Abstract
Introduction
Premature ventricular contractions (PVCs) frequently occur in patients with left ventricular dysfunction. However, there are limited data regarding the burden and morphologic characteristics of PVCs in patients receiving cardiac resynchronization therapy.
Methods and Results
Patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial‐Cardiac Resynchronization Therapy (MADIT‐CRT) with >5000 PVCs on a predevice implant 12‐lead, 24‐hour Holter were identified. The putative PVC site of origin for the most dominant PVC was characterized and their effects on clinical outcomes were evaluated. A total of 146 patients were identified to have >5000 PVCs on Holter of which 75 (51%) had PVCs originating from a non–outflow tract site. Other sites included the left ventricular outflow tract (LVOT), right ventricular outflow tract (RVOT), and the sinus of Valsalva. In multivariate analysis, the risk for HF/Deatd was similar in patients with Outflow tract PVCs when compared to patients with Non–outflow tract PVCs (HR 1.4, 95% CI 0.7–2.8, P = 0.3). The degree of echocardiographic reverse remodeling was similar in patients with outflow tract versus Non–outflow tract PVCs. One‐third of patients with nonischemic cardiomyopathy were found to have PVCs originating from the RVOT.
Conclusions
In patients with mild symptoms of heart failure, there is no difference in the risk of HF or death in patients with outflow versus non–outflow tract PVCs. One‐third of patients with NICM have frequent PVCs originating from the RVOT.
Keywords: noninvasive techniques, electrocardiography, clinical, implantable devices, biventricular pacing/defibrillation
INTRODUCTION
Frequent premature ventricular contractions (PVCs) have been shown to significantly decrease the effectiveness of cardiac resynchronization therapy (CRT) increasing the risk of heart failure or death.1, 2 In order to improve the response to biventricular pacing, antiarrhythmic drug therapies and/or catheter ablation of PVCs are considered for patients who have a high PVC burden. In patients deemed to be nonresponders to CRT, successful PVC ablation has been shown to improve left ventricular ejection fraction and New York Heart Association (NYHA) functional class.3, 4 However, the burden and morphology of PVCs in patients undergoing CRT has not been well characterized. We sought to evaluate the burden and electrocardiographic morphologies of PVCs in patients enrolled in the CRT‐D arm of Multicenter Automatic Defibrillator Implantation Trial‐Cardiac Resynchronization Therapy (MADIT‐CRT).5 We also aimed to determine the impact of outflow tract versus non–outflow tract PVCs on cardiac remodeling in patients undergoing CRT.
METHODS
Trial Design
The design and results from MADIT‐CRT have previously been published.5 Briefly, MADIT‐CRT sought to evaluate whether CRT‐D therapy would reduce the risk of death or heart failure in patients with NYHA class I or II heart failure symptoms, a left ventricular ejection fraction <30%, and a QRS duration >130 milliseconds when compared to ICD therapy alone. Between December 2004 to April 2008, 1820 patients were enrolled from 110 hospitals and were randomized in a 3:2 ratio to receive CRT‐D or ICD therapy. The MADIT‐CRT protocol was approved by the institutional research review board at each of the enrolling centers. Patients of either gender who were at least 21 years of age were enrolled if they had ischemic cardiomyopathy (NYHA functional class I or II) or nonischemic cardiomyopathy (NYHA functional class II only), normal sinus rhythm, a left ventricular ejection fraction of 30% or less, and a QRS duration of 130 milliseconds or greater.
Device Programming and Interrogation
Commercially available transvenous CRT‐D devices (Boston Scientific, Natick, MA, USA) were used in the trial and implanted according to standard techniques. Device testing and programming were performed as reported previously.6 Devices were programmed to monitor therapy, with a protocol recommendation to a setting of the ventricular tachycardia (VT) zone at 180 beats/min and the ventricular fibrillation (VF) zone at 210 beats/min. Sensitivity was programmed according to physician discretion. Detection times were 2.5 second for the VT zone and 1.0 second for the VF zone. The protocol recommended programming the VT zone first therapy to burst‐type antitachycardia pacing with 8 pulses at 88% of the measured cycle length with a 10‐millisecond decrement between bursts, then shock therapy. The remaining therapies were to be maximal energy shocks. All shocks were biphasic.
