Abstract
BACKGROUND
Renal inflammation and immune cell infiltration are characteristic of several forms of hypertension. Our laboratory has previously demonstrated that renal-inflammation-associated lymphangiogenesis occurs in salt-sensitive and nitric-oxide-inhibition-induced hypertension. Moreover, enhancing renal lymphatic density prevented the development of these two forms of hypertension. Here, we investigated the effects of angiotensin II-induced hypertension on renal lymphatic vessel density in male and female mice.
METHODS
Wild-type and genetically engineered male and female mice were infused with angiotensin II for 2 or 3 weeks. Isolated splenocytes and peritoneal macrophages from mice, and commercially available mouse lymphatic endothelial cells were used for in vitro studies.
RESULTS
Compared to vehicle controls, angiotensin II-infused male and female mice had significantly increased renal lymphatic vessel density in association with pro-inflammatory immune cells in the kidneys of these mice. Direct treatment of lymphatic endothelial cells with angiotensin II had no effect as they lack angiotensin II receptors; however, angiotensin II treatment of splenocytes and peritoneal macrophages induced secretion of the lymphangiogenic growth factor VEGF-C in vitro. Utilizing our genetic mouse model of inducible renal lymphangiogenesis, we demonstrated that greatly augmenting renal lymphatic density prior to angiotensin II infusion prevented the development of hypertension in male and female mice and this was associated with a reduction in renal CD11c+F4/80- monocytes.
CONCLUSION
Renal lymphatics play a significant role in renal immune cell trafficking and blood pressure regulation, and represent a novel avenue of therapy for hypertension.
Keywords: angiotensin II, blood pressure, hypertension, immunity, kidney, lymphatics
With the revision of the Hypertension Clinical Guidelines in 2017, nearly half of the adult population in the United States is classified as being hypertensive, and hypertension is the leading modifiable risk factor for deaths from cardiovascular diseases.1 Overactivation of the renin–angiotensin–system (RAS) is a powerful mediator of high blood pressure (BP) and its accompanying risks; accordingly, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers remain popular for the treatment of hypertension.2 Sex differences in hypertension are well documented; 3–5 men have a higher prevalence of hypertension than women from ages 45 to 54 years, whereas from age 75, more women become hypertensive. Despite improved awareness and treatment options, roughly 30%–60% of hypertensive patients do not achieve BP targets and lowering BP by just 10 mm Hg significantly reduces risks and improves health outcomes.6
Several studies over the past years have established that renal infiltration of immune cells and inflammation is linked to hypertension in humans and experimental models; experimentally reducing inflammation reduces hypertension, thus confirming this link.7–9 Renal infiltration of activated macrophages, dendritic cells, and T and B lymphocytes promote sodium retention, mediate renal injury and fibrosis, and elevate BP.10–12 Angiotensin II (Ang II), among other factors, is a powerful activator of the immune system, promoting pro-inflammatory cytokine production by activated immune cells.13 In turn, these cytokines lead to activation of RAS components, thus setting up a vicious cycle. Sex differences have also been noted in renal immune cell infiltration during hypertension. Increased pressor responses to Ang II in males is associated with greater T-cell infiltration in the kidney and perivascular adipose tissue.14,15 The mechanisms contributing to this differential response have not been fully elucidated, and gaining a better understanding of BP control in both males and females remains important.
Lymphatic vessels transport extravasated fluid, cells, and proteins out of the tissue interstitium to the draining lymph node, eventually returning it to the circulation, thus maintaining immune surveillance and tissue fluid balance.16,17 Inflammation-associated lymphangiogenesis is observed in many renal inflammatory diseases including renal carcinoma, unilateral ureteral obstruction, and diabetic nephropathy.18 Previous studies from our laboratory have demonstrated that lymphangiogenesis in the kidney is associated with hypertension in spontaneously hypertensive rats, and salt-sensitive hypertension and nitric-oxide-inhibition-induced hypertension in mice.19,20 Endogenous renal lymphangiogenesis accompanied immune cell accumulation and is likely a result of the increased physiological need for immune cell clearance during inflammation. In fact, accumulating monocytes have been identified as the source of the lymphangiogenic factor VEGF-C in the kidney upon injury.21 In all of these models, the extent of lymphangiogenesis, however, is limited and potentially insufficient to restore tissue homeostasis. We hypothesized that augmenting renal lymphatic density prior to the onset of hypertensive stimuli will prevent the development of hypertension. Using a genetic mouse model of inducible renal lymphangiogenesis, we demonstrated that enhancing renal lymphatic density prevented the development of salt-sensitive and nitric-oxide-inhibition-induced hypertension, and this was associated with a reduction in renal immune cell accumulation in both of these models.20
In this study, we examined the effects of Ang II-induced hypertension (A2HTN) on renal lymphatic vessels. As mentioned earlier, because sex differences have been documented in the pressor response to Ang II infusion and in renal accumulation of immune cells, we independently studied the effects of A2HTN on renal lymphatic density in male and female mice. In addition, we examined the direct and indirect effects of Ang II on lymphatic endothelial cells (LECs). We also tested the effects of genetically augmenting renal lymphatic vessel density on A2HTN in male and female mice. Our hypotheses were that male and female mice with A2HTN will demonstrate an increase in renal lymphatic vessel density, and that Ang II will directly induce the activation and adhesion of LECs. We also hypothesized that the genetic augmentation of renal lymphatic density will prevent the development of A2HTN in both male and female mice.
