Abstract
Introduction
Brain metastases are common in metastatic melanoma and radiosurgery is often utilized for local control. Immune checkpoint inhibitors (CPIs) play a central role in contemporary melanoma management; however, there is limited data exploring outcomes and potential toxicities for patients treated with CPIs and radiosurgery.
Methods
We retrospectively identified all consecutive cases of newly diagnosed melanoma brain metastases (MBM) treated with Gamma Knife radiosurgery at a single institution between 2012 and 2017, and included only patients that initiated CPIs within 8 weeks before or after radiosurgery.
Results
Thirty-eight patients were included with a median follow-up of 31.6 months. Two-year local control was 92%. Median time to out-of-field CNS and extra-CNS progression were 8.4 and 7.9 months, respectively. Median progression-free survival (PFS) was 3.4 months and median overall survival (OS) was not reached (NR). Twenty-five patients (66%) received anti-CTLA4 and 13 patients (34%) received anti-PD-1+/−anti-CTLA4. Compared with anti-CTLA4, patients that received anti-PD-1+/−anti-CTLA4 had significant improvements in time to out-of-field CNS progression (p = 0.049), extra- CNS progression (p = 0.015), and PFS (p = 0.043), with median time to out-of-field CNS progression of NR vs. 3.1 months, median time to extra-CNS progression of NR vs. 4.4 months, and median PFS of 20.3 vs. 2.4 months. Six patients (16%) developed grade ≥ 2 CNS toxicities (grade 2: 3, grade 3: 3, grade 4/5: 0).
Conclusions
Excellent outcomes were observed in patients that initiated CPIs within 8 weeks of undergoing radiosurgery for newly diagnosed MBM. There appears to be an advantage to anti-PD-1 or combination therapy compared to anti-CTLA4.
Keywords: Melanoma, Brain metastases, Radiosurgery, Immune checkpoint inhibitors, Anti-PD-1, Anti-CTLA4
Introduction
Brain metastases are a frequent occurrence in patients with metastatic melanoma, with historically poor outcomes [1]. More recently, the evolution of systemic therapy has resulted in improved survival [2, 3]. Patients with tumors harboring a BRAF mutation have benefited from the introduction of targeted agents against BRAF and MEK, [4–7] and central to the topic of this study, immune checkpoint inhibitors (CPIs) have dramatically improved survival for some patients [8–14]. Consistent with the findings of these landmark studies, there has been a rise in the use of immunotherapy between 2006 and 2015 with a commensurate decline in the use of cytotoxic chemotherapy in these patients [3]. Importantly, CPIs have documented activity in the central nervous system (CNS), [15–17] and emerging data suggest outcomes after radiosurgery might be improved in patients receiving CPIs [18–24]. Therefore, we reviewed our experience of patients with newly diagnosed melanoma brain metastases managed with CPIs and radiosurgery.
Materials and methods
We retrospectively reviewed the records of consecutive patients treated with Gamma Knife radiosurgery for melanoma brain metastases at a single institution between January 2012 and August 2017 to identify those meeting study inclusion criteria. Inclusion criteria were: (1) first presentation of melanoma brain metastases (patients with prior history of brain metastases were excluded) managed with radiosurgery, and (2) initiation of CPI within 8 weeks of radiosurgery. Patients that developed brain metastases while already receiving CPIs were excluded. Patients treated in a planned staged radiosurgery approach were also excluded from analysis for this study. Patients with progressive extracranial disease on the most recent cross-sectional imaging at the time of radiosurgery were categorized as having uncontrolled extracranial disease, while other disease states including stable disease, were categorized as controlled extracranial disease. Toxicity was scored according to the National Cancer Institute Common Toxicity Criteria for Adverse Events version 4.03 (NCI-CTCAE) [25].
Fisher’s exact tests were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) and were used to assess for associations between categorical variables (age, sex, KPS, BRAF mutation status, systemic disease status, number and volume of treated metastases) and class of CPI received. Graphpad Prism version 7.03 (GraphPad Software Inc., La Jolla, CA) was used for Kaplan–Meier survival analysis with log-rank comparison and for creation of the figures. The reverse Kaplan–Meier method was used for calculation of median follow-up [26].