Devices were interrogated quarterly, after which ICD data and disks were sent to the core laboratory for categorization and final adjudication of detected arrhythmias. An arrhythmia episode was defined when any type of therapy was rendered including antitachycardia pacing and shock. VT was defined as the ventricular rate up to 250 beats/min; VF was defined as ventricular rate faster than 250 beats/min with disorganized ventricular electrograms. Only appropriate therapy delivered for VT or VF was considered in this study.
Study End Points
The primary end point in MADIT‐CRT was heart failure event or death. A heart failure end point required signs and symptoms consistent with congestive heart failure requiring outpatient intravenous decongestive therapy or an augmented decongestive inpatient therapy. The great majority of heart failure events (87%) required inpatient admission and heart failure treatment. Secondary end points included in this analysis were the combined end point of VT or VF that required ICD therapy, and the combined end point of VT/VF or death. All end points were adjudicated by their respective end point adjudication committee that was unaware of study group assignments.
Holter Monitoring and PVC Characterization
All patients randomized to the CRT‐D arm of the trial underwent 12‐lead, 24‐hour Holter recording at time of enrollment. Holter ECGs were obtained using Mortara H12+ recorders (Milwaukee, WI, USA) and were analyzed centrally in a core ECG laboratory using the Mortara H‐Scribe scanning system (Milwaukee, WI, USA). Patients with greater than 5000 PVCs during the 24‐hour Holter recording were identified and the dominant PVC morphology was further characterized. These patients underwent detailed review of their dominant PVC morphology, as assessed by the 12‐lead Holter. We characterized the PVC morphology (right vs left bundle) and axis (right vs left; superior vs inferior). Using this information, we categorized PVCs based on their putative site of origin (right ventricular outflow tract [RVOT], left ventricular outflow tract [LVOT], sinus of Valsalva [SOV], or non–outflow tract; Figs. 1A, B).7, 8 We subsequently grouped PVCs originating from the RVOT, LVOT, and SOV as outflow tract PVCs and compared them to non–outflow tract PVCs. We also determined the presence or absence of VT that matched the dominant PVC morphology.
Figure 1.
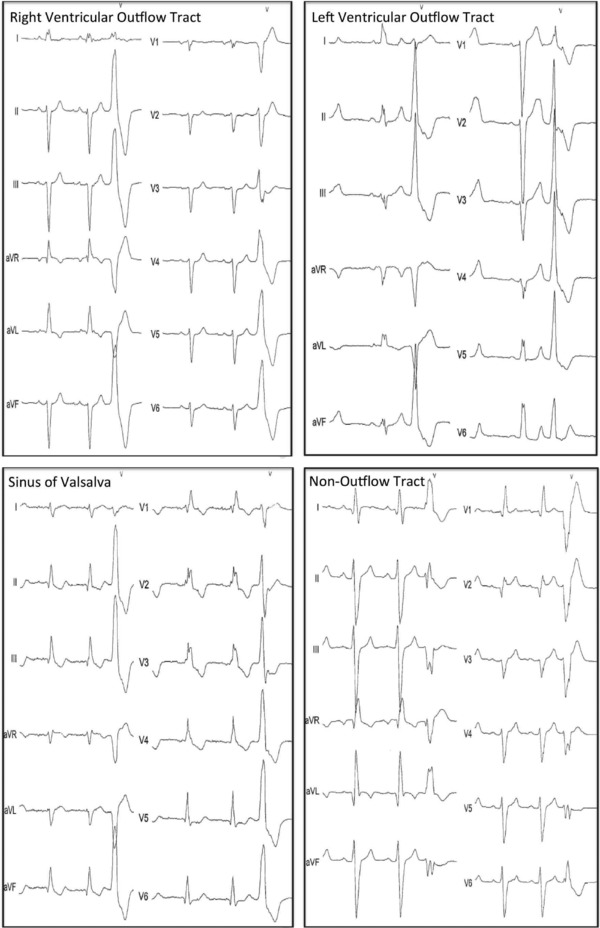
(A; Left) Right ventricular outflow tract premature ventricular beat. (Right) Left ventricular outflow tract beat. (B; Left) Sinus of Valsalva premature ventricular beat. (Right) Non–outflow tract premature ventricular beat.
