Abstract
Background
Cardiac resynchronization therapy (CRT) has been recommended for patients with symptomatic heart failure and a wide QRS. Fragmented QRS (fQRS) on a 12‐lead electrocardiography (ECG) has been shown to predict cardiac events. We aimed to investigate the relationship between resolution of fQRS and response to CRT.
Methods
Sixty‐seven consecutive patients (38 men, mean age 65 ± 11) with left bundle branch block and fQRS on ECG undergoing CRT were studied. The presence of fQRS was assessed using standardized criteria. Echocardiographic response to CRT was defined by a ≥15% reduction in left ventricular end‐systolic volume (LVESV) and resolution of fQRS was defined as decrease in number of leads with fQRS on ECG at 6 months follow‐up.
Results
Thirty‐nine patients (58%) had response to CRT. LVESV significantly decreased from 150 ± 64 to 100 ± 48 in responders (P = 0.001). There was not any significant decrease in nonresponders (LVESV; from 157 ± 70 to 153 ± 66, P = 0.45). The number of leads with fQRS was decreased from 4.4 ± 1.8 to 1.7 ± 1.6 in responder patients (P < 0.001). The number of leads with fQRS was not significantly changed in nonresponders. (4.2 ± 2.2 vs. 5.1 ± 2.4, P = 0.06). In multivariate analysis, significant associates of response to CRT was evaluated adjusting for etiology of cardiomyopathy, baseline QRS width, left ventricular ejection fraction, number of leads with fQRS and resolution of fQRS. Resolution of fQRS was the only predictor of response to CRT (OR 0.018, 95% CI, 0.004–0.083, P < 0.001).
Conclusions
After adjusting for potential confounders, resolution of fQRS, is associated with response to CRT.
Keywords: cardiac resynchronization therapy, fragmented QRS, resolution
Cardiac resynchronization therapy (CRT) is an effective treatment option for patients with severe symptomatic chronic heart failure (HF) and wide QRS complex on electrocardiography (ECG). Several studies demonstrated that CRT has been proven to reduce symptoms and HF hospitalizations, to improve exercise capacity, quality of life, and mortality.1, 2 Recently, fragmented QRS (fQRS), defined by unexpected deviations in the QRS morphology on a 12‐lead ECG has been shown to predict a various cardiac events in patients with LV dysfunction.3, 4, 5, 6 Furthermore, we have demonstrated the association between the number of leads with fQRS and response to CRT.7 We aimed to investigate the relationship between the resolution of fQRS following CRT and response to CRT.
METHODS
Patients
The study was a retrospective review of a prospectively collected group of consecutive patients with left bundle branch block (LBBB) and fQRS on baseline ECG who undergone CRT between 2008 and 2011 in our clinic. Patients with severe HF (New York Heart Association [NYHA] class III or IV) despite optimal medical therapy, left ventricular ejection fraction ≤35%, QRS duration ≥120 milliseconds, LBBB on ECG, and sinus rhythm were included. Patients with right bundle branch block and atrial fibrillation were excluded from the study. The etiology of HF was considered ischemic in the presence of significant coronary artery disease (>50% stenosis in ≥1 of the major coronary arteries) and/or a history of myocardial infarction or previous revascularization. Optimal pharmacological treatment before and after pacemaker implantation was given to all patients. At baseline and after 6 months, a clinical evaluation was performed. NYHA score was used to classify HF symptoms.
The study was approved by the Local Ethical Committee, and written informed consent was obtained from all patients.
Electrocardiography
fQRS with LBBB was defined as various RSR' patterns with or without a Q wave, with >2 R waves (R') or >2 notches in the R wave, or >2 notches in the downstroke or upstroke of the S wave, in two contiguous leads corresponding to a major coronary artery territory.8 Two independent clinicians blinded to study design, echocardiographic findings and follow‐up data interpreted the resting and 6th month 12‐lead ECGs (0.5–150 Hz, 25 mm/s, 10 mm/mV). There was a 98% of concordance for fQRS and resolution of fQRS. In case of disagreement, the final diagnosis provided with the mutual consent. The resolution of fQRS was defined as decrease in number of leads with fQRS on ECG at 6 months follow‐up (Fig. 1).
Figure 1.