METHODS
A detailed description of the methods used is provided in the Supplementary Data.
RESULTS
Renal lymphatic vessel density is increased in male mice with A2HTN
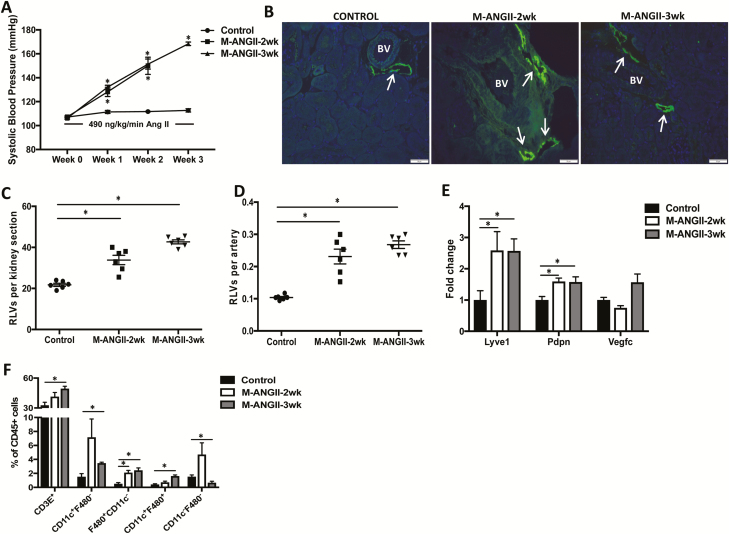
To examine changes in renal lymphatic density induced by A2HTN, male mice were infused with saline (control) or Ang II for 2 or 3 weeks. As expected, Ang II-infused mice were hypertensive at weeks 2 and 3 (Figure 1a; P < 0.05). Cross-sections of kidneys isolated from these mice were stained with antibodies against the lymphatic vessel markers LYVE-1 (Figure 1b) and podoplanin (not shown). Compared to controls, A2HTN male mice at 2 and 3 weeks had a visible increase in the number of lymphatic lumina (Figure 1b). This was then quantified by counting every LYVE-1+, lumen-containing vessel found around the cortical interlobular arteries in each kidney section. The mean number of lymphatic vessels per kidney section and per artery were significantly increased in A2HTN male mice at 2 and 3 weeks compared to controls (Figure 1c,d; P < 0.01). A corresponding increase in renal gene expression of Lyve1 and Pdpn (P < 0.05) further supported these data; however, there was no significant change in gene expression of the lymphangiogenic signal Vegfc (Figure 1e, P = 0.28). We hypothesized that this increase in renal lymphatic vessel density was an inflammation response and thereby associated with renal immune cell accumulation. To test this hypothesis, we performed flow cytometry analysis on the kidneys of these mice. For the gating strategy used, see Supplementary Figure 1a. A2HTN male mice had significant increases in F4/80+CD11c− monocytes at 2 weeks, and CD3E+ T cells, CD11c+F4/80−, F4/80+CD11c−, and CD11c+F4/80+ monocyte populations at 3 weeks, while CD11c−F4/80− cells were decreased at week 3 (Figure 1f; P < 0.05). Renal mRNA expression of the pro-inflammatory markers Tnfa, Il1b, and Mcp1 were also increased in A2HTN male mice at 2 and 3 weeks (Supplementary Figure 2a; P < 0.01). Together, these results suggest that A2HTN male mice demonstrate an increase in renal lymphatic vessel density in association with renal accumulation of pro-inflammatory immune cells and a corresponding increase in renal gene expression of pro-inflammatory cytokines.
Figure 1.
RLV density is increased in male mice with angiotensin II-induced hypertension: (a) systolic blood pressure measures in male mice infused with saline (Control) or Ang II for 2 (M-ANGII-2wk) or 3 (M-ANGII-3wk) weeks. (b) LYVE-1 (lymphatic vessel endothelial hyaluronan receptor 1) immunofluorescence on kidney sections from Control, M-ANGII-2wk, and M-ANGII-3wk mice. Scale bars = 50 μm. Renal interlobular lymphatic density as determined by mean number of LYVE-1+, lumen-containing lymphatic vessels (RLVs) (c) per kidney section and (d) per artery. (e) Gene expression changes in lymphatic vessel markers in kidneys from Control, M-ANGII-2wk, and M-ANGII-3wk mice. (f) Immune cell populations expressed as percentage of CD45+ cells in kidneys of Control, M-ANGII-2wk, and M-ANGII-3wk mice as determined by flow cytometry. Results are expressed as mean ± SEM (n = 6 per group), and statistical analyses were performed with Student’s t test. *P < 0.05 vs. Control mice. Abbreviations: Ang II, angiotensin II; BV, blood vessel; RLV, renal lymphatic vessel.