Echocardiography Protocol
Echocardiograms were obtained according to a study‐specific protocol, and were performed before device implantation and at 1‐year after CRT‐D implant. Echocardiographic parameters were measured in a core echocardiography laboratory according to established American Society of Echocardiography protocols. Left ventricular volumes were measured using Simpson's method of disks in the apical four‐ and two‐chamber views and averaged.
Statistical Analysis
Baseline clinical characteristics among patients with different site of PVC origin were compared with the Wilcoxon rank‐sum test for continuous variables and the chi‐square test for categorical variables. Kaplan‐Meier estimates of survival were used to evaluate the association between outflow and non–outflow tract PVCs and outcomes. All P‐values are 2‐tailed. All analyses were on an intention‐to‐treat basis. Analyses were performed with version 4.0 of the MADIT‐CRT database using SAS software (version 9.2; SAS Institute, Cary, NC, USA)
RESULTS
Of the 947 patients enrolled in the CRT‐D arm of the trial, 146 patients (15%) were identified to have >5000 PVCs during a 24‐hour period. Of the 146 patients, 75 (51%) had PVCs originating from a non–outflow tract site. Baseline patient characteristics according to those with outflow tract versus non–outflow tract PVCs are shown in Table 1 Patients in the two groups were similar with respect to baseline echocardiographic parameters, comorbid conditions, and baseline electrocardiographic findings. The only difference observed between the two groups was in the number of patients with ischemic cardiomyopathy and NYHA class II symptoms and in the number of patients with nonischemic cardiomyopathy and NYHA class II symptoms.
Table 1.
Baseline Characteristics of Patients with Outflow versus Non–Outflow Tract PVCs
| Parameters | Outflow | Non–outflow | P Value |
|---|---|---|---|
| (n = 71) | (n = 75) | ||
| Age, years | 66.9±10 | 66.7 ± 9.6 | 0.86 |
| Gender, female | 11 (15) | 8 (11) | 0.39 |
| Cardiomyopathy Type | |||
| Ischemic, NYHA class I | 10 (14) | 12 (16) | 0.75 |
| Ischemic, NYHA class II | 25 (35) | 41 (55) | 0.02 |
| Nonischemic, NYHA class II | 36 (51) | 22 (29) | 0.01 |
| Electrocardiogram | |||
| QRS duration | 154.3 ± 18.6 | 150.4 ± 17.8 | 0.20 |
| Left bundle branch block | 47 (66) | 20 (27) | <0.001 |
| Comorbidities | |||
| Hypertension | 50 (70) | 43(57) | 0.10 |
| Diabetes | 25 (35) | 29(39) | 0.67 |
| Prior myocardial infarction | 27 (39) | 39(54) | 0.06 |
| Medications | |||
| ACE‐inhibitor or ARB | 64 (90) | 70(93) | 0.48 |
| Beta‐blockers | 63 (89) | 65(87) | 0.70 |
| Calcium blockers | 6(8) | 7(9) | 0.85 |
| Digoxin | 19 (27) | 15(20) | 0.33 |
| Statins | 53 (75) | 56(75) | 0.99 |
| Echocardiography | |||
| Left atrial volume | 95.1 ± 24.2 | 96.9 ± 21.3 | 0.38 |
| Ejection fraction,% | 24.1 ± 5.7 | 23.9 ± 5.1 | 0.67 |
| LVEDV indexed | 119.4 ± 26.2 | 123.9 ± 29.0 | 0.36 |
| LVESV indexed | 84.8 ± 21.1 | 88.6 ± 22.9 | 0.26 |
ACE = angiotensin‐converting enzyme; ARBs = angiotensin receptor blockers; LVEDV = left ventricular end diastolic volume; LVESV = left ventricular end systolic volume; LVEDV = left ventricular end diastolic volume; LVESV = left ventricular end systolic volume; NYHA = New York Heart Association.
