES
- 1. Slaughter MS, Rogers JG, Milano C A, et al. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med 2009;361(23):2241–2251. [DOI] [PubMed] [Google Scholar]
- 2. Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail 2012;5(2):241–248. [DOI] [PubMed] [Google Scholar]
- 3. Stewart GC, Stevenson LW. Keeping left ventricular assist device acceleration on track. Circulation 2011;123(14):1559–1568; discussion 1568. [DOI] [PubMed] [Google Scholar]
- 4. John R. Current axial‐flow devices–the HeartMate II and Jarvik 2000 left ventricular assist devices. Semin Thorac Cardiovasc Surg 2008;20(3):264–272. [DOI] [PubMed] [Google Scholar]
- 5. Fonseca C, Mota T, Morais H, et al. The value of the electrocardiogram and chest X‐ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail 2004;6(6):807–812, 821–822. [DOI] [PubMed] [Google Scholar]
- 6. Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368(17):1585–1593. [DOI] [PubMed] [Google Scholar]
- 7. Saxon LA, Stevenson WG, Middlekauff HR, et al. Increased risk of progressive hemodynamic deterioration in advanced heart failure patients requiring permanent pacemakers. Am Heart J 1993;125(5 Pt 1):1306–1310. [DOI] [PubMed] [Google Scholar]
- 8. Barold SS, Herweg B. Usefulness of the 12‐lead electrocardiogram in the follow‐up of patients with cardiac resynchronization devices. Part II Cardiol J 2011;18(6):610–624. [DOI] [PubMed] [Google Scholar]
- 9. Barold SS, Herweg B. Usefulness of the 12‐lead electrocardiogram in the follow‐up of patients with cardiac resynchronization devices. Part I Cardiol J 2011;18(5):476–486. [DOI] [PubMed] [Google Scholar]
- 10. Boineau JP. Diagnosis of multiple infarcts from complex electrocardiograms during normal rhythm, left bundle‐branch block, and ventricular pacing. J Electrocardiol 44(6):605–610. [DOI] [PubMed] [Google Scholar]
- 11. Cleland JGF, Daubert J‐C, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352(15):1539–1549. [DOI] [PubMed] [Google Scholar]
- 12. Mollo R, Cosenza A, Coviello I, et al. A novel electrocardiographic predictor of clinical response to cardiac resynchronization therapy. Europace 2013;15(11):1615–1621. [DOI] [PubMed] [Google Scholar]
- 13. Kandala J, Upadhyay GA, Altman RK, et al. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. Eur Heart J 2013;34(29):2252–2262. [DOI] [PubMed] [Google Scholar]
- 14. Lund LH, Gabrielsen A, Tirén L, et al. Derived and displayed power consumption, flow, and pulsatility over a range of HeartMate II left ventricular assist device settings. ASAIO J 58(3):183–190. [DOI] [PubMed] [Google Scholar]
- 15. Chinitz JS, Cooper JM, Verdino RJ. Electrocardiogram voltage discordance: interpretation of low QRS voltage only in the limb leads. J Electrocardiol 41(4):281–286. [DOI] [PubMed] [Google Scholar]
- 16. Lok SI, Martina JR, Hesselink T, et al. Single‐centre experience of 85 patients with a continuous‐flow left ventricular assist device: Clinical practice and outcome after extended support. Eur J Cardiothorac Surg 2013;44(3):e233–e238. [DOI] [PubMed] [Google Scholar]
- 17. Yuan N, Arnaoutakis GJ, George TJ, et al. The spectrum of complications following left ventricular assist device placement. J Card Surg 2012;27(5):630–638. [DOI] [PubMed] [Google Scholar]