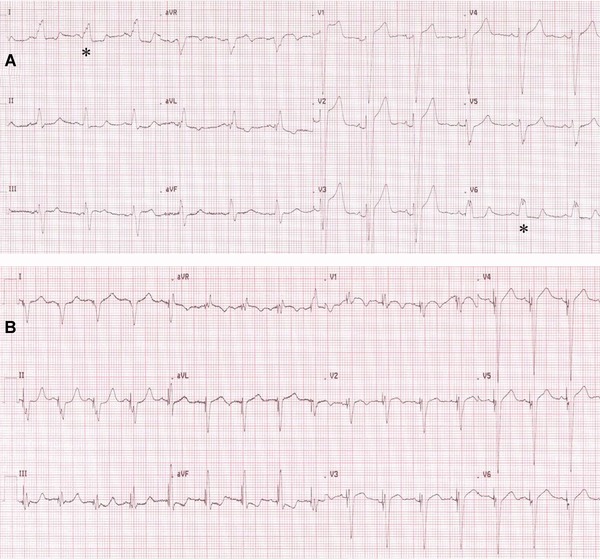
(A) Example of fragmented QRS before CRT. Asterisks denote fragmented QRS.
(B) Resolution of fragmented QRS after CRT.
Cardiac Resynchronization Therapy Device Implantation
Infraclavicular approach was held for all pacemaker implantations. Right atrial and right ventricular leads were implanted transvenously. Left ventricular leads were positioned transvenously through the coronary sinus in a lateral or posterolateral vein. The atrioventricular delay was optimized using Doppler echocardiographic measurements of transmitral flow 1 week after implantation.
Echocardiography
Transthoracic echocardiography was performed for each patient before and 6 months after implantation. Patients were imaged in the left lateral decubitus position with a commercially available system (VIVID 7, General Electric‐Vingmed Ultrasound, Horten, Norway). Images were obtained with a 2.5‐MHz broadband transducer at a depth of 16 cm in the parasternal and apical views (standard long‐axis, 2‐ and 4‐chamber images). Standard two‐dimensional and color Doppler data triggered to the QRS complex were saved in cine‐loop format. LV volumes were calculated using Teicholz method. LVEF was calculated from the conventional apical 2‐ and 4‐chamber images using the biplane Simpson's technique.9
All echocardiographic measurements after CRT implantation were made with the device in active pacing mode. Echocardiographic response to CRT was defined by a ≥15% reduction in left ventricular end‐systolic volume at 6 months follow‐up.10
Statistical Analysis
All statistical analyses were performed with the statistical software program SPSS V.21.0 (IBM Corporation, Armonk, NY, USA). Continuous data were expressed as mean (SD). Categorical variables were compared by the chi‐square or Fisher's exact test. The differences in baseline clinical, electrocardiographic, and echocardiographic findings between responder and nonresponder patients were compared by Mann‐Whitney U test. Paired sample t‐test or Wilcoxon signed‐rank test was used to compare the clinical, electrocardiographic, and echocardiographic variables before and after CRT. Variables associated with CRT response in univariate analysis were entered into a forward stepwise logistic regression model. A value of P < 0.05 was considered statistically significant.
RESULTS
A total of 67 patients (38 men; mean age 65 ± 11 years) were included in the study. Baseline data of the study population are shown in Table 1. A biventricular implantable cardioverter defibrillator (InSync ICD, Medtronic Inc, Minneapolis, MN, USA) was implanted in 46 patients and a biventricular pacemaker (InSync III, Medtronic Inc.) in 21 patients. The baseline clinical, electrocardiographic, and echocardiographic characteristics of patients with CRT responders and nonresponders showed no statistically significant difference (Table 2). All patients had fQRS. Response to CRT developed in 39 patients (58%). Twenty‐three patients in the responder patient group had fQRS at 6 months follow‐up. Twenty‐eight patients do not respond to CRT. Twenty‐six patients in the nonresponder patient group had fQRS at 6 months follow‐up. fQRS was present in the limb, precordial leads, or in both of the leads. fQRS was present in the limb, precordial and both limb and precordial leads were 49%, 18%, and 33% in responder patient group, respectively. In nonresponder patient group, fQRS was present in 32%, 29%, and 39% in the limb, precordial and both limb and precordial leads, respectively. Left ventricular end‐systolic volume significantly decreased from 150 ± 64 to 100 ± 48 in CRT responders (P = 0.001). There was not any significant decrease in CRT nonresponders (LVESV; from 157 ± 70 to 153 ± 66, P = 0.45). The number of leads with fQRS was decreased from 4.4 ± 1.8 to 1.7 ± 1.6 in responder patients (P < 0.001). The number of leads with fQRS was increased in nonresponder patients, however not significantly changed (4.2 ± 2.2 vs. 5.1 ± 2.4, P = 0.06). Comparison of baseline and 6 months of clinical, electrocardiographic, and echocardiographic measurements in CRT responder and nonresponder patients are outlined in Table 3.