Primary and Secondary End Points in Univariate Analyses
The cumulative probabilities for the primary end point of heart failure or death, and secondary end points of VT/VF and the combined end point of VT/VF or death by outflow tract versus non–outflow tract PVC site origin are presented in Figure 2 There were no significant differences between the two groups for the end points of HF/Death, VT/VF, and VT/VF or death.
Figure 2.
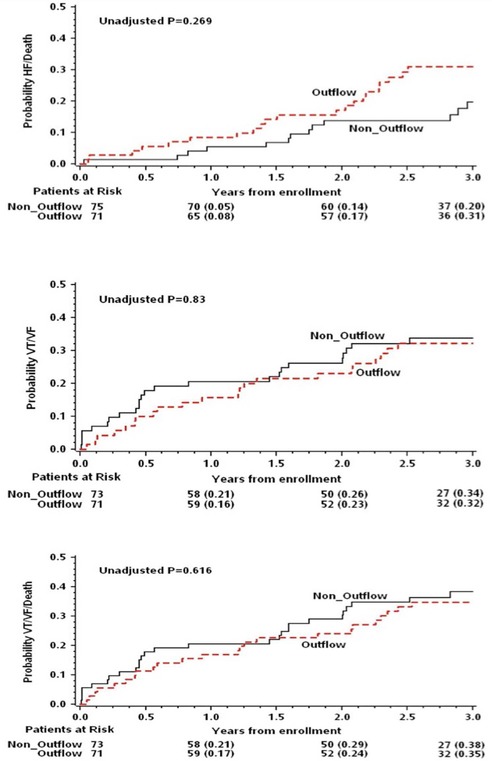
Kaplan‐Meier estimates of clinical end points by premature ventricular contraction from the outflow tracts versus non–outflow tract sites.
Primary and Secondary End Points in Multivariate Analyses
In multivariate analysis, the risk for HF/Death was similar in patients with outflow tract PVCs compared to those with non–outflow tract PVCs. There were no differences in risk for the end points of VT/VF and VT/VF or death between the two groups (Table 2).
Table 2.
Multivariate Analysis: Risk of Heart Failure or Death, VT/VF, and VT/VF or Death in Patients with Outflow Tract versus Non–Outflow Tract PVCs
| End Point | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Heart failure or death (39 events) | 1.4 | 0.7–2.8 | 0.3 |
| VT/VF (48 events) | 1.0 | 0.6–1.8 | 1.0 |
| VT/VF or death (53 events) | 0.9 | 0.5–1.6 | 0.7 |
Adjusted for ischemic etiology of cardiomyopathy, left ventricular ejection fraction and presence of left bundle branch block.
VT = ventricular tachycardia; VF = ventricular fibrillation.
PVCs in Ischemic versus Nonischemic Cardiomyopathy
Among patients with >5000 PVCs, 88 (60%) had ICM and 58 (40%) had NICM. The median total PVC count in patients with ICM was 8807 as compared to 8950 in patients with NICM. Characteristics of PVCs among patients with ICM compared to NICM are shown in Table 2 The dominant PVC accounted for 66% of all observed PVCs in patients with ICM and for 70% of the total PVC burden in patients with NICM, P = NS. Patients with NICM were more likely to experience PVCs originating from the RVOT, whereas non–outflow tract PVCs were more commonly seen in patients with ICM (Table 3).
Table 3.
Characteristic of Premature Ventricular Contractions (PVC) in Patients with Ischemic versus Nonischemic Cardiomyopathy
| PVC Characteristics | Ischemic | Nonischemic | P Value |
|---|---|---|---|
| (n = 88) | (n = 58) | ||
| Dominant PVC burden | 5707 | 6253 | NS |
| Total PVC burden | 8807 | 8950 | NS |
| Morphology | |||
| Left bundle branch block | 34(39) | 33(57) | 0.03 |
| Right bundle branch block | 53(60) | 25(43) | 0.04 |
| Axis | |||
| Inferior | 48(55) | 42(72) | 0.03 |
| Superior | 40(45) | 16(28) | 0.03 |
| Left | 49(56) | 22(38) | 0.04 |
| Right | 39(44) | 36(62) | 0.04 |
| Site of origin | |||
| Right ventricular outflow tract | 11(13) | 19(33) | 0.003 |
| Left ventricular outflow tract | 15(17) | 9(16) | NS |
| Sinus of Valsalva | 9(10) | 8(14) | NS |
| Non–outflow tract | 53(60) | 22(38) | 0.008 |
| Ventricular tachycardia (VT) | |||
| Non‐sustained VT | 68(77) | 48(83) | NS |
| PVC match to nonsustained VT | 26(30) | 18(31) | NS |
| Multiple nonsustained VT episodes | 40(45) | 29(50) | NS |
Echocardiographic Response to CRT According to PVC Site of Origin
At baseline, left ventricular ejection fraction, left atrial volume, left ventricular end‐diastolic volume (LVEDV) indexed to body surface area (BSA), left ventricular end‐systolic volume (LVESV) indexed to BSA were similar between patients with outflow tract and non–outflow tract PVCs (Table 1). At 1‐year follow‐up, the degree of reverse remodeling measured by echocardiography was similar between the two groups (Fig. 3).