Renal lymphatic vessel density is increased in female mice with A2HTN
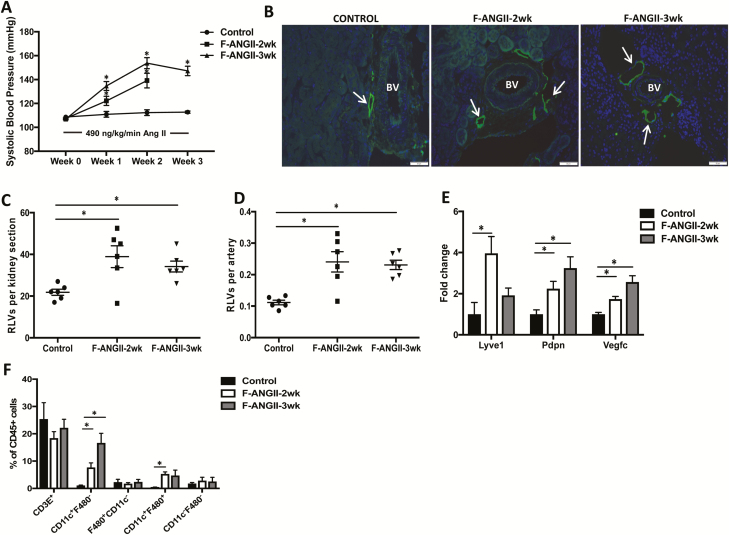
As noted earlier, A2HTN females exhibit differences in immune activation and inflammatory milieu. Hence, we determined whether A2HTN in female mice was associated with an increase in renal lymphatic vessel density. Female mice were similarly infused with saline or Ang II for 2 or 3 weeks, and as expected, these mice were hypertensive although to a lesser degree than males (Figure 2a; P < 0.05). Similar to males, females with A2HTN had an increase in renal lymphatic vessel staining as demonstrated by immunofluorescence for LYVE-1 (Figure 2b) and podoplanin (not shown). Quantification of these vessels demonstrated that the mean number of lymphatic vessels per kidney section and per artery were significantly higher in A2HTN females at 2 and 3 weeks compared to controls (Figure 2c,d; P < 0.05). In addition to the increased mRNA expression of Lyve1 and Pdpn, A2HTN females also upregulated their expression of Vegfc in the kidney (Figure 2e; P < 0.05). Upon analysis of renal immune cell populations, we noted that A2HTN females had significantly increased renal CD11c+F4/80− and CD11c+F4/80+ monocytes at 2 weeks, and that the CD11c+F4/80− monocytes remained elevated at 3 weeks (Figure 2f; P < 0.05). The renal immune cell accumulation was also associated with a significant increase in gene expression of Tnfa, Il1b, and Mcp1 with a trend toward increased Il6 mRNA levels in the kidneys (Supplementary Figure 2b; P < 0.05). These data collectively suggest that A2HTN female mice demonstrate an increase in renal lymphatic vessel density in association with renal accumulation of CD11c+ monocytes and pro-inflammatory cytokines.
Figure 2.
RLV density is increased in female mice with angiotensin II-induced hypertension: (a) systolic blood pressure measures in female mice infused with saline (Control) or angiotensin II for 2 (F-ANGII-2wk) or 3 (F-ANGII-3wk) weeks. (b) LYVE-1 (lymphatic vessel endothelial hyaluronan receptor 1) immunofluorescence on kidney sections from Control, F-ANGII-2wk, and F-ANGII-3wk mice. Scale bars = 50 μm. Renal interlobular lymphatic density as determined by mean number of LYVE-1+, lumen-containing lymphatic vessels (c) per kidney section and (d) per artery. (e) Gene expression changes in lymphatic vessel markers in kidneys from Control, F-ANGII-2wk, and F-ANGII-3wk mice. (f) Immune cell populations expressed as percentage of CD45+ cells in kidneys of Control, F-ANGII-2wk, and F-ANGII-3wk mice as determined by flow cytometry. Results are expressed as mean ± SEM (n = 6 per group), and statistical analyses were performed with Student’s t test. *P < 0.05 vs. Control mice. Abbreviations: BV, blood vessel; RLV, renal lymphatic vessel.
Ang II-activated splenocytes induce LEC activation and inflammation
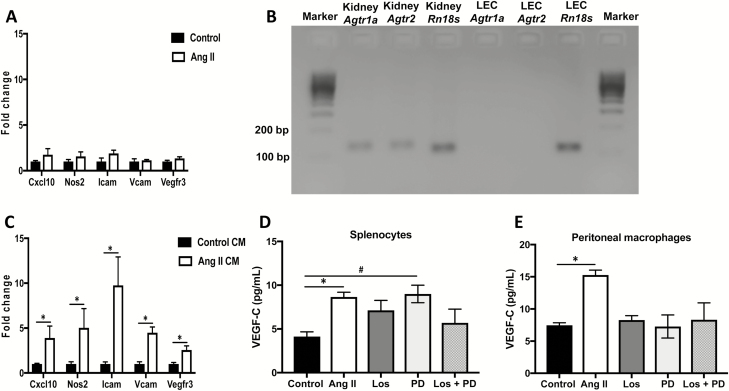
It has been reported that the concentration of Ang II in the renal interstitium is several fold higher than in the plasma.22 As this interstitial fluid comes in direct contact with LECs, we first tested whether Ang II could directly act on LECs to induce their activation. Primary mouse LECs treated with vehicle or Ang II for 24 hours demonstrated no difference in gene expression for various markers involved in LEC activation, adhesion, and proliferation (Figure 3a). Furthermore, no detectable transcript levels of the Ang II receptors Agtr1a or Agtr2 were found in these LECs (Figure 3b), suggesting that interstitial Ang II does not directly act on LECs.