Results
Thirty-eight patients meeting our pre-specified inclusion criteria were identified with a median follow-up of 31.6 months. Twenty-one patients (55%) were under 70 years old, 24 patients (63%) were male, and the majority of patients (82%) had a Karnofsky Performance Status (KPS) of 90–100. Twelve patients (32%) had tumors with a BRAF mutation. Twenty-six patients (68%) had uncontrolled extracranial disease. Twenty-four patients (63%) were treated to three or less brain metastases and 14 patients (37%) were treated to four or more brain metastases (maximum: 12). Twenty-two patients (58%) were treated to a total volume of brain metastases of less than 1 cc, and 16 patients (42%) were treated to a total volume of brain metastases of 1 cc or more. Twenty-five patients (66%) received anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) agents alone and 13 patients (34%) received anti-programmed cell death protein-1 (PD1) alone (n = 9) or combination anti-PD-1 and anti-CTLA4 therapy (n = 4). There were no significant baseline differences between groups receiving anti-CTLA4 agents alone or anti-PD-1 with or without anti-CTLA4. (Table 1).
Table 1.
Patient characteristics
| Variable | All patients | Anti-PD-1 ± anti-CTLA4 | Anti-CTLA4 | p value |
|---|---|---|---|---|
| N = 38 | N = 13 | N = 25 | ||
| Age | 0.689 | |||
| < 70 | 21 (55%) | 11 (85%) | 19 (76%) | |
| ≥ 70 | 17 (45%) | 2 (15%) | 6 (24%) | |
| Sex | 1.00 | |||
| Female | 14 (37%) | 5 (38%) | 9 (36%) | |
| Male | 24 (63%) | 8 (62%) | 16 (64%) | |
| KPS | 0.672 | |||
| ≤ 70 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 80 | 7 (18%) | 3 (23%) | 4 (16%) | |
| ≥ 90 | 31 (82%) | 10 (77%) | 21 (84%) | |
| BRAF mutation | 0.270 | |||
| No | 26 (68%) | 7 (54%) | 19 (76%) | |
| Yes | 12 (32%) | 6 (46%) | 6 (24%) | |
| Uncontrolled extracranial disease | 0.270 | |||
| No | 12 (32%) | 6 (46%) | 6 (24%) | |
| Yes | 26 (68%) | 7 (54%) | 19 (76%) | |
| Number of treated brain metastases | 1.00 | |||
| 1–3 | 24 (63%) | 8 (62%) | 16 (64%) | |
| ≥4 | 14 (37%) | 5 (38%) | 9 (36%) | |
| Volume of treated brain metastases | 0.742 | |||
| < 1 cc | 22 (58%) | 7 (54%) | 15 (60%) | |
| ≥ 1 cc | 16 (42%) | 6 (46%) | 10 (40%) |
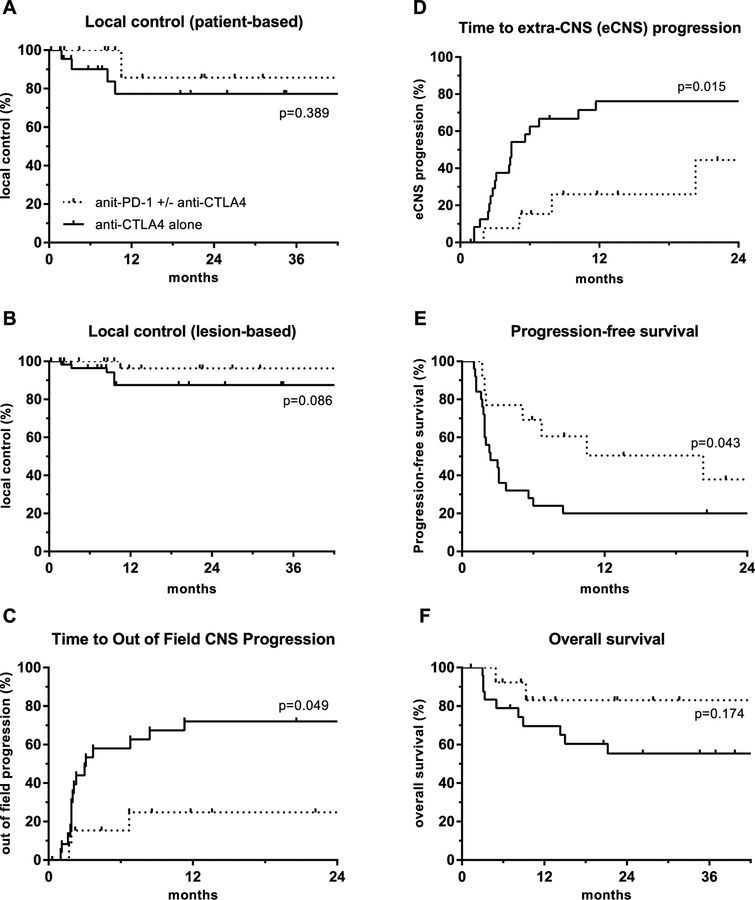