Figure 3.
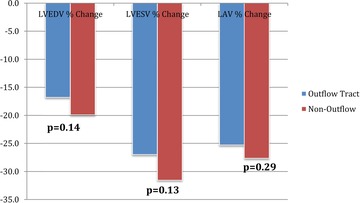
Echocardiographic reverse remodeling in patients with outflow versus non–outflow tract PVCs.
DISCUSSION
The primary finding of this MADIT‐CRT substudy is that of the enrolled patients in the trial 15% had a PVC burden exceeding 5000 PVCs on 24‐hour Holter monitoring. The predominant PVC morphologies could be electrocardiographically characterized to originate from one of the four putative sites, that being to the RVOT, LVOT, SOV, or a non–outflow tract site. The primary end point of heart failure or death and secondary end points were similar when comparing patients with outflow tract PVCs to those with non–outflow tract PVCs.
Frequent PVCs result in dyssynchronous ventricular activation and contraction and a high PVC burden can lead to PVC‐induced cardiomyopathy.9, 10 In such patients, successful PVC ablation has been shown to improve LV function and in some cases complete recovery of LV function has been reported.11, 12, 13 Our study provides important information about the burden and electrocardiographic patterns of PVCs in patients with mild heart failure symptoms undergoing CRT‐D therapy. Although the clinical outcomes were similar between PVCs originating from the outflow versus non–outflow tract, significant differences in PVC morphologies were seen in patients with ischemic and nonischemic cardiomyopathy.
Patients with ICM more commonly experienced non–outflow tract PVCs consistent with the postinfarction substrate that would be expected in these patients. However, as reported in other studies, patients with NICM were more likely to have PVCs originating from the RVOT.11, 14 In our study, one‐third of patients with NICM were found to have frequent PVCs originating from the RVOT. Frequent PVCs originating from the RVOT have been recognized as a possible cause of unexplained cardiomyopathy wherein its successful treatment with catheter ablation can result in recovery of LV function.13 Interestingly, nearly one‐third of patients in both groups had NSVT, with a morphology matching the predominant PVC and this may have implications when trying to decide on whether ablation of these PVC sites may improve a patient's VT burden.
Although CRT algorithms have been developed to deliver fusion pacing on PVCs in an effort to maximize the amount of biventricular pacing,15 frequent PVCs often result in symptoms requiring intervention. Medical therapy including antiarrhythmic drug therapy is often prescribed to patients in an attempt to suppress PVCs, particularly in symptomatic patients. However, the use of antiarrhythmic drugs themselves poses the risk of proarrhythmia and recently catheter ablation has been shown to be more effective in reducing the burden of PVCs when compared to antiarrhythmic drug therapy.16 Additional research is required to better determine those patients with LV dysfunction in whom elimination of PVCs with antiarrhythmic drug therapy and/or catheter ablation should be considered to improve cardiac function.
Our study population comprises patients from a larger study population of MADIT‐CRT patients and therefore it is subject to all the limitations inherent in performing substudy analyses. Patients were categorized and analyzed according to the dominant PVC morphology at baseline, however, this may not be representative of the patients’ chronic PVC burden and overtime the dominant PVC morphology may have changed. Patients in this study did not undergo electrophysiology study and mapping of their PVCs therefore the precise sites of PVC origin for these patients are unknown. Finally, the small sample size of patients in each of the PVC groups may have been inadequate to detect any meaningful differences in the end points.