Figure 3.
Angiotensin II-activated immune cells induce LEC activation and inflammation: (a) Gene expression changes in LECs treated with vehicle (saline) or Ang II for 24 hours. (b) Gel electrophoresis of PCR products from mouse kidney (positive control) and primary mouse LECs amplified for Agtr1a and Agtr2. Rn18s was used as housekeeping gene. (c) Gene expression changes in LECs treated for 24 hours with conditioned media from saline-treated or Ang II-treated splenocytes. VEGF-C levels in conditioned media derived from (d) splenocytes or (e) peritoneal CD11b+ cells treated with saline, Ang II, Ang II + Los, Ang II + PD, or Ang II + Los + PD for 48 hours as measured by ELISA. All experiments were performed in triplicate. Results are expressed as mean ± SEM, and statistical analyses were performed with Student’s t test. #0.05 < P < 0.1 vs. Control; *P < 0.05 vs. Control or Control CM. Abbreviations: Ang II, angiotensin II; CM, conditioned media; LEC, lymphatic endothelial cell; PCR, polymerase chain reaction.
As there were no direct effects of Ang II on LECs, we then determined the effects of Ang II-treated immune cells on LECs. Splenocytes were treated with vehicle control (saline) or Ang II for 48 hours and conditioned media (CM) were collected. In order to elicit an acute response to Ang II treatment in vitro, and as females are known to have a dampened immune response to Ang II, only splenocytes isolated from male mice were used. Compared to control CM-treated LECs, Ang II CM-treated LECs had increased gene expression of the inflammatory markers Cxcl10 and Nos2, the adhesion molecules Icam and Vcam, and the VEGF-C/VEGF-D receptor Vegfr3 (Figure 3c; P < 0.01). To identify whether Ang II induces immune cells to secrete VEGF-C, splenocytes and peritoneal CD11b+ cells were isolated and treated with Ang II. Treatment with Ang II induced elevated secretion of the VEGFR-3 ligand VEGF-C as measured by ELISA (Figure 3d,e; P < 0.05). Furthermore, inhibition of the Ang II receptors AT1aR and AT2R with losartan (Los) and PD123319 (PD), respectively, blocked the secretion of VEGF-C (Figure 3d,e). These results demonstrate that although interstitial Ang II does not directly act on LECs, it induces activation of LECs through its effects on immune cells and primarily CD11b+ cells.
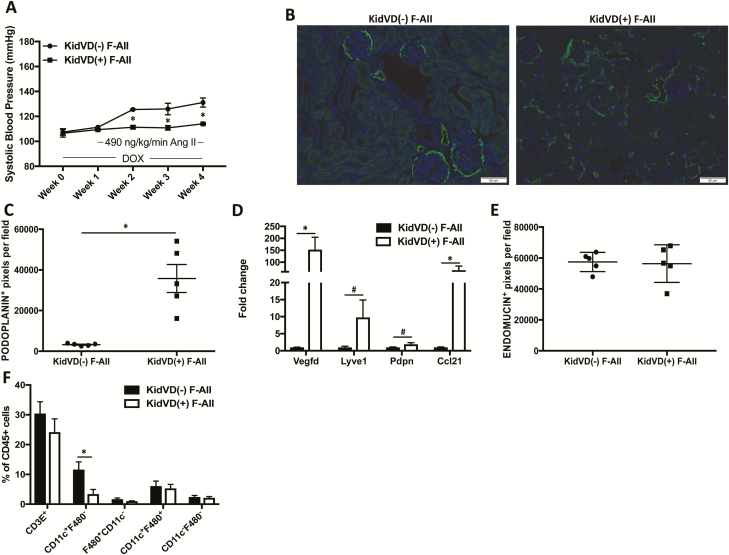
Genetic augmentation of renal lymphatic vessel density prevents the development of A2HTN in male mice
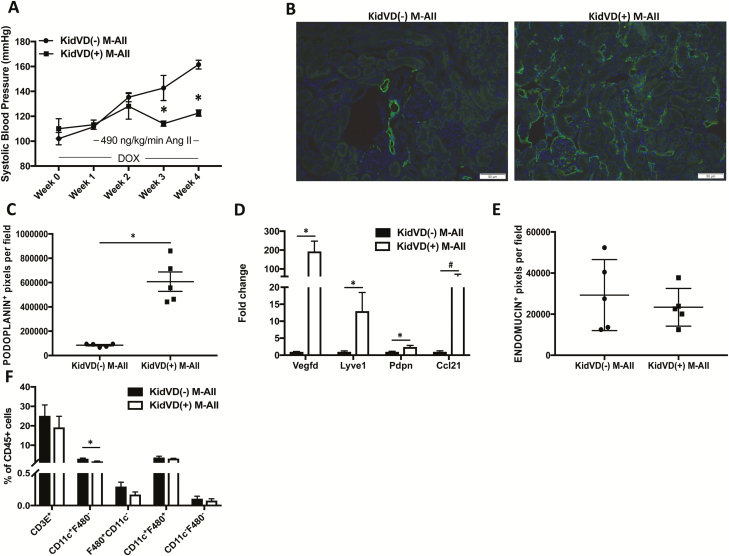
We used our genetic mice that undergo doxycycline (DOX)-inducible, kidney-specific VEGF-D overexpression (KidVD) that leads to massive lymphangiogenesis to determine the effects of enhanced renal lymphatic density on A2HTN.23 Administration of DOX a week before Ang II infusion induced the overexpression of the VEGFR-3-specific ligand VEGF-D and led to augmentation of renal lymphatic density in KidVD(+) male mice. KidVD(−) male mice infused with Ang II became hypertensive starting at week 1 and remained hypertensive throughout the 3-week Ang II infusion. However, the enhanced renal lymphatic density in KidVD(+) male prevented the development of A2HTN (Figure 4a). Renal immunofluorescence for podoplanin (Figure 4b), podoplanin+ pixel density (Figure 4c; P < 0.001), and gene expression of associated lymphatic vessel markers (Figure 4d; P < 0.05) supported the augmented renal lymphatic density. Gene expression of the LEC-derived immune cell trafficking chemokine Ccl21 also tended to be increased in KidVD(+) males (Figure 4d; P = 0.08). The observed BP response was not due to alterations in microvessel density, as identified by quantification of endomucin+ pixel density (Figure 4e). In association with the attenuation of BP, KidVD(+) male mice demonstrated a significant decrease in renal CD11c+F4/80− monocytes (Figure 4f; P < 0.05). Gene expression of Tnfa and Il1b that were previously elevated in the A2HTN males were normalized in Ang II-treated KidVD(+) males (Supplementary Figure 2c). In addition, although wild-type male mice given Ang II had significantly elevated levels of the injury markers Fn1 and Lcn2, these were normalized in KidVD(+) mice (Supplementary Figure 3). These data demonstrate that augmentation of renal lymphatic vessel density in male KidVD(+) mice prevented the development of A2HTN, and this was associated with a reduction in renal CD11c+F4/80− monocytes.
Figure 4.
Genetic augmentation of renal lymphatic vessel density prevents the development of angiotensin II-induced hypertension in male mice: (a) Systolic blood pressure measures in KidVD(−) and KidVD(+) male mice infused with Ang II for 3 weeks (KidVD(−) M-ANGII and KidVD(+) M-ANGII). (b) Podoplanin immunofluorescence on kidney sections from KidVD(−) M-ANGII and KidVD(+) M−ANGII mice. Scale bars = 50 μm. (c) Renal lymphatic density in KidVD(−) M-ANGII and KidVD(+) M-ANGII mice as measured by podoplanin+ pixel density. (d) Gene expression changes in lymphatic vessel markers in kidneys from KidVD(−) M-ANGII and KidVD(+) M-ANGII. (e) Renal microvessel density in KidVD(−) M-ANGII and KidVD(+) M-ANGII mice measured by endomucin+ pixel density. (f) Immune cell populations expressed as percentage of CD45+ cells in kidneys of KidVD(−) M-ANGII and KidVD(+) M-ANGII mice as determined by flow cytometry. Results are expressed as mean ± SEM (n = 5 per group), and statistical analyses were performed with Student’s t test or one-way ANOVA. #0.05 < P < 0.1 vs. KidVD(−) M-ANGII mice; *P < 0.05 vs. KidVD(−) M-ANGII mice. Abbreviations: Ang II, angiotensin II; DOX, doxycycline.
Genetic augmentation of renal lymphatic vessel density prevents the development of A2HTN in female mice
Given the possible contribution of different immune mechanisms to hypertension and associated lymphangiogenesis in male and female mice, we determined if augmented renal lymphatic vessel density could prevent A2HTN in female mice. DOX was administered to female KidVD(−) and KidVD(+) mice for a week, following which these mice received DOX and Ang II for 3 weeks. Similar to males, KidVD(−) female mice developed hypertension, but KidVD(+) female mice did not develop A2HTN (Figure 5a). The expansion of renal lymphatic density in KidVD(+) females was confirmed by immunofluorescence for podoplanin (Figure 5b), podoplanin+ pixel quantification across kidney sections (Figure 5c; P < 0.001), and quantitative polymerase chain reaction for Vegfd, Lyve1, and Pdpn (Figure 5d; P < 0.01). KidVD(+) females also had significantly increased renal mRNA expression of Ccl21 (Figure 5d; P < 0.01). Quantification of endomucin+ pixel density revealed no changes in microvasculature density between these mice (Figure 5e). We hypothesized that the enhanced renal lymphatic density would aid in the exfiltration of immune cells. Indeed, similar to KidVD(+) males, KidVD(+) female mice had a significant reduction in renal CD11c+F4/80− monocytes (P < 0.05); however, there were no changes in the other immune cell populations analyzed (Figure 5f). Furthermore, mRNA levels of Tnfa, Il1b, Mcp1, and Il6 were normalized in Ang II-treated KidVD(+) female mice compared to wild-type mice given Ang II (Supplementary Figure 2d). As expected, although wild-type female mice given Ang II did not have significantly increased gene levels of Fn1 and Lcn2, they tended toward an increase, whereas mRNA levels of these renal injury markers were not elevated in KidVD(+) female mice (Supplementary Figure 3b). Thus, augmentation of renal lymphatic vessel density in female mice prevented the development of A2HTN in association with a reduction in renal CD11c+F4/80− monocytes.