For the entire cohort, 2-year patient-based and lesion-based local control were 81% and 92%, respectively. The median time to out-of-field CNS and extra-CNS (eCNS) progression were 8.4 and 7.9 months, respectively. Median progression-free survival (PFS) was 3.4 months and median overall survival (OS) was not reached (NR). (Fig. 1). Seventeen of 38 patients (45%) received salvage CNS therapies after initial radiosurgery which are detailed in supplemental table 1. Compared with anti-CTLA4, patients that received anti-PD-1 or combination therapy had improved time to out-of-field CNS progression (median NR vs. 3.1 months), time to eCNS progression (median NR vs. 4.4 months), and PFS (median 20.3 vs. 2.4 months). These measures were significantly different between patients that received anti-CTLA4 and patients that received anti-PD-1 or combination therapy: out-of-field CNS progression (p = 0.049), eCNS progression (p = 0.015), and PFS (p = 0.043). There was a trend towards improved OS (p = 0.174) (Fig. 2).
Fig. 1.
Kaplan-meier analysis of a patient-based local control, b lesion-based local control, c out of field central nervous system (CNS) progression, d extra-CNS (eCNS) progression, e progression-free survival, and f overall survival, for patients receiving immune checkpoint inhibitors within 8 weeks of stereotactic radiosurgery
Fig. 2.
Kaplan-meier analysis of a patient-based local control, b lesion-based local control, c out of field central nervous system (CNS) progression, d extra-CNS (eCNS) progression, e progression-free survival, and f overall survival, for patients receiving immune checkpoint inhibitors within 8 weeks of stereotactic radiosurgery, stratified by class of immune checkpoint inhibitor
We also explored the impact of timing of CPIs with radiosurgery. Outcomes were compared for patients that received an infusion of CPI within 7 days before or after radiosurgery with patients receiving CPIs outside of that window. As shown in supplemental table 2, there were no significant baseline differences between these groups. PFS and OS were not significantly different for patients that received an infusion of CPI within 7 days before or after radiosurgery (p = 0.072 and p = 0.065, respectively). Yet, we observed trends towards improved PFS (median 7.3 vs. 2.2 months) and OS (median NR vs. 21.2 months) (Supplemental Fig. 1).
Radiosurgery and immune checkpoint inhibition was well tolerated overall. Three patients (8%) experienced grade two CNS toxicities (hemorrhagic conversion of treated brain metastases in all three patients). Three patients (8%) experienced grade three CNS toxicities (radiation necrosis which was confirmed on final pathology in all three patients) (Table 2). An example of the progressive radiographic changes observed in these patients is shown in supplemental Fig. 2. Grade three CNS toxicities were observed only in patients receiving anti-CTLA4 agents. There were no recorded grade four or five CNS toxicities.
Table 2.