CONCLUSION
In patients with mild symptoms of heart failure and frequent ventricular ectopy, the predominant PVC morphology can be characterized electrocardiographically to a putative site of origin, specifically the LVOT, RVOT, SOV, or non–outflow tract sites. There is no difference in the risk of HF or death in patients with outflow versus non–outflow tract PVCs. Finally, one‐third of patients with NICM have frequent PVCs originating from the RVOT.
MADIT‐CRT was supported by unrestricted research grant from Boston Scientific to the University of Rochester Medical Center
REFERENCES
- 1. Mittal S, Aktas MK, Moss AJ, et al. The impact of nonsustained ventricular tachycardia on reverse remodeling, heart failure, and treated ventricular tachyarrhythmias in MADIT‐CRT. J Cardiovasc Electr 2014;25:1082–1087. [DOI] [PubMed] [Google Scholar]
- 2. Ruwald MH, Mittal S, Ruwald AC, et al. Association between frequency of atrial and ventricular ectopic beats and biventricular pacing percentage and outcomes in patients with cardiac resynchronization therapy. J Am Coll Cardiol 2014;64:971–981. [DOI] [PubMed] [Google Scholar]
- 3. Herczku C, Kun C, Edes I, et al. Radiofrequency catheter ablation of premature ventricular complexes improved left ventricular function in a non‐responder to cardiac resynchronization therapy. Europace 2007;9:285–288. [DOI] [PubMed] [Google Scholar]
- 4. Lakkireddy D, Di Biase L, Ryschon K, et al. Radiofrequency ablation of premature ventricular ectopy improves the efficacy of cardiac resynchronization therapy in nonresponders. J Am Coll Cardiol 2012;60:1531–1539. [DOI] [PubMed] [Google Scholar]
- 5. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. New Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 6. Moss AJ, Brown MW, Cannom DS, et al. Multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy (MADIT‐CRT): Design and clinical protocol. Ann Noninvasive Electrocardiol 2005;10:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bala R, Marchlinski FE. Electrocardiographic recognition and ablation of outflow tract ventricular tachycardia. Heart Rhythm 2007;4:366–370. [DOI] [PubMed] [Google Scholar]
- 8. Yamada T, Yoshida N, Murakami Y, et al. Electrocardiographic characteristics of ventricular arrhythmias originating from the junction of the left and right coronary sinuses of valsalva in the aorta: The activation pattern as a rationale for the electrocardiographic characteristics. Heart Rhythm 2008;5:184–192. [DOI] [PubMed] [Google Scholar]
- 9. Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865–869. [DOI] [PubMed] [Google Scholar]
- 10. Lee GK, Klarich KW, Grogan M, et al. Premature ventricular contraction‐induced cardiomyopathy: A treatable condition. Circulation 2012;5:229–236. [DOI] [PubMed] [Google Scholar]
- 11. Bogun F, Crawford T, Reich S, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: Comparison with a control group without intervention. Heart Rhythm 2007;4:863–867. [DOI] [PubMed] [Google Scholar]
- 12. Mountantonakis SE, Frankel DS, Gerstenfeld EP, et al. Reversal of outflow tract ventricular premature depolarization‐induced cardiomyopathy with ablation: Effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm 2011;8:1608–1614. [DOI] [PubMed] [Google Scholar]
- 13. Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005;112:1092–1097. [DOI] [PubMed] [Google Scholar]
- 14. Kanei Y, Friedman M, Ogawa N, et al. Frequent premature ventricular complexes originating from the right ventricular outflow tract are associated with left ventricular dysfunction. Ann Noninvasive Electrocardiol 2008;13:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aktas MK, Jeevanantham V, Sherazi S, et al. Effect of biventricular pacing during a ventricular sensed event. Am J Cardiol 2009;103:1741–1745. [DOI] [PubMed] [Google Scholar]
- 16. Ling Z, Liu Z, Su L, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: Prospective randomized study. Circulation 2014;7:237–243. [DOI] [PubMed] [Google Scholar]