Figure 5.
Genetic augmentation of renal lymphatic vessel density prevents the development of angiotensin II-induced hypertension in female mice: (a) Systolic blood pressure measures in KidVD(−) and KidVD(+) female mice infused with Ang II for 3 weeks (KidVD(−) F-ANGII and KidVD(+) F-ANGII). (b) Podoplanin immunofluorescence on kidney sections from KidVD(−) F-ANGII and KidVD(+) F-ANGII mice. Scale bars = 50 μm. (c) Renal lymphatic density in KidVD(−) F-ANGII and KidVD(+) F-ANGII mice as measured by podoplanin+ pixel density. (d) Gene expression changes in lymphatic vessel markers in kidneys from KidVD(−) F-ANGII and KidVD(+) F-ANGII. (e) Renal microvessel density in KidVD(−) F-ANGII and KidVD(+) F-ANGII mice measured by endomucin+ pixel density. (f) Immune cell populations expressed as percentage of CD45+ cells in kidneys of KidVD(−) F-ANGII and KidVD(+) F-ANGII mice as determined by flow cytometry. Results are expressed as mean ± SEM (n = 5 per group), and statistical analyses were performed with Student’s t test or one-way ANOVA. #0.05 < P < 0.1 vs. KidVD(−) F-ANGII mice; *P < 0.05 vs. KidVD(−) F-ANGII mice. Abbreviations: Ang II, angiotensin II; DOX, doxycycline.
DISCUSSION
In this study, we demonstrated that renal lymphatic vessel density and immune cell numbers are increased in kidneys of male and female mice with A2HTN. We also demonstrated that Ang II does not have direct effects on LECs because they lack Ang II receptors, but acts on immune cells, primarily CD11b+ cells, to induce the secretion of the lymphangiogenic growth factor VEGF-C, and this process is Ang II receptor dependent. In addition, CM from Ang II-activated immune cells induced the transcription of the lymphangiogenic growth factor receptor Vegfr3, adhesion molecules Icam and Vcam, and inflammatory markers Cxcl10 and Nos2 in LECs. Finally, we demonstrated that augmenting renal lymphatic density prevented the development of A2HTN in male and female mice, and in both cases, we observed a reduction in renal CD11c+F4/80− monocyte accumulation.
In general, female mice have a greater expression and activation of the nonclassical RAS (Ang type II receptor, Mas receptor, ACE2), which is thought to underlie their differential response to Ang II.24 The kidneys in particular might play an essential role in mediating these differences as kidney transplant from female to male mice attenuated Ang II-induced increases in BP.25 In our study, although associative, we noted differences in renal immune cell accumulation between males and females. Although males showed significant increases in T cell and CD11c+F4/80−, F4/80+CD11c−, and CD11c+F4/80+ monocytes after 3 weeks of Ang II, females only exhibited an increase in CD11c+F4/80− monocytes at this time point. Studies have demonstrated that females have attenuated renal T-cell accumulation and have been shown to be partially protected from the pro-hypertensive effects of T cells in A2HTN. The female hormonal milieu has been suggested to suppress T-cell mediated reactions, although this remains unclear. Once activated, monocytes secrete pro-inflammatory cytokines and activate T cells. Naïve T cells polarize to Th1 or Th17 cells, which in turn secrete cytokines and cause sodium retention and hypertension.26 Although Tnfa, Il1b, and Mcp1 gene expression in the kidneys was upregulated by 2 weeks of Ang II infusion in males, these cytokines were increased mostly only by the 3rd week in females. The mechanisms behind this discrepancy are currently unknown; nevertheless, our study further corroborates the existence of sex-specific regulation of immune mechanisms during A2HTN.