Table 1.
Patient Characteristics (n = 67)
| Age (years) | 65 ± 11 |
| Men (n/%) | 38/57% |
| Etiology | |
| Nonischemic (n/%) | 45/67% |
| Ischemic (n/%) | 22/33% |
| Hypertension (n/%) | 43/64% |
| Diabetes (n/%) | 23/34% |
| NYHA (mean) | 3.1 ± 0.4 |
| Number of leads with fQRS | 4.3 ± 1.9 |
| Number of patients with fQRS in anterior leads | 32 |
| Number of patients with fQRS in inferior leads | 36 |
| Number of patients with fQRS in lateral leads | 30 |
| QRS (mm) | 137 ± 15 |
| LV EF (%) | 22 ± 6 |
| Use of ACE‐inhibitors or ARB (n/%) | 59/92% |
| Use of beta‐blocker | 54/88% |
| Use of diuretic | 61/81% |
ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; fQRS = fragmented QRS; LV EF = left ventricular ejection fraction; NYHA = New York Heart Association.
Table 2.
Baseline Clinical, Electrocardiographic, and Echocardiographic Characteristics of Responder and Nonresponder Patients
| Responder (n = 39) | Nonresponder | P | |
|---|---|---|---|
| Age (years) | 65 ± 10 | 66 ± 12 | 0.16 |
| NYHA | 3 ± 0.5 | 3.1 ± 0.3 | 0.89 |
| Ischemic CMP (n/%) | 10/26 | 12/43 | 0.09 |
| QRS (milliseconds) | 138 ± 17 | 131 ± 14 | 0.07 |
| Number of leads with fQRS | 4.4 ± 1.8 | 4.2 ± 2.2 | 0.56 |
| Number of patients with fQRS in anterior leads | 17 | 15 | 0.42 |
| Number of patients with fQRS in inferior leads | 21 | 15 | 0.98 |
| Number of patients with fQRS in lateral leads | 18 | 12 | 0.79 |
| LVEDD (mm) | 66 ± 9 | 67 ± 10 | 0.93 |
| LVESD (mm) | 56 ± 8 | 55 ± 13 | 0.51 |
| LAD (mm) | 43 ± 3 | 45 ± 7 | 0.19 |
| RVD (mm) | 24 ± 2 | 25 ± 2 | 0.07 |
| LVEF (%) | 23 ± 6 | 22 ± 7 | 0.64 |
| LVEDV (mm3) | 209 ± 76 | 225 ± 81 | 0.52 |
| LVESV (mm3) | 144 ± 53 | 147 ± 75 | 0.97 |
CMP = cardiomyopathy; fQRS = fragmented QRS; LAD = left atrial diameter; LVEDD = left ventricular end‐diastolic diameter; LVEDV = left ventricular end‐diastolic volume; LVEF = left ventricular ejection fraction; LVESD = left ventricular end‐systolic diameter; LVESV = left ventricular end‐systolic volume;NYHA = New York Heart Association; RVD = right ventricular diameter.
Table 3.