Details of grade 2 or higher central nervous system (CNS) toxicities
| NCI CTCAE v4.03 grade | Toxicity | N (%) | Class of immune checkpoint inhibitor |
|---|---|---|---|
| Grade 2 | Hemorrhagic conversion of treated metastases | 3 (8%) | Anti-CTLA4:0 |
| Anti-PD-1:1 | |||
| Anti-PD-1 + anti-CTLA4: 2 | |||
| Grade 3 | Radiation necrosis | 3 (8%) | Anti-CTLA4: 3 |
| Anti-PD-1: 0 | |||
| Anti-PD-1 + anti-CTLA4: 0 | |||
| Grade 4 | N/A | 0 (0%) | N/A |
| Grade 5 | N/A | 0 (0%) | N/A |
Discussion
The landscape of systemic therapy for metastatic melanoma has undergone tremendous evolution over the last several years. Between BRAF and MEK targeted therapies and immune checkpoint inhibition, there has been an unprecedented improvement in outcomes from this disease [4–14]. Nevertheless, brain metastases remain common, and radiosurgery continues to be an important part of the management strategy for CNS disease in patients with melanoma brain metastases. In melanoma radiosurgery series from the era prior to targeted agents and immunotherapy, median OS for patients with brain metastases was on the order of a few months with only about 25% of melanoma patients surviving 1 year from radiosurgery [27–31]. Importantly, in our series of patients treated between 2012 and 2017 with CPIs and radiosurgery, median OS was not reached at a median follow-up of 31.6 months. Similarly, in the updated prognostic assessment for melanoma patients with brain metastases by Sperduto et al, the median OS for patients with the most favorable prognostic features was 34 months [2].
Our study focused on overall outcomes and toxicities for patients treated with CPIs and radiosurgery, and on the comparison between patients receiving anti-CTLA4 and those receiving anti-PD-1 or combination therapy. Therefore, we did not include a comparison to patients that were managed with radiosurgery without CPIs. Other groups have addressed this question and have demonstrated that receipt of CPIs and CNS radiation is associated with improved outcomes compared with CNS radiation alone [22–24]. Silk et al retrospectively compared outcomes for 33 patients that received ipilimumab and CNS radiation with 37 patients that received CNS radiation without ipilimumab. Median OS was 18.3 months in the ipilimumab group and 5.3 months in the no ipilimumab group with a hazard ratio for OS of 0.43 (p = 0.005) [23]. Similarly, Knisely et al compared 27 patients that received ipilimumab and CNS radiation to 50 patients that received CNS radiation without ipilimumab. Median OS was 21.3 months in the ipilimumab group and 4.9 months in the no ipilimumab group (p = 0.044) [22]. Finally, Stokes et al utilized the National Cancer Database to address this question and found that among 1287 patients with melanoma brain metastases treated with CNS radiation, 185 also received immunotherapy, and receipt of immunotherapy was found to be associated with improved overall survival (HR 0.57, 95% CI 0.47–0.70, p < 0.01) [24].
Again one of the main aims of our study was to evaluate outcomes for patients that received anti-PD-1 or combination anti-PD-1 and CTLA4 compared with patients that received only drugs targeting CTLA4. We found that anti-PD-1 or combination immune checkpoint inhibition was associated with superior time to out-of-field CNS progression, time to eCNS progression, and PFS, with trends towards improved local control and OS. Interestingly, as a possible mechanism to support our clinical findings of improved outcomes with PD-1 blockade, preclinical studies have shown that radiation upregulates PD-L1 and can synergize with anti-PD-L1 therapy [32, 33]. Further, our clinical findings are consistent with those previously reported by other groups [18, 20]. Ahmed et al presented the results of 96 patients that were treated with radiosurgery for melanoma brain metastases within 3 months of receiving systemic therapy, including anti-PD-1, anti-CTLA4, BRAF/MEK inhibitors, or conventional chemotherapy. Distant brain metastases control was worse on multivariate analysis for patients receiving chemotherapy compared to anti-PD-1 (HR 3.1, 95% CI 1.5–6.6, p = 0.001) but not for chemotherapy compared to anti-CTLA4 (HR 1.4, 95% CI 0.76–2.7, p = 0.26) [18]. Choong et al reviewed the outcomes of 108 patients with melanoma brain metastases treated with radiosurgery and anti-CTLA4, anti-PD-1, or BRAF+/−MEK inhibitors between 2010 and 2015, and found superior median brain control in patients receiving anti-PD-1 compared to anti-CTLA4 (12.7 vs. 7.5 months). Median OS was also longer in patients receiving anti-PD-1 compared to anti-CTLA4 (20.4 vs. 7.5 months) [20].