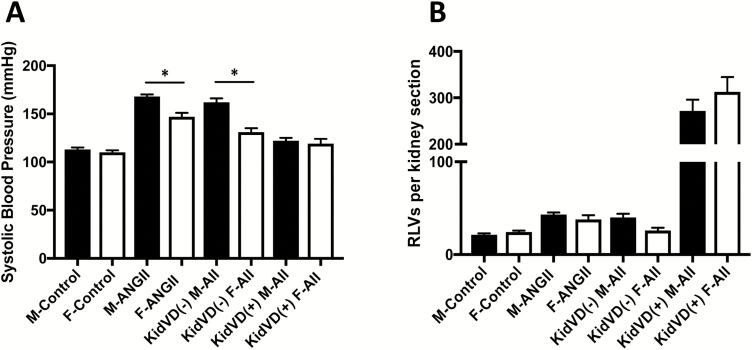
Regardless of the difference in pressor response to Ang II infusion, A2HTN male and female mice both had an almost 2-fold increase in the number of renal lymphatic vessels per artery (Figure 6), suggesting that the lymphangiogenesis is caused by other stimuli and not the BP itself. In the kidney, activated macrophages and tubular epithelial cells could secrete VEGF-C and contribute to lymphangiogenesis.27 As renal interstitial Ang II levels are several fold higher than that in the plasma,22 we determined whether Ang II has direct effects on LECs in vitro. The lack of changes in gene expression indicates that Ang II does not directly act on LECs, likely due to the absence of its receptors on LECs. However, CM from Ang II-stimulated splenocytes induced the transcription of Cxcl10, Nos2, Icam, and Vcam in LECs. Ang II has been shown to induce immune cell proliferation and their secretion of pro-inflammatory cytokines.28,29 When activated by these cytokines, LECs increase their expression of adhesion molecules and chemokines, and this has been shown to aid leukocyte transmigration across the lymphatic endothelium.30,31 In addition, LECs also increased their transcription of the VEGF-C receptor Vegfr3 when treated with Ang II CM. Inflammatory stimuli have been shown to induce VEGF-C secretion by immune cells, and hence, to identify whether Ang II stimulates immune cells to secrete VEGF-C, splenocytes and peritoneal CD11b+ cells were stimulated with Ang II in vitro. Treatment with Ang II induced VEGF-C secretion by both splenocytes and peritoneal CD11b+ cells. Inhibiting AT1R or both AT1R and AT2R, but not AT2R alone, on splenocytes diminished this response, whereas blocking either of the receptors or both prevented the increase in VEGF-C secretion by CD11b+ cells. Whether Ang II receptor stimulation directly induces VEGF-C secretion or activates the TGFβ or connective tissue growth factor pathway to induce VEGF-C will be examined.
Figure 6.
Sex-specific differences in blood pressure but not in renal lymphatic density in mice: (a) Systolic blood pressure measures after 3 weeks of saline or angiotensin II treatment in wild-type and KidVD male and female mice. (b) Renal interlobular lymphatic density as determined by mean number of LYVE-1+, lumen-containing lymphatic vessels (RLVs) per kidney section after 3 weeks of saline or angiotensin II treatment in wild-type and KidVD male and female mice. Results are expressed as mean ± SEM (n = 5–6 per group), and statistical analyses were performed with Student’s t test. *P < 0.05 vs. same treatment/genotype male mice. Abbreviations: Ang II, angiotensin II; RLV, renal lymphatic vessel.
Although inflammation-associated lymphangiogenesis is a common event in chronic inflammation, the consequences of these newly grown vessels seem to be tissue- and context-dependent.32,33 In the context of hypertension, our laboratory has previously demonstrated that renal inflammation induces lymphangiogenesis and that further augmenting renal lymphatic density prevents the development of nitric-oxide-inhibition-induced and salt-sensitive hypertension.19,20 Besides immune activation, sex differences may also exist in the regulation of lymphatic responses. Recently, it was shown that estradiol promotes the transcriptional upregulation of Vegfd, Vegfr3, and Lyve1, and confers a protective effect against lymphedema.34 Here, we report that females, but not males, have an increased gene expression of Vegfc in the kidney during Ang II infusion. Whether females have a more robust lymphangiogenic response to Ang II-induced renal inflammation and whether this underlies their different susceptibilities to Ang II-induced responses remain to be determined. Overall, these differences highlight the need to independently investigate BP regulation in both males and females. Nonetheless, augmenting renal lymphatic density using our genetic mouse model was able to prevent the development of A2HTN in both male and female mice. It should be noted that while the tail-cuff method is sufficient to detect large differences in BP as seen in this study, it would be beneficial to obtain more accurate BP values using the telemetry approach. The enhanced lymphatics could aid in the clearance of interstitial immune cells, as evidenced by reduced accumulation of renal CD11c+F4/80− monocytes in both males and females.
In conclusion, A2HTN induces an increase in renal lymphatic vessel density in male and female mice. Ang II induces secretion of the lymphangiogenic growth factor VEGF-C by activated immune cells in vitro and could potentially aid lymphangiogenesis. Further augmentation of renal lymphatic density beyond pathological levels prevents the development of A2HTN in males and females and might represent a novel mechanism of regulation of BP.
Supplementary Material
ACKNOWLEDGMENTS
We thank Andrea J. Reyna and Sheridan Barajas for their assistance with animal husbandry. This work was funded by an American Heart Association Predoctoral Fellowship (#18PRE33990461) to D.B., and an American Heart Association Transformational Project Award (18TPA34170266) and National Institutes of Health RO1 (DK120493) to B.M.M. J.M.R. is supported by an American Heart Association Grant in Aid (17GRNT33671220).