Comparison of Baseline and 6 Months of Clinical, Electrocardiographic, and Echocardiographic Measurements in Responder and Nonresponder Patients
| Nonresponder | Responder | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | P | Baseline | 6 months | P | |
| NYHA (mean) | 3 ± 0.2 | 3 ± 0.4 | 0.18 | 3 ± 0.5 | 2.3 ± 0.5 | 0.001 |
| QRS (milliseconds) | 131 ± 14 | 125 ± 22 | 0.06 | 139 ± 17 | 107 ± 21 | 0.001 |
| Number of leads with fQRS | 4.2 ± 2.2 | 5.1 ± 2.4 | 0.007 | 4.4 ± 1.8 | 1.7 ± 1.6 | 0.015 |
| Number of patients with fQRS in anterior leads | 15 | 16 | 0.57 | 17 | 9 | 0.01 |
| Number of patients with fQRS in inferior leads | 15 | 19 | 0.16 | 21 | 14 | 0.33 |
| Number of patients with fQRS in lateral leads | 12 | 15 | 0.27 | 18 | 11 | 0.006 |
| LVEDD (mm) | 67 ± 10 | 66 ± 10 | 0.09 | 66 ± 9 | 62 ± 9 | 0.001 |
| LVESD (mm) | 56 ± 12 | 55 ± 10 | 0.63 | 56 ± 8.4 | 50 ± 8.6 | 0.001 |
| LAD (mm) | 45 ± 8 | 45 ± 6 | 0.87 | 43 ± 3.4 | 41 ± 4.2 | 0.002 |
| RVD (mm) | 25 ± 2 | 27 ± 3 | 0.003 | 24 ± 2 | 22 ± 2 | 0.001 |
| LVEF (n/%) | 22 ± 6 | 23 ± 6 | 0.06 | 23 ± 6 | 36 ± 10 | 0.001 |
| LVEDV (mm3) | 225 ± 81 | 220 ± 78 | 0.45 | 209 ± 76 | 167 ± 59 | 0.001 |
| LVESV (mm3) | 157 ± 70 | 153 ± 66 | 0.46 | 150 ± 64 | 100 ± 48 | 0.001 |
fQRS = fragmented QRS; LAD = left atrial diameter; LVEDD = left ventricular end‐diastolic diameter; LVEDV = left ventricular end‐diastolic volume; LVEF = left ventricular ejection fraction; LVESD = left ventricular end‐systolic diameter; LVESV = left ventricular end‐systolic volume; NYHA = New York Heart Association; RVD = right ventricular diameter.
In multivariate analysis, significant associates of response to CRT was evaluated adjusting for etiology of cardiomyopathy, baseline QRS width, baseline left ventricular ejection fraction, number of leads with fQRS, and resolution of fQRS. Resolution of fQRS was the only predictor of response to CRT (OR 0.018, 95% CI, 0.004–0.083, P < 0.001).
DISCUSSION
CRT is a treatment option in selected HF patients. Acute hemodynamic and chronic beneficial effects of CRT has been shown previously.11, 12, 13 However, despite current selection criteria, approximately one‐third of patients with CHF and QRS duration >120 milliseconds do not respond to CRT, suggesting that QRS duration alone may not be a definite inclusion criteria for patient selection.14
ECG, which is an inexpensive, widely, and easily accessible examination remains an essential and required criteria in the inclusion of patients to CRT. Furthermore, ECG evaluation of CRT patients after implantation also gives strong clues of response.15
It is shown that response rate to CRT in HF patients with QRS duration >150 milliseconds and LBBB is more than patients with a QRS duration between 120 and 150 milliseconds and RBBB and nonLBBB.16, 17, 18, 19, 21 In our study, 58% of patients responded to CRT. Our response ratio is lower than the previous studies. The mean QRS duration of our patients was 137 ± 15 milliseconds, which could explain the lower response to CRT. Also, all of our patients had fQRS on baseline ECG, which has been shown to be associated with previous myocardial infarction, ventricular enlargement, and decreased LVEF.20, 21 The presence of LBBB and fQRS on ECG in all of our patient group gives us the opportunity to find other electrocardiographic parameters like number of leads with fQRS that may affect the response to CRT.
In our study, response to CRT after 6 months follow‐up was significantly more common in patients with resolution of fQRS (89% vs. 12%, P = 0.001). Myocardial scar that causes heterogeneous ventricular activation and dyssynchronous contraction results in fragmentation in QRS complex.20 CRT is associated with both mechanical and electrical reverse remodeling in responder patients.22 Reduction of number of leads with fQRS may be associated with more homogeneous ventricular activation and synchronous contraction. Thus, electrical reverse remodeling and reverse remodeling at tissue level can be reasons of this reduction.
We acknowledged that the present study has some limitations. First, the data were obtained from a single‐center in nonrandomized design. Second, because of the small number of the study sample, the presented data need to confirm in larger study population to acquire more significance. Third, we have not investigated the intraventricular dyssynchrony and presence of myocardial scar. Forth, we have investigated the relationship between resolution the fQRS and CRT response at 6 months of follow‐up not the acute effects of CRT on resolution of fQRS during implantation procedure, which could give us information about the optimal lead position. Further studies are needed to confirm our findings.
There is no financial support to declare.