In addition to a comparison of anti-PD-1 and anti-CTLA4, we also set out to examine the impact of the timing of radiosurgery and receipt of immune checkpoint inhibition on outcomes. In one prior study by Ahmed et al. [19], of 26 patients treated with radiosurgery and the anti-PD-1 agent, nivolumab, on univariate cox regression for overall survival there was not a statistically significant difference between patients that were treated with radiosurgery before or during nivolumab compared with those that underwent radiosurgery after nivolumab (HR 0.71, 95% CI 0.16–2.24, p = 0.58). However, Kiess et al, in their study of ipilimumab and radiosurgery, showed that patients that received radiosurgery before or during ipilimumab had improved overall survival and out-of-field recurrence-free survival compared with patients that received radiosurgery greater than one month after the last infusion of ipilimumab [21]. As a result of the selection criteria for our study, our cohort was limited to the better performing arms of the study by Kiess etal (radiosurgery before or during treatment with CPI), and thus the excellent outcomes observed in our cohort appear to support their findings. We further aimed to determine if we could identify an optimal timing for radiosurgery with infusion of CPI within our cohort. We observed trends towards superior PFS and OS in patients that received infusion of a CPI within 7 days before or after radiosurgery compared with patients receiving CPIs outside of this window. Although these findings did not reach statistical significance, possibly due to sample size, the magnitude of difference is observed is notable.
Beyond efficacy, it is also important to note that the toxicity profile for patients in our study was acceptable. The overall rate of grade 2 or higher CNS toxicity was 16% (8% grade 2, 8% grade 3, and no grade 4 or 5 CNS toxicities). Many of the previously cited studies also reported toxicities for comparison. Silk et al reported comparable toxicities in their ipilimumab group in respect to their comparison cohort, with only 3.9% of patients experiencing intratumoral hemorrhage in the ipilimumab group and no radiation necrosis in the ipilimumab group [23]. In the study of 26 patients by Ahmed et al that received anti-PD-1 and radiosurgery, only one patient experienced grade 2 headache managed with steroids. There were no other reported toxicities [19]. Kiess et al, however, do report that 15.2% of the 46 patients in their study of radiosurgery and ipilimumab experienced grade 3 or 4 CNS toxicities,[21] higher than the rates of grade ≥ 3 CNS toxicity we observed. One possible explanation for this variation is that their study included only anti-CTLA4, whereas 24% of our cohort received anti-PD-1 alone. Importantly, all three grade 3 toxicities in our study were observed in patients that received anti-CTLA4. We did not observe any grade 3 toxicities in patients that received anti-PD-1.
Importantly, our study does have several salient limitations. This is a retrospective study and the conclusions are limited by selection bias inherent to studies of this nature. Further, while the number of patients included in our analysis is comparable to existing published series, the overall cohort size is nonetheless small, and from a single institution, further limiting the potential generalizability of our findings. Despite these limitations, this study adds to the emerging body of evidence supporting a benefit to immune checkpoint inhibition and radiosurgery for melanoma brain metastases. Further, we observed a benefit to anti-PD-1 therapy compared to anti-CTLA4, which is concordant with the limited existing data on this topic. Additional studies exploring this paradigm, with a specific emphasis on class of CPI and timing with radiosurgery, are warranted.