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Whelton PK, Carey RM, Aronow WS, CaseyDE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, SmithSC, Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, WilliamsKA, Sr, Williamson JD, WrightJT, Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension 2018; 71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 2. Case DB, Wallace JM, Keim HJ, Weber MA, Sealey JE, Laragh JH. Possible role of renin in hypertension as suggested by renin-sodium profiling and inhibition of converting enzyme. N Engl J Med 1977; 296:641–646. [DOI] [PubMed] [Google Scholar]
- 3. Boynton RE, Todd RL. Blood pressure readings of 75,258 university students. Arch Intern Med (Chic) 1947; 80:454–462. [DOI] [PubMed] [Google Scholar]
- 4. Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension 2015; 65:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, MohlerER, III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee ; Stroke Statistics Subcommittee. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 2016; 133:e38–360. [DOI] [PubMed] [Google Scholar]
- 6. Braam B, Taler SJ, Rahman M, Fillaus JA, Greco BA, Forman JP, Reisin E, Cohen DL, Saklayen MG, Hedayati SS. Recognition and management of resistant hypertension. Clin J Am Soc Nephrol 2017; 12:524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2010; 298:R1136–R1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sommers SC, Relman AS, Smithwick RH. Histologic studies of kidney biopsy specimens from patients with hypertension. Am J Pathol 1958; 34:685–715. [PMC free article] [PubMed] [Google Scholar]
- 10. De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 2005; 25:2106–2113. [DOI] [PubMed] [Google Scholar]
- 11. Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A, Drummond GR. Obligatory role for B cells in the development of angiotensin II-dependent hypertension. Hypertension 2015; 66:1023–1033. [DOI] [PubMed] [Google Scholar]
- 12. Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep 2017; 21:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satou R, Penrose H, Navar LG. Inflammation as a regulator of the renin-angiotensin system and blood pressure. Curr Hypertens Rep 2018; 20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 2014; 64:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 2014; 64:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abouelkheir GR, Upchurch BD, Rutkowski JM. Lymphangiogenesis: fuel, smoke, or extinguisher of inflammation’s fire? Exp Biol Med (Maywood) 2017; 242:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balasubbramanian D, Lopez Gelston CA, Rutkowski JM, Mitchell BM. Immune cell trafficking, lymphatics and hypertension. Br J Pharmacol 2019; 176:1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yazdani S, Navis G, Hillebrands JL, van Goor H, van den Born J. Lymphangiogenesis in renal diseases: passive bystander or active participant? Expert Rev Mol Med 2014; 16:e15. [DOI] [PubMed] [Google Scholar]
- 19. Kneedler SC, Phillips LE, Hudson KR, Beckman KM, Lopez Gelston CA, Rutkowski JM, Parrish AR, Doris PA, Mitchell BM. Renal inflammation and injury are associated with lymphangiogenesis in hypertension. Am J Physiol Renal Physiol 2017; 312:F861–F869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopez Gelston CA, Balasubbramanian D, Abouelkheir GR, Lopez AH, Hudson KR, Johnson ER, Muthuchamy M, Mitchell BM, Rutkowski JM. Enhancing renal lymphatic expansion prevents hypertension in mice. Circ Res 2018; 122:1094–1101. [DOI] [PubMed] [Google Scholar]
- 21. Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, Lee S, Park SK, Lee SY, Kang MJ, Moon WS, Kim HJ, Jeong YB, Sung MJ, Kim W. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int 2013; 83:50–62. [DOI] [PubMed] [Google Scholar]
- 22. Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 2002; 39:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lammoglia GM, Van Zandt CE, Galvan DX, Orozco JL, Dellinger MT, Rutkowski JM. Hyperplasia, de novo lymphangiogenesis, and lymphatic regression in mice with tissue-specific, inducible overexpression of murine VEGF-D. Am J Physiol Heart Circ Physiol 2016; 311:H384–H394. [DOI] [PubMed] [Google Scholar]
- 24. Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens 2018; 31:1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang L, Wang X, Qu HY, Jiang S, Zhang J, Fu L, Buggs J, Pang B, Wei J, Liu R. Role of kidneys in sex differences in angiotensin II-induced hypertension. Hypertension 2017; 70:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Norlander AE, Madhur MS. Inflammatory cytokines regulate renal sodium transporters: how, where, and why? Am J Physiol Renal Physiol 2017; 313:F141–F144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki Y, Ito Y, Mizuno M, Kinashi H, Sawai A, Noda Y, Mizuno T, Shimizu H, Fujita Y, Matsui K, Maruyama S, Imai E, Matsuo S, Takei Y. Transforming growth factor-beta induces vascular endothelial growth factor-C expression leading to lymphangiogenesis in rat unilateral ureteral obstruction. Kidney Int 2012; 81:865–879. [DOI] [PubMed] [Google Scholar]
- 28. Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest 1999; 104:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hahn AW, Jonas U, Bühler FR, Resink TJ. Activation of human peripheral monocytes by angiotensin II. FEBS Lett 1994; 347:178–180. [DOI] [PubMed] [Google Scholar]
- 30. Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med 2006; 203:2763–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem 2007; 55:721–733. [DOI] [PubMed] [Google Scholar]
- 32. Huggenberger R, Siddiqui SS, Brander D, Ullmann S, Zimmermann K, Antsiferova M, Werner S, Alitalo K, Detmar M. An important role of lymphatic vessel activation in limiting acute inflammation. Blood 2011; 117:4667–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, Laakkonen P, Petrova T, Langer B, Raab I. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 2004; 15:603–612. [DOI] [PubMed] [Google Scholar]
- 34. Morfoisse F, Tatin F, Chaput B, Therville N, Vaysse C, Métivier R, Malloizel-Delaunay J, Pujol F, Godet AC, De Toni F, Boudou F, Grenier K, Dubuc D, Lacazette E, Prats AC, Guillermet-Guibert J, Lenfant F, Garmy-Susini B. Lymphatic vasculature requires estrogen receptor-α signaling to protect from lymphedema. Arterioscler Thromb Vasc Biol 2018; 38:1346–1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.