REFERENCES
- 1. Bleeker GB, Schalij MJ, Nihoyannopoulos P, et al. Left ventricular dyssynchrony predicts right ventricular remodeling after cardiac resynchronization therapy. J Am Coll Cardiol 2005;46:2264–2269. [DOI] [PubMed] [Google Scholar]
- 2. Delnoy PP, Ottervanger JP, Luttikhuis HO, et al. Sustained benefit of cardiac resynchronization therapy. J Cardiovasc Electrophysiol 2007;18(3):298–302. [DOI] [PubMed] [Google Scholar]
- 3. Cheema A, Khalid A, Wimmer A, et al. Fragmented QRS and mortality risk in patients with left ventricular dysfunction. Circ Arrhythm Electrophysiol 2010;3:339–344. [DOI] [PubMed] [Google Scholar]
- 4. Korhonen P, Husa T, Konttila T, et al. Fragmented QRS in prediction of cardiac deaths and heart failure hospitalizations after myocardial infarction. Ann Noninvasive Electrocardiol 2010;15:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pei J, Li N, Gao Y, et al. The J wave and fragmented QRS complexes in inferior leads associated with sudden cardiac death in patients with chronic heart failure. Europace 2012;14(8):1180–1187. [DOI] [PubMed] [Google Scholar]
- 6. Chatterjee S, Changawala N. Fragmented QRS complex: A novel marker of cardiovascular disease. Clin Cardiol 2010;33:68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Celikyurt U, Agacdiken A, Sahin T, et al. Number of leads with fragmented QRS predicts response to cardiac resynchronization therapy. Clin Cardiol 2013;36(1):36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol 2008;1:258–268. [DOI] [PubMed] [Google Scholar]
- 9. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 10. Auger D, van Bommel RJ, Bertini M, et al. Prevalence and characteristics of patients with clinical improvement but not significant left ventricular reverse remodeling after cardiac resynchronization therapy. Am Heart J 2010;160:737–743. [DOI] [PubMed] [Google Scholar]
- 11. Breithardt OA, Stellbrink C, Franke A, et al. Acute effects of cardiac resynchronization therapy on left ventricular Doppler indices in patients with congestive heart failure. Am Heart J 2002;143:34–44. [DOI] [PubMed] [Google Scholar]
- 12. Bhatia V, Bhatia R, Dhindsa S, et al. Cardiac resynchronization therapy in heart failure: Recent advances and new insights. Indian Pacing Electrophysiol J 2003;3:129–142. [PMC free article] [PubMed] [Google Scholar]
- 13. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 14. Geng J, Wu B, Zheng L, et al. Heart failure patients selection for cardiac resynchronization therapy. Eur J Intern Med 2011;22:32–38. [DOI] [PubMed] [Google Scholar]
- 15. Rickard J, Popovic Z, Verhaert D, et al. The QRS narrowing index predicts reverse left ventricular remodeling following cardiac resynchronization therapy. Pacing Clin Electrophysiol 2011;34(5):604–611. [DOI] [PubMed] [Google Scholar]
- 16. Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol 2008;52:1834–1843. [DOI] [PubMed] [Google Scholar]
- 17. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 18. Abraham TP, Olsen NT. QRS width and mechanical dyssynchrony for selection of patients for cardiac resynchronization therapy: One can't do without the other. JACC Cardiovasc Imaging 2010;3:141–143. [DOI] [PubMed] [Google Scholar]
- 19. Hsing JM, Selzman KA, Leclercq C, et al. Paced left ventricular QRS width and ECG parameters predict outcomes after cardiac resynchronization therapy: PROSPECT‐ECG substudy. Circ Arrhythm Electrophysiol 2011;4(6):851–857. [DOI] [PubMed] [Google Scholar]
- 20. Basaran Y, Tigen K, Karaahmet T, et al. Fragmented QRS complexes are associated with cardiac fibrosis and significant intraventricular systolic dyssynchrony in nonischemic dilated cardiomyopathy patients with a narrow QRS interval. Echocardiography 2011;28:62–68. [DOI] [PubMed] [Google Scholar]
- 21. Sha J, Zhang S, Tang M, et al. Fragmented QRS is associated with all‐cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol 2011;16:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sebag FA, Martins RP, Defaye P, et al. Reverse electrical remodeling by cardiac resynchronization therapy: Prevalence and clinical impact. J Cardiovasc Electrophysiol 2012;23(11):1219–1227. [DOI] [PubMed] [Google Scholar]