Supplementary Material
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest Karl Lewis: Consultant: Roche, Genentech. David Raben: Consultant: Astra Zeneca; Advisory Boards: Merck and EMD Serono and Genentech. All remaining authors have declared no conflicts of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11060-018-2930-5) contains supplementary material, which is available to authorized users.
References
- 1.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J (2011) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, Shih HA, Bangdiwala A, Shanley R, Lockney NA (2017) Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA). Int J Rad Oncol Biol Phys 99:812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, Shih HA, Bangdiwala A, Shanley R, Lockney NA (2017) The prognostic value of BRAF, cKIT and NRAS mutations in melanoma patients with brain metastases. Int J Rad Oncol* Biol* Phys 98(5): 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauschild A, Grob J-J, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Kaempgen E (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380:358–365 [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, De Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob J-J (2015) Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444–451 [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L (2015) Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30–39 [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O’day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD (2015) Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16:908–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330 [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB (2012) Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med 2012: 2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (Check-Mate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384 [DOI] [PubMed] [Google Scholar]
- 14.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, Marasco A, Rivoltini L, Simeone E, Nicoletti SV (2012) Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. lancet Oncol 13:879–886 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L (2016) Pembrolizumab for patients with melanoma or nonsmall-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, Wolchok JD, Clark JI, Sznol M, Logan TF (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13:459–465 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed K, Abuodeh Y, Echevarria M, Arrington J, Stallworth D, Hogue C, Naghavi A, Kim S, Kim Y, Patel B (2016) Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol 27:2288–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed K, Stallworth D, Kim Y, Johnstone P, Harrison L, Caudell J, Yu H, Etame A, Weber J, Gibney G (2015) Clinical outcomes of melanoma brain metastases treated with stereotactic radiation and anti-PD-1 therapy. Ann Oncol 27:434–441 [DOI] [PubMed] [Google Scholar]
- 20.Choong ES, Lo S, Drummond M, Fogarty GB, Menzies AM, Guminski A, Shivalingam B, Clarke K, Long GV, Hong AM (2017) Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer 75:169–178 [DOI] [PubMed] [Google Scholar]
- 21.Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, Chan TA, Yamada Y, Beal K (2015) Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Rad Oncol* Biol* Phys 92:368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL (2012) Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 117:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD (2013) Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes WA, Binder DC, Jones BL, Oweida AJ, Liu AK, Rusthoven CG, Karam SD (2017) Impact of immunotherapy among patients with melanoma brain metastases managed with radiotherapy. J Neuroimmunol 313:118–122 [DOI] [PubMed] [Google Scholar]
- 25.(2010) Common Terminology Criteria for Adverse Events (CTCAE), v4.03. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute [Google Scholar]
- 26.Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–346 [DOI] [PubMed] [Google Scholar]
- 27.Chang EL, Selek U, Hassenbusch SJ III, Maor MH, Allen PK, Mahajan A, Sawaya R, Woo SY (2005) Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery 56:936–945 [PubMed] [Google Scholar]
- 28.Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, Hwu P, Bedikian A (2011) Prognostic factors for survival in melanoma patients with brain metastases. Cancer 117:1687–1696 [DOI] [PubMed] [Google Scholar]
- 29.Liew DN, Kano H, Kondziolka D, Mathieu D, Niranjan A, Flickinger JC, Kirkwood JM, Tarhini A, Moschos S, Lunsford LD (2011) Outcome predictors of Gamma Knife surgery for melanoma brain metastases. J Neurosurg 114:769–779 [DOI] [PubMed] [Google Scholar]
- 30.Mori Y, Kondziolka D, Flickinger JC, Kirkwood JM, Agarwala S, Lunsford LD (1998) Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Rad Oncol Biol Phys 42:581–589 [DOI] [PubMed] [Google Scholar]
- 31.Selek U, Chang EL, Hassenbusch SJ, Shiu AS, Lang FF, Allen P, Weinberg J, Sawaya R, Maor MH (2004) Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Rad Oncol* Biol* Phys 59:1097–1106 [DOI] [PubMed] [Google Scholar]
- 32.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu Y-X (2014) Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM (2015) Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.